Abstract
Emergency department (ED) lenght of stay of the patients requiring admission to the intensive care units has increased gradually in recent years. Mechanical ventilation is an integral part of critical care and mechanically ventilated patients have to be managed and monitored by emergency physicians for longer than expected in EDs. This early period of care has significant impact on the outcomes of these patients. Therefore, emergency physicians should have comprehensive knowledge of mechanical ventilation. This review will summarize the current literature of the basic concepts, appropriate clinical applications, monitoring parameters, components and mechanisms of mechanical ventilation in the ED.
Keywords: Mechanical ventilation, Emergency department, Mechanical ventilation parameters, Mechanical ventilation modes, Critical care
1. Introduction
Mechanical ventilation (MV) is a procedure that should be applied in intensive care units (ICU) where the patients will be strictly monitored by experienced teams. However, many patients in need of intensive care are obliged to be monitored outside of the ICUs. In the USA, the patients admitted to the ICUs between 2008 and 2009 increased by 48.8% compared to the past six years.1 Annually over 240,000 patients in the USA needs mechanical ventilation in the emergency department (ED) which is 0.23% of all ED admissions. A one-quarter of these patients remain in the ED for more than 5 hours, and their mortality is significantly higher than the other patients admitted to the ICU.2 In Japan, it is reported that 46.4% of patients3 requiring MV are monitored in non-ICU settings. EDs is one of these places that frequently monitor mechanically ventilated patients. The increase of the length of stay in the ED leads to an increase in mortality and MV duration of the patients.4,5
The number of EDs with special care areas such as critical care units or ED intensive care units (ED-ICUs) for treating critically ill patients are increasing. Even though, these areas are uncommon, they should be properly equipped and staffed when available6 because inadequacy of these units is an important problem which negatively affects the long-term outcome of critically ill patients. For instance, acute lung injury has been reported in %9.5 of the non-trauma patients that MV is applied to in the EDs.7 However, the lung protective strategy is rarely being used and the tidal volumes applied to patients are highly variable.8 Hyperoxemia incidence of the MV-initiated patients in the ED is reported as %43.5, and the mortality is increased in these patients.9
In conclusion, emergency physicians are obliged to have the required skill set to follow patients in MV, even if it is short-term, because it affects the patient outcome. Therefore, all emergency physicians should be well informed about MV settings and their monitoring. This review will summarize the current literature of the basic concepts, appropriate clinical applications, monitoring parameters, components and mechanisms of MV in ED. However, even if its basic application is not changed, the use of MV and monitoring of the patient can vary in line with the facilities of the given ED existing equipment, and experience of the team. In accordance with a protocol, it is reported that monitoring of the patients in MV positively affects clinical outcomes.10 Therefore, EDs should consider using a mechanical ventilator protocol designed for mechanically ventilated patients in each hospital.
2. Features of mechanical ventilators
Although mechanical ventilators are generally classified as negative and positive pressure ventilators, negative pressure ventilators are rarely used today. The first positive pressure ventilators appeared in the 1940s. However, they began to become widespread after the reduction of mortality in mechanically ventilated patients in the polio epidemics of the 1950s.11 The developments in engineering enabled the switch from the devices that initially only guaranteed the set tidal volume at a certain respiratory rate to the devices used today, which monitor the patient's condition and respiratory dynamics and adjust the respiratory parameters to the patient's needs.
Today, physicians use a wide range of devices in the hospital and pre-hospital settings. Considering the purposes of use and characteristics, we can classify the devices we use in the invasive mechanical ventilation in the EDs in three groups;
2.1. Manual ventilators
Manual ventilation is usually performed with a balloon valve mask in EDs, also anesthetic type breathing circuit can be used in practice. It is frequently used during the transfer to the hospital and during the transport from the EDs to the ICUs and diagnostic procedures. Complications such as hyperventilation and respiratory alkalosis might occur during manual ventilation,12 and it might cause lung damage due to excessive ventilation.13
2.2. Portable ventilators
These are the preferred instruments for the transport of mechanically ventilated patients. Portable devices can be examined in three groups; [1] automatic resuscitator; typically gas powered, provides a set breathing frequency and pressure, and the only alarm is for high pressure; [2] simple portable ventilator: they have partial security systems, and the tidal volume can be adjusted; [3] sophisticated portable ventilator; these are portable ventilators with different modes and monitoring features and allow spontaneous breathing.14
The connections of the portable ventilators are often simple. Reusable circuits or disposable circuits are available for use. With reusable circuits, a filter change is needed in each patient. Despite the convenient use of the portable ventilators during transports, they operate with a battery which is an important handicap and it should be checked before transport and a balloon valve mask should be available. These devices are often used with oxygen tubes. In addition to the patient's needs during their use, there must be enough oxygen supply to provide the bias flow through the breathing circuit and to control the ventilator cycle itself.15
2.3. Intensive care ventilators
These devices, which were initially only volume-controlled and could not able to detect the triggered breaths of the patients, did not have a monitor or alarm. In the second-generation devices, the trigger of the patients was possible, and some parameters such as respiratory rate could be monitored. Shortly after, intermittent positive pressure ventilation (IMV) began to be used, and pressure-controlled and pressure-assisted ventilation was used in clinical practice over time. Third generation devices using microprocessors were produced with technological developments. Flow triggering was introduced in these devices, gas delivery, and monitoring was possible, and synchronizedintermittent mechanical ventilation (SIMV) was applied, and pressure assistance could be given. Today's complex and versatile devices are known as fourth generation devices, and the most important feature of these devices is that a wide variety of modes have been brought into use by companies.16
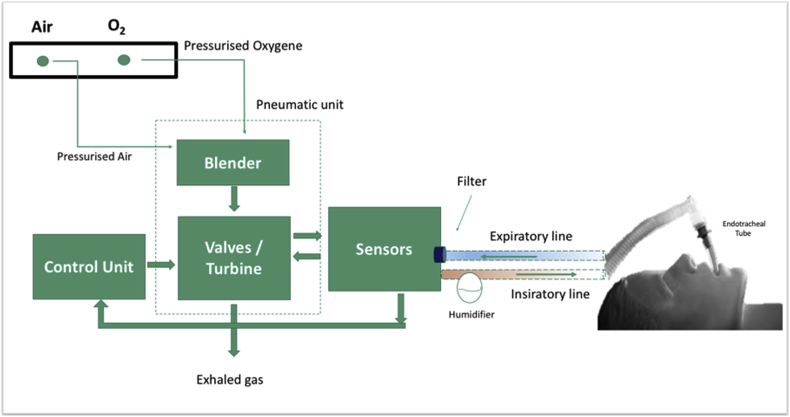
Current ventilators roughly consist of a control unit, monitor and alarm/warning panels and most of them are based on closed-loop circuit control. The initiator of inspiration might be the pressure or flow in the respiratory tract, whereas the terminator is one of the factors such as set pressure, volume, time and flow. At the end of the inspiration, the gas flow stops, and the expiratory phase begins. The monitoring characteristics of the devices and the complexity of the modes particular to the companies are generally variable. By means of the use of microprocessors with technological advances, these complex modes and monitors, which are developed for safer breathing of the patients, increasing patient-device compliance, better gas exchange and facilitating the work of the users, the basic equipment and connections of the devices are similar although it might be confusing for new users (Fig. 1). The devices cycles are generally classified from inspiratory phase to expiratory phase rotation methods; The machine's signal to terminate the inspiratory activity is either a preset volume (volume-cycled ventilation), a predetermined pressure limit (pressure-cycled ventilation) or a preset time factor (time-cycled ventilation).
Fig. 1.
The mechanical structure and connections of a mechanical ventilator (Adopted from Ref. 11).
3. Mechanical ventilator connections and basic parameters
The breathing circuits that connect the patient with the mechanical ventilator need their own sensor and are therefore they can be suitable for brands or general use. For this reason, physicians need to get acquainted of the connections of the device and the additional consumable materials needed. Ventilator circuits are generally single-use and dual-limb. Especially in portable devices, reusable single-limb circuits requires a filter change. Respiratory system filters can be of bacterial filters, heat and moisture exchangers or combined type and are important not only for holding the bacteria but also for particulates so as to ensure safer breathing of the patient and protect the device, personnel and of course the patient. These can be attached to the expiratory inlet of the device to prevent the contamination of the device, to the inspiratory outlet to prevent infection of the patient with contaminated gas from the machine or to the Y line of the breathing circuit and to the inlet of the pressured gas source to prevent both. Although there are studies on the effectiveness of the use of filters to protect circuits from bacterial contamination, there is no evidence that the use of filters protects from ventilator-associated pneumonia (VAP). For this reason, the recommendations on the use of filters vary between countries. British Society for Antimicrobial Chemotherapy recommends the use of appropriate filters to protect circuits from bacterial contamination and following of the national guidelines with regard to the use of expiratory filters for patients suffering from highly communicable infections.17
Humidification of the gas inspired by mechanical ventilation is standard in practice. This can be done actively or passively. In active humidification, the inspiration air is passed through or above a heated water bath. In passive humidification, the humidity taken from the patient in expiration is given back to the patient in inspirium. For this purpose, heat and moisture exchangers (HMEs) with bacteria filter feature are connected between the intubation tube and the Y connection of the breathing circuit which can be used safely without being changed to 48 h. The incidence of VAP is reported to be less in passive humidification than the active humidification.18 Using semi-recumbent position (30-45°) after the patient connected to the mechanical ventilator is another method to prevent VAP. This position must also be maintained during the transfer of the patient.
The parameters set on the control panel vary according to the breathing pattern (mode) selected and the device used. It should be noted that the values measured in the control unit may differ from the values measured in the monitoring panel. The basic parameters of invasive mechanical ventilation:
3.1. The fraction of Inspired Oxygen (FiO2)
In all mechanical ventilators, oxygen given by inspiration can be adjusted. The level is between 21% and 100% (0.21–1.0). Since the patients are frequently intubated due to hypoxia in the ED, it can be initially selected as 100%. To avoid oxygen toxicity, it should be titrated according to oxygen saturation and PaO2 measurements and should be reduced below 50% if possible. It is not possible to mention a single target value for ED patients, but oxygen saturation should be greater than 88–94%, PaO2> 60 mmHg, although this may vary depending on the medical cause. Liberal oxygen delivery is harmful,19 and it is reported that high FiO2, low or high PaO2 in the first 24 h increases mortality in mechanical ventilation.20
3.2. Respiratory Rate (RR or frequency: f) and Minute Ventilation
Respiratory rate set in MV is the target respiratory rate for the patient in the absence of spontaneous breathing in CMV and A/C mode, and the number of respirations to be synchronized with both the target minimum respiratory rate and patient effort in SIMV mode. The frequency for a normal patient can be set at 12–16/min but should be adjusted according to the patient's metabolic status. For example, a tachypneic patient with metabolic acidosis should initially be simulated and respiratory rate kept high.
The frequency value seen on the monitor panel is the sum of the patient's spontaneous breaths given/assisted by the mechanical ventilator. Therefore, the patient's tidal volume (VT) and frequency should be observed carefully. In IMV, normal minute ventilation is 7–10 L/min (Minute ventilation [VE or Vexp] = VT X f). In patients with septic and/or metabolic acidosis who are likely to breathe in high frequency with spontaneous breathing effort, or in patients with an insufficient tidal volume such as ARDS, minute ventilation should be meticulously monitored, and VT and frequency should be readjusted if necessary.
3.3. Inspirium: Expiratory Rate (I:E)
A breath cycle in IMV is an entire duration of the inspiration and the expiratory time (Ti + Te). The monitoring or appropriate adjustment of these durations is also important for the effective ventilation of the patient. The inspiratory/expiratory ratio (I: E ratio) in a normal patient is 1:2. Extension of expiration is important for CO2 excretion and the extension of inspiration is important for oxygenation. In the case of obstructive type diseases with -hypercapnic respiratory failure or risk, the ratio of I:E should be changed to 1:3 and above in favor of expiration; in patients with oxygenation problems such as ARDS, the I:E ratio should be changed to 1:1 and above in favor of inspiration. The I: E ratio might vary with many interventions:
-
•
Changing frequency or minute ventilation
-
•
Changing Ti and Texp or adding an inspiratory pause
-
•
Changing the respiratory waveform: The expiratory length in the square form is extended. Therefore, it should be preferred in obstructive respiratory failure.
-
•
Changing the flow rate: Most ventilators allow us to change the flow rate according to the need of the patient. 60L/min is sufficient in many patients. When the flow rate is increased, the set tidal volume is reached more quickly, the inspiratory time is shortened, the expiratory is extended, and the risk of developing auto-PEEP is reduced. For this reason, the flow rate is increased in obstructive type respiratory failures and decreased when the inspiration is demanded to extend.
3.4. Trigger and Trigger sensitivity
A breath can be triggered by ventilator or by the patient. The trigger is the setting that determines the initiation of this breath. When ventilation performed only by the device and carried out with rescue breaths, the frequency determined for the patient determines the initiation time of respiration which is called time-triggered ventilation. With current devices, patient-triggered respiration can take place with four methods; pressure triggering, flow triggering, volume triggering, or shape-signal triggering.21 ICU ventilators are often operated with pressure or flow trigger which facilitates breathing with pressure or flow changes in respiratory tract. The pressure trigger sensitivity is often set in the range of -1 to -3 cmH2O, and the flow trigger sensitivity is often set in the range of 2–3 L/min. If it is too high, the device is hardly stimulated, and the patient's respiratory workload is increased. If it is too low, the device might be stimulated more than necessary. Flow trigger is more sensitive, it detects the patient's effort more easily, and the patient can trigger the device with less effort.
3.5. Tidal Volume (VT)
Conventionally, initial VT was recommended as 8–10 mL/kg [predicted body weight-PBW] in cases other than ARDS, but this approach shown to be not correct in the recent years. In a recent study, it has been reported that the use of low VT in undamaged lungs has no effect on ventilator-free days,22 however, initial ventilation of both damaged and undamaged lungs with 6–8 mL/kg PBW is recommended in the EDs.23, 24, 25 Additionally, it should be considered that many patients with mechanical ventilation indication in the ED have damaged the lung. It is reported that the use of low VT in MV in patients with ARDS in the ED has a positive effect on mortality and ventilator-free days.10 Some authors have recommended that the lung protective ventilation strategy starting from 6 to 8 mL/kg PBW on non-ARDS patients, 6 mL/kg PBW on ARDS and adjusting VT according to plateau pressure has a positive effect on the reduction of mortality and on ventilator-free days.10,26
When determining VT, the predicted body weight can be determined by calculating the ideal weight of the patient according to gender and height.27
| [PBW = ♂50.0 + 0.905 × (height in cm) −152.4; ♀ 45.5 + 0.905 × (height in cm) −152.4]. |
3.6. Airway Pressure
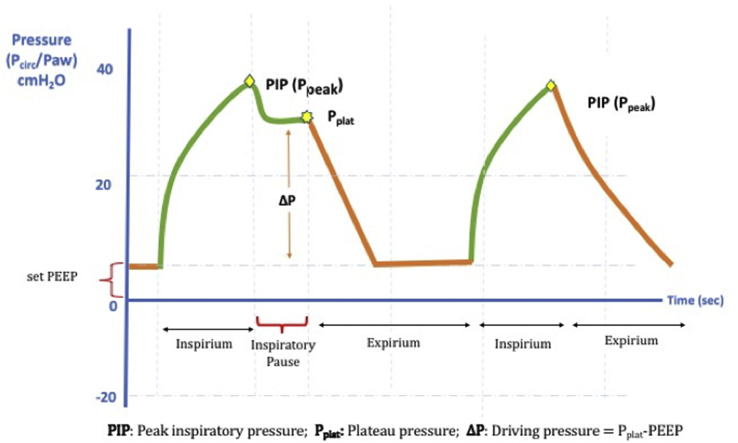
During mechanical ventilation, there are five different airway pressures, which can be externally adjusted and occur during breathing. One of them is PEEP, which is one of the main determinants of oxygenation. The others are the pressures that we need to follow from the monitor during breathing and the pressure that is used in patient's management (Fig. 2).
Fig. 2.
The pressures measured in mechanical ventilation. Note that inspiratory pause is applied for the plateau pressure measurement.
3.6.1. Positive end-expiratory pressure (PEEP)
PEEP is the positive pressure at the end of expiration for preventing damaged airways and collapse of alveoli, keeping them open, and increasing/facilitating oxygenation. Initially, it is set to 5–8 cmH2O and can be increased 2 cmH2O at 10–15min intervals according to the patient's need.21 High PEEP might cause negative cardiovascular findings due to the increase of intrathoracic pressure and intracranial pressure due to the decrease of venous turn. In patients with ARDS with resistant hypoxemia, oxygenation is improved if high PEEP is applied. Fuller et al. used a protocol where they proved the efficacy of lung protective ventilation in ED; if the estimated BMI> 30, 8 cmH2O PEEP applied and if the BMI> 40, 10 cmH2O PEEP applied and it has been reported that high PEEP application has a positive effect on the prognosis of patients with ARDS in ED.10
3.6.2. Peak inspiratory pressure (Ppeakor PIP)
PIP is the maximum airway pressure that occurs during inspiration. PIP is the sum of the pressures that occur with the resistance of the patient's lungs and the pressures throughout the entire circuit. It can be read in real-time on the mechanical ventilator screen during each breath. High readings might indicate that there is high pressure in small airways or a problem that raises pressure throughout the circuit. PIP should be < 35–40 cmH2O.
3.6.3. Plateau pressure (Pplat)
Pplat is the inspiratory pressure measured in the airway at the end of inspiration. Unlike PIP, it indicates only alveolar pressure and is therefore purely indicative of alveolar elasticity. This parameter should be monitored to avoid barotrauma. Pplat is measured by pressing the inspiratory pause key for a few seconds, thus preventing the initiation of expiration at the end of the inspiration (Fig. 2). It can therefore only be measured during volume-targeted (VC) ventilation. High Pplat is an indication that the tidal volume given to the patient is high and should be reduced. In order to avoid barotrauma, it should be kept below 30–35 cmH2O. In ARDS patients, it is tried to be kept below 30 cmH2O.
3.6.4. Driving Pressure (ΔP)
Driving pressure is the difference between Pplat and PEEP. Higher ΔP related to increased mortality, suggesting that it can be used for prognosis determination with higher accuracy than the other parameters in the recent meta-analyses. The static compliance of the respiratory system is the ratio of VT to ΔP (CRS= VT/ΔP). For this reason, ΔP represents VT corrected according to static compliance, and it might be a better method to monitor as a safety limit during mechanical ventilation. There is insufficient evidence to determine a specific cut-off when using it in monitoring. However, 15 cmH2O can be determined not as a target but a safety limit. If ΔP ≥ 15 cmH2O, recruitment maneuvers, prone position, neuromuscular blockade and limitation of VT with 6 mL/kg PBW in ARDS patients; decrease of VT to 5–6 mL/kg PBW and the optimization of PEEP in the other restrictive lung diseases is reported as an approach, yet, the evidence of such an approach is not sufficient (28).
4. Mechanical ventilation modes
Mechanical ventilator modes can be divided into volume-targeted ventilation, pressure-targeted ventilation, and dual-controlled modes. Pressure-targeted and volume-targeted modes allow targeted pressure or tidal volume adjustment, but most devices provide a function through control of the flow, not through volume or pressure.
4.1. Volume-Targeted Ventilation
Volume-Targeted Ventilation also named as volume preset, volume control, volume assist or volume cycled ventilation. When volume-controlled ventilation is set, each breath delivered by the device is supplied with the preset inspiratory flow-time. Since the area under the flow-time curve defines the volume, VT remains constant and is not affected by the patient's effort. The square waveform or decelerating inspiratory flow for the inspiratory flow-time profile are commonly used. For volume targeted ventilation, apart from FiO2, PEEP, trigger mode and sensitivity,1 VT2; inspiratory waveform3; mandatory respiratory rate [f]4; and timing such as inspiratory/expirium ratio or inspiratory time/total breathing time and inspiratory time (Ti) is set.29
4.2. Pressure-Targeted Ventilation
Pressure-Targeted Ventilation also named as pressure-cycled, pressure-limited, pressure-control, pressure-assist, or pressure-targeted ventilation.23 The pressure control is determined by the user, and the device generates inspiration in the patient's airway and cuts off the gas flow when the pressure limit is reached. The generated VT and inspiratory flow are might vary depending on the respiratory resistance, impedance and inspiratory effort of the patient. If the airway resistance increases depending on the patient's lung and chest wall resistance, the inspiration time (Ti) and VT are reduced; as a result, VT does not reach a fixed value and is variable. This might lead to reduced minute ventilation, hypoxemia and carbondioxide retention.29 Although the risk of barotrauma is considered to be low due to the control of the target pressure, the variable VT is an important problem for patients who are targeted for lung protective ventilation, such as ARDS patients. There is insufficient information about the use and advantages of pressure targeted or volume targeted methods in patients with Acute lung injury and ARDS.30
For Pressure-Targeted ventilation, apart from FiO2, PEEP, trigger mode and sensitivity, [1] respiratory rate [f]; [2] inspiratory pressure (pressure high or peak airway pressure [Paw]); [3] inspiration time [Ti] or I/E ratio is adjusted.
4.3. Frequently Used MV Modes
A typical example of volume-controlled ventilation is Controlled Mechanical Ventilation (CMV). It is named as V-CMV, P-CMV depending on being volume targeted or pressure targeted. Volume controlled CMV is the simplest form of mechanical ventilation. The determined tidal volume is given to the patient with a set respiratory rate. This mode is time-triggered and time-cycled, ignoring spontaneous breathing. Therefore, it can only be used in paralyzed, sedated or apneic patients. It creates problems such as coughing, incoordination with the mechanical ventilator ('fighting the vent') on the patients who are awake even slightly. This mode is not suitable for long-term ventilation because it may cause atrophy in the respiratory muscles. A/C and SIMV are the most commonly used modes in EDs.
4.3.1. Assist-Control Ventilation (A/C)
In Assist/Control mode ventilation (A/C), the tidal volume/peak pressure level and the respiration rate is determined, and the patient is provided with a guaranteed tidal volume or intrinsic pressure (pressure targeted or volume targeted). The ventilator guarantees that each triggered breath reaches the desired volume or pressure. Its main feature is that each breath triggered or generated by the device is the same. The patient can determine his own respiratory rate, but the breaths are tidal volume or Paw controlled or supported. It guarantees the user-defined respiratory frequency when it is not triggered by the patient. For this reason, each breath is time-cycled (controlled) or volume/pressure-cycled (assisted).
Since it can be triggered and supported by the patient, it requires less workload for the patient, and it is easy for the user to prevent respiratory acidosis and alkalosis. However, it might cause breath stacking and auto-PEEP development on the patient with tachypnea since there might not be enough time for exhalation or respiratory alkalosis. It requires strict monitoring to prevent plateau pressure and dynamic hyperinflation to prevent barotrauma.31
4.3.2. Synchronized Intermittent Mechanical Ventilation (SIMV)
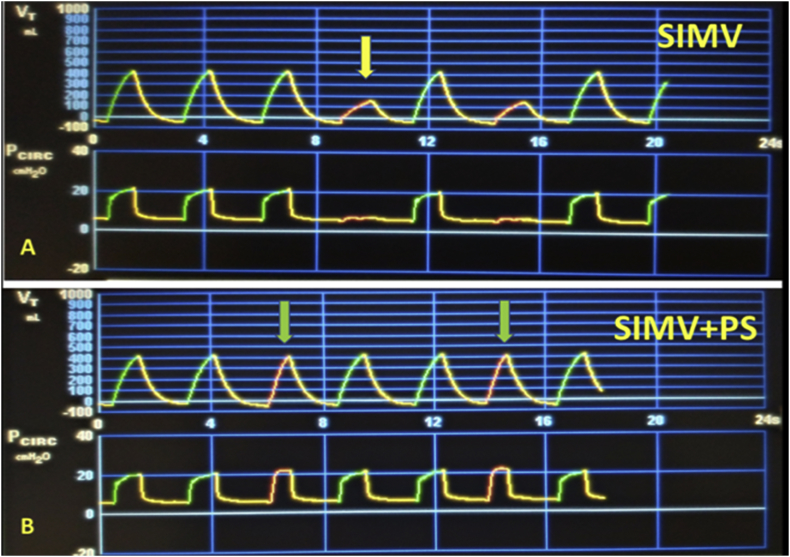
SIMV allows synchronizing between the mandatory breath set for the patient and the mandatory respiration of the device to support these breaths to the desired parameters. Its main feature is being synchronized. In SIMV, when the patient-assisted breaths are in the assist window (in the number of frequencies that is set), it provides respiration guaranteeing the volume or pressure target (Fig. 3). If the patient does not have breathing effort in the time window determined by frequency, he guarantees the breath at the determined frequency. Due to synchronization, the patients who are awake breathe relatively more comfortably.
Fig. 3.
Volume and Pressure to time waveforms during volume targeted SIMV and SIMV + PS ventilations. Note that when there is no pressure support, spontaneous breaths occur without support (yellow arrow) and when there is support, they occur with increased tidal volume (green arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.3.3. SIMV-PS
If no pressure support is additionally added to SIMV, breaths greater than the frequency setting are not supported. In SIMV-PS respiration, a PS is determined in addition to the standard SIMV settings to increase the volume of the patient's spontaneous breaths or to overcome the resistance of the endotracheal tube. In this case, the patient can adjust his own breathing rate and make pressure-assisted breaths (Fig. 3). Apart from the weaning phase, the use of SIMV is often done with PS support.
4.3.4. Pressure-Support Ventilation (PSV)
PSV is a mode that provides partial ventilation support and is usually the preferred mode in the weaning phase. When patients are not sedated, paralyzed, and retain their respiratory dynamics this mode is frequently used. This mode is come into prominence in intensive care units by preventing the development of atrophy of the respiratory muscles due to its little need for sedation. In this mode, each respiratory cycle is triggered by the patient and supported by the mechanical ventilator. The trigger of the respiratory cycles that reach the desired/limited pressure setting by the patient that is more comfortable and generates a respiratory dynamic that requires less sedation. Its main feature is that the device only controls the pressure support level.
5. Which mode should we use in ED?
It is difficult to answer such a question with a single answer. Numerous factors may be determinative, such as the device to be used, the user's experience, the patient's medical condition and the expected time for MV. The A/C mode, which is present in almost all ventilators in the EDs, is a good initial mode in patients with metabolic and respiratory acidosis. Volume controlled A/C ventilation is the most preferred mode in EDs, ARDS patients and the other indications8,10,26 and it is recommended as a first choice by some authors.24,32 In the USA, it is the most preferred approach in intensive care units33 and A/C volume-controlled mod is used in the original study testing low tidal volume in the ARDS patients.34 In this mode, the patient can control each breath by self-initiating, and if only breathing effort is slower than the determined frequency, the ventilator will give the mandatory patient breath, thus creating a more natural cycle of ventilation. However, in this mode, all parameters except the initiation of respiration are controlled by the device, so it can be challenging for the patient if he wants to breathe more than the specified volume, or if the patient's own inspiratory tendency is greater than the number of breaths determined.25
SIMV is the preferred mode of initial MV by some physicians, probably because it requires less sedation and allows the patient to have spontaneous respiratory efforts. In this mode, when the breaths below the specified frequency are not supported, the patient needs to create sufficient inspiratory force to overcome the resistance of the breathing circuit and the intubation tube. However, adding additional pressure support to the patient's spontaneous breaths can help to cope with this problem.25 This application is named SIMV-pressure support ventilation. It is reported that physicians in Australia and New Zealand use this mode most frequently.35 In the PRoVENT study evaluating the MV practice in 16 countries in ARDS patients, SIMV and pressured controlled ventilation are reported as the most frequently used modes.36 Ortiz et al. reported that SIMV is used more frequently in North America when compared to different regions and is often used in trauma, less severe diseases and postoperative patients. In the same study, there was no difference between the duration and mortality of mechanical ventilation of patients who were ventilated with SIMV-PS and AC.37
In conclusion, the mode in the ED should be selected according to the medical team's experience and the medical condition of the patients, however, VC-A/C ventilation might be the first choice in most cases.
6. Monitoring the patient in MV
Fuller et al.10,26 reported that mechanical ventilator adjustment and low tidal volume ventilation resulted in improvement in survival and ventilator-free days in accordance with a protocol after selecting a suitable mode for the patient in the ED. The reason for the monitoring of mechanical ventilation in the ED may be various conditions such as COPD, asthma, trauma, poisoning, and ARDS. And it is not possible for a single protocol to be used of the standard patient monitoring parameters for all patient groups. The initial settings can be used in different patient groups and what should be considered in monitoring are summarized in Fig. 2. However, these settings should only be considered as guides and should be arranged for each patient according to the monitored patient's hemodynamic findings, current clinical condition and the parameters monitored in the mechanical ventilator. The effectiveness and changes in the vital findings of respiration should be monitored for a while without leaving the patient's bedside once the MV is initiated in the ED.
In addition to continuous cardiac monitoring and oxygen saturation monitoring, continuous capnography should be part of standard care. Capnography provides the advantages of mechanical ventilation, monitorization of the severity of pulmonary disease, monitoring of metabolic status as well as immediate recognition of obstruction in the tube or circuit.38 Capnography is an ideal method for evaluating patients with ventilation, perfusion or metabolic problems. However, patients with intubation indications in the ED often have multiple problems. EtCO2 of a patient with perfusion problems decreases, but it may increase in patients with ventilation problems. Flow rate decreases with low VT, which might lead to a decrease in EtCO2,39 therefore, the estimation of PaCO2 by EtCO2 measurement is a controversial issue. Razi et al. reported that the difference between PaCO2 and EtCO2 during respiration with SIMV was 3.37 ± 7.93 mmHg.40 In contrast, other studies showed, PaCO2 was found to be roughly 7 mmHg higher than EtCO2 in patients with MV, while the correlation was better during elective surgery and worse in patients with severe lung diseases.41 EtCO2 concordance with PaCO2 is worse in different PEEP levels, sedation levels, the presence of spontaneous breaths, severe thoracic trauma, poor perfusion, hypotension or severe lung diseases.42,43 As a result, capnography should be evaluated together with the other factors of the patient in the monitoring of the patient with MV.
The peak airway pressure should be monitored in the MV. Peak pressure can be seen on all ventilator monitors. The plateau pressure indicates the elasticity of the chest wall and the lung parenchyma. The plateau pressure can be monitored by adding inspiratory pause (0.5 s) in volume-controlled ventilation. Plateau pressure and driving pressure are used as a guide for determining the volume to be given to the patient. ARDSnet study reported that if VT application was started with 6 mL/kg PBW VT and decreased by 1 mL/kg VT (minimum 4 mL/kg PBW) in case of plateau pressure> 30 cmH2O, a 22% reduction in mortality compared to the traditional VT application and ventilator-free days were improved.44 In studies evaluating patients with acute lung injury, it's emphasized that low VT application is not frequent, even though VT was set according to keeping plateau pressure below 30 cmH2O.45 Recent studies have shown that in non-ARDS patients in the ED, initiation of VT 6–8 mL/kg PBW and Plateau pressure of 30 cmH2O are targeted and, if necessary, with adjustment of VT, providing a significant improvement in the prognosis of lung protective ventilation patients.10,26
The driving pressure (ΔP), calculated by the difference between plateau pressure and PEEP, is one of the parameters that can be used in patient management currently. High driving pressure predicts ARDS development in critically ill patients in ICU.46 Even though the evidence is insufficient, the authors state that keeping the driving pressure under 15 cm H2O can be used as a safety limit if the driving pressure≥15cmH2O, reduction in VT and PEEP optimization can be considered.31
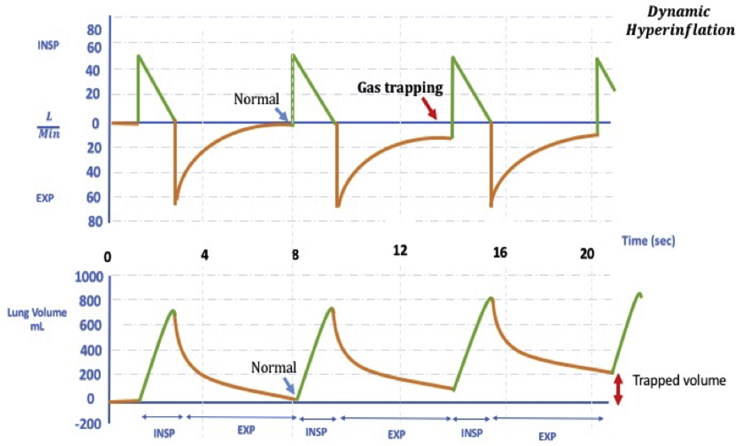
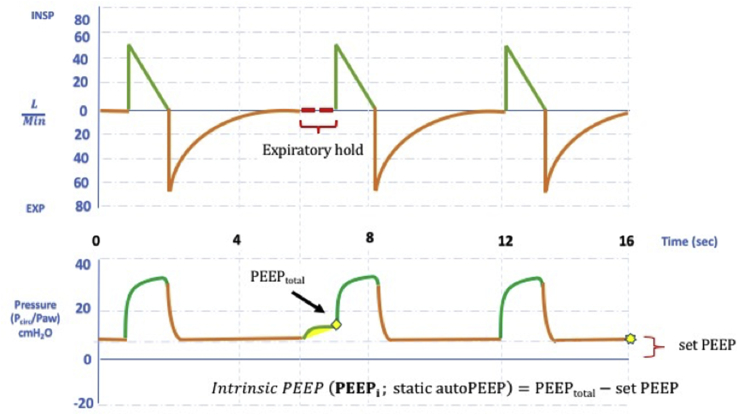
Dynamic hyperinflation is an important problem in the monitoring of patients with obstructive respiratory failure in the ED. Dynamic hyperinflation is defined as an increase in end-expiratory volume as a result of constant inadequate expiration (Fig. 4). Dynamic hyperinflation is associated with alveolar autogenerated PEEP known as auto-PEEP or intrinsic PEEP.47 This might lead to increased intrathoracic pressure, decreased venous return, reduced preload, decreased left ventricular compliance, and increased right ventricular afterload.48 If it is not noticed, the patient might develop cardiovascular collapse as a result. If the expiratory flow does not reach the zero point in the flow-time curve, the presence of dynamic hyperinflation should be considered. Static auto-PEEP can be measured by occlusion maneuver at the end of the expirium (Fig. 5). In the presence of dynamic hyperinflation, the time allocated for expiration should be considered to be less than necessary. If the patient is breathing with mandatory breaths, the frequency should be decreased; the patient's respiratory effort might be decreased due to opioids in the spontaneous triggering ventilation. Sedation and neuromuscular blockers (NMB) will also reduce minute ventilation if administrated. High flow rate in targeted volume ventilation, square waveform or reduction of target volume are other maneuvers to extend expiration. PEEP titration according to measured intrinsic PEEP reduces the patient's effort to trigger the ventilator. If the patient develops severe hemodynamic impairment or arrest, the patient should be immediately separated from the ventilator, and a full exhalation should be allowed.49
Fig. 4.
Dynamic Hyperinflation. Note that the flow-time waveform at the top does not return to point 0 at the end of expirium.
Fig. 5.
Flow-time and pressure- time waveforms during the measurement of static autoPEEP (PEEPi) with expiratory hold.
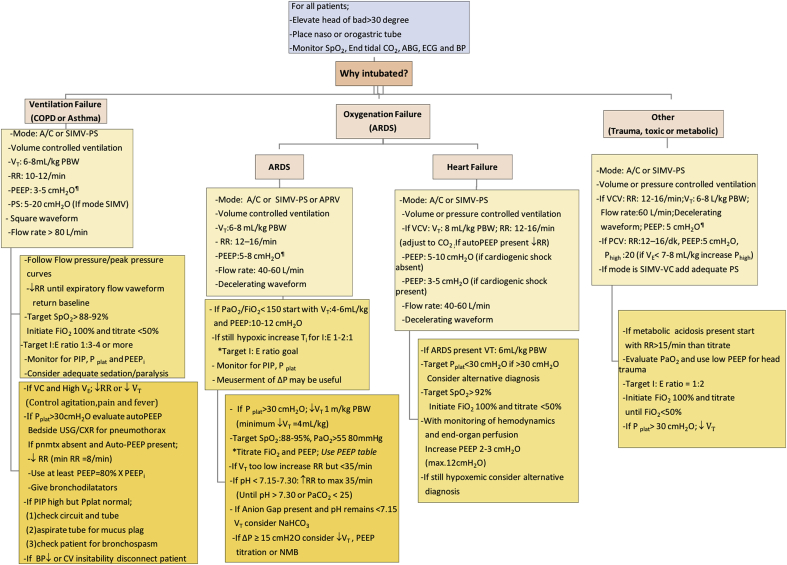
Algorithm for monitoring parameters and approach to deteriorated patients in MV summarized in Fig. 6.
Fig. 6.
Initial ventilator settings in different clinical situations and monitoring tips.10,21,25 ABG; arteriel blood gas; BP: blood pressure; A/C: Asist controlled ventilation; SIMV: Synchronized Intermittent-Mandatory Ventilation; APRV: Airway Pressure Release Ventilation; PS: Pressure support; PVC: pressured controlled ventilation; VCV: volume controlled ventilation; I:E: Inspiration/expiration; VT: Tidal volume; RR: Respiratory rate; PS: Pressure support or Pressure above PEEP; PIP: Peak airway pressure; Pplat: Plateau pressure; PEEPi: intirinsic PEEP; NMB:neuromuscular blocker. ¶ Consider high PEEP in patients with stiff chest wall [eg. LOV-ED study protocol; if BMI>30 set PEEP to 8 cmH2O; if BMI>40 set PEEP to 10 cmH2O]. Δ Instead of 100%, FiO2 can initiate 30–40% and than titrate to achieve target SpO2 and PaO2 in the ED.10
7. Sedation and analgesia
Sedation and analgesia applied to the patient in MV reduces discomfort from the intubation tube, protects the patient from self-injury, corrects the patient-ventilator relationship and reduces pain during interventions. For this reason, the continuous sedative infusion is a frequently used practice, but there is no standard for drug administration and it varies between regions. Fentanyl, morphine, and hydromorphone as analgesics; sedative drugs such as benzodiazepines and barbiturates are frequently used.50 Continuous propofol infusion is also one of the most frequently used applications, and it is reported that patients with mechanical ventilation with propofol infusion compared with midazolam have less mortality, patients are removed from the intensive care unit earlier, and mechanical ventilation duration is shortened.51 NMBs are especially used in patients with oxygenation problems. Only nondepolarizing NMBs are used in intensive care with a bolus administration or continuous infusion. Concurrent sedation is important in patients using NMB, and therefore it is recommended to use a method that monitors the sedation depth such as bispectral index (BIS) in these patients.50
The effect of sedation depth on the prognosis in MV and sedation applications, which were frequently performed at the first 48–96 h, were evaluated in several studies and it was reported that patients who underwent deep sedation had higher mortality, longer duration of MV and ICU stay.52 However, the majority of studies on this subject are ICU studies. In one study, deep sedation was applied at 64% in the ED, and mortality in these patients was reported to be greater than in patients undergoing mild sedation.53 It is an important problem to provide and sustain the sedation of a patient that mechanically ventilated in the ED. In intensive care practice, the application of analgesia-first sedation, the use of propofol and dexmedetomidine instead of benzodiazepine, and the application of routine mild sedation are recommended.54 In conclusion, although regular and targeted sedation level is difficult to monitor due to the more chaotic environment of the EDs than the intensive care units and the rapid changes in the hemodynamic status of the patients, the application of mild sedation in the ED is the best approach unless otherwise is proven by the further evidence (see Table 1).
Table 1.
| ANALGESIA | Fentanyl | Remifentanil | Morphine | Hydromorphone |
|---|---|---|---|---|
| Intermittent Dosing | 0.35–0.5 μg/kg IV q0.5–1 h | N/A | 2–4 mg IV q1–2 h | 0.2–0.6 mg IV q1–2 h |
| IV Infusion Rates | 0.7–10 μg/kg/h | 0.5–1.5 μg/kg/h | 2–30 mg/h | 0.5–3 mg/h |
| SEDATION | Midazolam | Propofol | Dexmedetomidine | Ketamine |
| Loading dose | 0.01–0.05 mg/kg | 5 μg/kg/min over 5 min | 1 μg/kg over 10 min | 0.1–0.5 mg/kg IV |
| Maintenance dose | 0.02–0.1 mg/kg/h | 5–50 μg/kg/min | 0.2–0.7 μg/kg/h | 0.05–0.4 mg/kg/h |
| PARALYSIS | Cis-Atracurium | Atracurium | Rocuronium | Vecuronium |
| Initial Bolus | 0.15 mg/kg or 15 mg | 0.4–0.5 mg/kg | 0.6 mg/kg (1mg/kg for RSI) | 0.8–0.1 mg/kg |
| Starting Infusion Rates | 3 μg/kg/min | 11–13 μg/kg/min | 10–12 μg/kg/min | 1 μg/kg/min |
Conflicts of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
Fig. 1; Reproduced with permission of the © ERS 2019: Breathe 13 (2) 8498; DOI: 10.1183/20734735.007817 Published 8 June 2017.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
References
- 1.Mullins P.M., Goyal M., Pines J.M. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad Emerg Med. 2013;20(5):479–486. doi: 10.1111/acem.12134. [DOI] [PubMed] [Google Scholar]
- 2.Easter B.D., Fischer C., Fisher J. The use of mechanical ventilation in the ED. Am J Emerg Med. 2012;30(7):1183–1188. doi: 10.1016/j.ajem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Iwashita Y., Yamashita K., Ikai H. Epidemiology of mechanically ventilated patients treated in ICU and non-ICU settings in Japan: a retrospective database study. Crit Care. 2018;22(1):329. doi: 10.1186/s13054-018-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angotti L.B., Richards J.B., Fisher D.F. Duration of mechanical ventilation in the emergency department. West J Emerg Med. 2017;18(5):972. doi: 10.5811/westjem.2017.5.34099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung S.C., Kung C.T., Hung C.W. Determining delayed admission to the intensive care unit for mechanically ventilated patients in the emergency department. Crit Care. 2014;18(4):485. doi: 10.1186/s13054-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingart S.D., Sherwin R.L., Emlet L. ED intensivists and ED intensive care units. Am J Emerg Med. 2013;31(3):617–620. doi: 10.1016/j.ajem.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M., Houseman D., Johnson N.J. Prevalence of acute lung injury among medical patients in the emergency department. Acad Emerg Med. 2012;19(9):1011–1018. doi: 10.1111/j.1553-2712.2012.01429.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuller B.M., Mohr N.M., Dettmer M. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page D., Ablordeppey E., Wessman B.T. Emergency department hyperoxia is associated with increased mortality in mechanically ventilated patients: a cohort study. Crit Care. 2018;22(1):9. doi: 10.1186/s13054-017-1926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller B.M., Ferguson I.T., Mohr N.M. A quasi-experimental, before-after trial examining the impact of an emergency department mechanical ventilator protocol on clinical outcomes and lung-protective ventilation in acute respiratory distress syndrome. Crit Care Med. 2017;45(4):645. doi: 10.1097/CCM.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellaca R.L., Veneroni C., Farre R. Trends in mechanical ventilation: are we ventilating our patients in the best possible way? Breathe (Sheff) 2017;13(2):84–98. doi: 10.1183/20734735.007817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst J.M., Davis K., Jr., Branson R.D. Comparison of blood gases during transport using two methods of ventilatory support. J Trauma. 1989;29(12):1637–1640. doi: 10.1097/00005373-198912000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Holets S.R., Davies J.D. Should a portable ventilator be used in all in-hospital transports? Respir Care. 2016;61(6):839–853. doi: 10.4187/respcare.04745. [DOI] [PubMed] [Google Scholar]
- 14.Blakeman T.C., Branson R.D. Evaluation of 4 new generation portable ventilators. Respir Care. 2013;58(2):264–272. doi: 10.4187/respcare.01994. [DOI] [PubMed] [Google Scholar]
- 15.Fludger S., Klein A. Portable ventilators. Cont Educ Anaesth Crit Care Pain. 2008;8(6):199–203. [Google Scholar]
- 16.Kacmarek Robert M. The mechanical ventilator: past, present, and future. Respir Care. 2011;56(8):1170–1180. doi: 10.4187/respcare.01420. [DOI] [PubMed] [Google Scholar]
- 17.Masterton R.G., Galloway A., French G. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2008;62(1):5–34. doi: 10.1093/jac/dkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess D.R., Kallstrom T.J., Mottram C.D. Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir Care. 2003;48(9):869–879. [PubMed] [Google Scholar]
- 19.Chu D.K., Kim L.H., Young P.J. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge E., Peelen L., Keijzers P.J. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6):156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sassoon C.S. Triggering of the ventilator in patient-ventilator interactions. Respir Care. 2011;56(1):39–51. doi: 10.4187/respcare.01006. [DOI] [PubMed] [Google Scholar]
- 22.Simonis F.D., Neto A.S., Binnekade J.M. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. Jama. 2018;320(18):1872–1880. doi: 10.1001/jama.2018.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jairo I., Santanilla . Mechanical ventilation. In: Roberts J.R., Hedges J.R., editors. Roberts and Hedges' Clinical Procedures in Emergency Medicine E-Book. Elsevier Health Sciences; 2013. pp. 160–180. [Google Scholar]
- 24.Weingart S.D. Managing initial mechanical ventilation in the emergency department. Ann Emerg Med. 2016;68(5):614–617. doi: 10.1016/j.annemergmed.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel R., Mallemat H. Emergency department treatment of the mechanically ventilated patient. Emerg Med Clin N Am. 2016;34(1):63–75. doi: 10.1016/j.emc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Fuller B.M., Ferguson I.T., Mohr N.M. Lung-protective ventilation initiated in the emergency department (LOV-ED): a quasi-experimental, before-after trial. Ann Emerg Med. 2017;70(3):406–418. doi: 10.1016/j.annemergmed.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva P.L., Rocco P.R. The basics of respiratory mechanics: ventilator-derived parameters. Ann Transl Med. 2018;6(19) doi: 10.21037/atm.2018.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bugedo Guillermo, Retamal Jaime, Bruhn Alejandro. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care. 2017;21(1):199. doi: 10.1186/s13054-017-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosier J.M., Hypes C., Joshi R. Ventilator strategies and rescue therapies for management of acute respiratory failure in the emergency department. Ann Emerg Med. 2015;66(5):529–541. doi: 10.1016/j.annemergmed.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Holets Steven R., Hubmayr Rolf D. Setting the ventilator. In: Tobin, Martin J., editors. Principles and Practice of Mechanical Ventilation. McGraw-Hill; New York: 2013. [Google Scholar]
- 31.Chacko B., Peter J.V., Tharyan P. Pressure‐controlled versus volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD008807.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpio A.L.M., Mora J.I. StatPearls [Internet] StatPearls Publishing; 2018. Ventilation assist control. [Google Scholar]
- 33.Jabaley C.S., Groff R.F., Sharifpour M. Modes of mechanical ventilation vary between hospitals and intensive care units within a university healthcare system: a retrospective observational study. BMC Res Notes. 2018;11(1):425. doi: 10.1186/s13104-018-3534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 35.Rose L., Presneill J.J., Johnston L. Ventilation and weaning practices in Australia and New Zealand. Anaesth Intensive Care. 2009;37(1):99–107. doi: 10.1177/0310057X0903700117. [DOI] [PubMed] [Google Scholar]
- 36.Neto A.S., Barbas C.S., Simonis F.D. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med. 2016;4(11):882–893. doi: 10.1016/S2213-2600(16)30305-8. [DOI] [PubMed] [Google Scholar]
- 37.Ortiz G., Frutos-Vivar F., Ferguson N.D. Outcomes of patients ventilated with synchronized intermittent mandatory ventilation with pressure support: a comparative propensity score study. Chest. 2010;137(6):1265–1277. doi: 10.1378/chest.09-2131. [DOI] [PubMed] [Google Scholar]
- 38.Walsh B.K., Crotwell D.N., Restrepo R.D. Capnography/Capnometry during mechanical ventilation. Respir Care. 2011;56(4):503–509. doi: 10.4187/respcare.01175. [DOI] [PubMed] [Google Scholar]
- 39.Long B., Koyfman A., Vivirito M.A. Capnography in the emergency department: a review of uses, waveforms, and limitations. J Emerg Med. 2017;53(6):829–842. doi: 10.1016/j.jemermed.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Razi E., Moosavi G.A., Omidi Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res. 2012;1(2):58. doi: 10.5812/atr.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nassar B.S., Schmidt G.A. Estimating arterial partial pressure of carbon dioxide in ventilated patients: how valid are surrogate measures? Ann Am Thorac Soc. 2017;14(6):1005–1014. doi: 10.1513/AnnalsATS.201701-034FR. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.W., Hong Y.S., Han C. Concordance of end-tidal carbon dioxide and arterial carbon dioxide in severe traumatic brain injury. J Trauma Acute Care Surg. 2009;67(3):526–530. doi: 10.1097/TA.0b013e3181866432. [DOI] [PubMed] [Google Scholar]
- 43.Kerr M.E., Zempsky J., Sereika S. Relationship between arterial carbon dioxide and end-tidal carbon dioxide in mechanically ventilated adults with severe head trauma. Crit Care Med. 1996;24(5):785–790. doi: 10.1097/00003246-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 45.Jaswal D.S., Leung J.M., Sun J. Tidal volume and plateau pressure use for acute lung injury from 2000 to present: a systematic literature review. Crit Care Med. 2014;42(10):2278. doi: 10.1097/CCM.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blondonnet R., Joubert E., Godetet Driving pressure and acute respiratory distress syndrome in critically ill patients. Respirology. 2019;24(2):137–145. doi: 10.1111/resp.13394. [DOI] [PubMed] [Google Scholar]
- 47.Natalini G., Tuzzo D., Rosano A. Assessment of factors related to auto-PEEP. Respir Care. 2016;61(2):134–141. doi: 10.4187/respcare.04063. [DOI] [PubMed] [Google Scholar]
- 48.Blanch L., Bernabé F., Lucangelo U. Measurement of air trapping, intrinsic positive end-expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respir Care. 2005;50(1):110–124. [PubMed] [Google Scholar]
- 49.Berlin D. Hemodynamic consequences of auto-PEEP. J Intensive Care Med. 2014;29(2):81–86. doi: 10.1177/0885066612445712. [DOI] [PubMed] [Google Scholar]
- 50.Fierro M.A., Bartz R.R. Management of sedation and paralysis. Clin Chest Med. 2016;37(4):723–739. doi: 10.1016/j.ccm.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Lonardo N.W., Mone M.C., Nirula R. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med. 2014;189(11):1383–1394. doi: 10.1164/rccm.201312-2291OC. [DOI] [PubMed] [Google Scholar]
- 52.Stephens R.J., Dettmer M.R., Roberts B.W. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018;46(3):471–479. doi: 10.1097/CCM.0000000000002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens R.J., Ablordeppey E., Drewry A. Analgosedation practices and the impact of sedation depth on clinical outcomes among patients requiring mechanical ventilation in the ED: a cohort study. Chest. 2017;152(5):963–971. doi: 10.1016/j.chest.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barr J., Fraser G.L., Puntillo K. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]