Abstract
Trier Social Stress Test (TSST) is an experimental psychological test that induces changes in autonomic, endocrinological and immunological activity. Two measures used to evaluate the inflammatory activity induced by this test are the interleukin 6 (IL-6), a cytokine sensitive to changes in sympathetic nervous activity, and the mean arterial pressure (MAP), a measure sensitive to changes in autonomic activity. This study had two goals: first, the study examined whether TSST increases IL-6 and MAP levels; second, pre- and post-TSST IL-6 levels were compared for participants whose IL-6 levels increased or decreased due to the TSST. Saliva samples of IL-6 and MAP were taken from 42 participants clinically healthy, without psychiatric history, and data were analysed via quantile comparisons. The results showed that TSST did not lead to an increase in sympathetic activity as indexed by IL-6. Instead, TSST led to increases in MAP. Also, there were significant differences between the IL-6 distributions of people whose IL-6 levels changed from low to high (63%) and from high to low (37%) before and after the TSST. These findings suggest that the TSST will not have the same effect on all participants; that is, individual differences can be assessed using a biomarker to identify people with specialized psychological care needs.
Keywords: Neuroscience, Physiology, Clinical psychology, Psychology
1. Introduction
The Trier Social Stress Test (TSST) paradigm has been used as a laboratory test to induce acute psychological stress, whose physiological effects have been documented in several investigations (Allen et al., 2014). Some studies have focused on the sympathetic autonomic activity and the inflammatory response of interleukin 6 (IL-6) pre-post TSST (Cooper et al., 2015; Lockwood et al., 2017).
This is of special relevance due to the multiple properties of IL-6 on the effects of its prolonged concentration in the organism for the development of physical diseases (Schett, 2018) and affective disorders (Hodes et al., 2016). In people who have suffered some traumatic experience, the level of concentration of IL-6 rises after the stressor and is significantly higher compared to people who have not suffered traumatic experiences (Carpenter et al., 2010).
However, due to the logarithmic transformations of the data to obtain a normal distribution of IL-6 (see examples of this practice in Miyamoto et al., 2013), its predictive value is lost as an individual biomarker of the possible autonomic-inflammatory alteration in a person with psychological distress. For example, a study by Rodríguez-Medina et al. (2018) showed that IL-6 levels influence autonomic regulatory capacity and only in those with low or moderate concentration of IL-6 relaxation strategies decreased sympathetic activity, but not in people with an elevated level of this cytokine. This study illustrates the clinical utility of considering the value of IL-6 as a biomarker of inflammatory activity without resorting to a logarithmic transformation.
This means that there could be differences in the level of IL-6 concentration before and after TSST, although this experimental task usually increases sympathetic activity. Sympathetic autonomic activity can be evaluated by changes in mean arterial blood pressure (MAP) too. MAP accounts for both systolic and diastolic pressures, and its use has been suggested to index the cardiovascular effect of TSST (Allen et al., 2014).
The aim of the present investigation is to explore the distribution of IL-6 before and immediately after TSST in a group of clinically healthy, normotensive participants, with no history of affective disorders. The objective of the present study was to explore if the TSST has the same effect for all the participants on the IL-6 and MAP.
2. Methods
2.1. Participants
Forty-two undergraduate students (27 males and 15 females), from the Autonomous University of Querétaro, voluntarily participated in the study. The median age was 23 ± 3.70 years. For this study, it was considered that the participant was free of any biological influence that could alter the value of IL-6. As an inclusion criterion, it was established that participants were normotensive. The exclusion criteria were: to consume drugs for three days prior to the study and not have to fast for at least 3 h before the study. The study was of a non-invasive kind and it abided by the Helsinki declaration. The study was approved by Clínica de Enfermería de la UAQ, San Juan del Río and all participants gave informed consent.
2.2. Instruments and materials
Citizen Digital Baumanometer CH-656C. A device that records arterial pressure. For this investigation, the mean arterial pressure (MAP) was used. MAP was estimated via the formula . When participants arrived at the stress test room, they remained there for 10 min. Subsequently, their MAP was recorded pre - post stress test.
Interleukin 6 salivary. Salivette kit for IL-6 was used to collect a non-invasive sample of the proinflammatory cytokine. The salivary samples were obtained according to the instructions of Sarstedt, Newton, NC, and centrifuged at 3000 rpm for 15 min. The concentration level of IL-6 was determined by an ELISA kit based on the instructions of Salimetrics State College PA, at a dilution of 1:5. IL-6 concentrations were determined by using a luminescence counter at 450 nm with a secondary filter of 620–630 nm.
Trier Social Stress Test (TSST). Acute psychosocial stress induction protocol. It consists of four phases: 1) Rest in the room; 2) instructions are given to the participant to prepare a speech, during 3 min, that describes why he is a good candidate for an ideal work. This speech will last for 5 min and will be evaluated by a panel of expert judges in public speaking; 3) verbal presentation of the speech during 5 min; and 4) the participant is instructed to perform an arithmetic task, for 5 min, that consists of counting from 17 to 17 backward from 1021 (if the participant makes a mistake he must restart from 1021).
2.3. Procedure
According to the TSST protocol (Allen et al., 2014), we used a pre-post-test design to collect the salivary sample of IL-6 and the mean arterial pressure. Participants were scheduled to participate in the study between 9:00am and 11:00am for three consecutive days. The study took approximately 25 min per participant.
2.4. Statistical analyses
Traditional parametric analyses compare data set's means and estimate standard deviations; which are unbiased estimators of location and scale for normally distributed data. When data do not meet parametric assumptions (i.e. normality and homogeneity of variance), transformations (usually, logarithmic) are applied. In this study we used a robust statistics approach that enables analysing data without the need of relying on those assumptions. Specifically, we used a technique that allows comparing the quantiles of the distributions of two dependent groups. Such technique, thus, goes beyond traditional means' estimation/comparison by tackling the entire data's distribution. We focused on the distributions' quartiles. That is, the .25 (left tail of a data's distribution), .5 (or median), and .75 (right tail of a data's distribution) quantiles of the distributions of interests were pairwise-compared at a .05 alpha level. The analyses were performed in R via the ‘Dqcomhd’ function in the package WRS2 (see p. 210–212 in Wilcox, 2017 for details). When the associated p-values were very close to 0, these were reported as p < .0001; otherwise, the exact p-values were reported.
Robust estimation of location and scale were used to describe vectors of data; specifically, the median (Mdn) and the median absolute deviation (MAD) were reported as Mdn ± MAD.
Results were visualised via empirical cumulative distribution function plots (ECDF plots). These plots show the proportion of scores (shown in the Y axis) that are less than or equal to each score (shown in the X axis).
3. Results
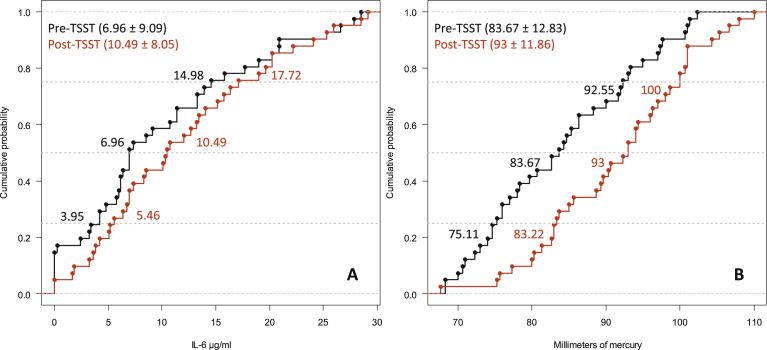
Data from one participant had to be removed as his IL-6 value in the post-TSST condition was an outlier (Mdn ± 32.93 MAD); so the data from 41 participants were analysed. Fig. 1 shows that while there was not a significant rightward shift in the distribution of IL-6 values after TSST (Q.25: diff = -1.90, 95%CI [-4.87, .68], p = .16; Q.50: diff = -2.92, 95%CI [-5.31, .04], p = .06; Q.75: diff = -2.15, 95%CI [-5.60, 1.40], p = .27), there was a significant complete rightward shift in the distribution of MAP values after TSST (Q.25: diff = -8.51, 95%CI [-11.81, -5.62], p < .0001; Q.50: diff = -9.10, 95%CI [-12.88, -5.58], p < .0001; Q.75: diff = -6.76, 95%CI [-10.52, -2.81], p < .0001).
Fig. 1.
ECDF plots of the IL-6 distributions pre- and post-TSST (A) and the MAP distributions pre- and post-TSST (B). The horizontal dotted grey lines cut through the distributions' quartiles (i.e. .25, .5, and .75).
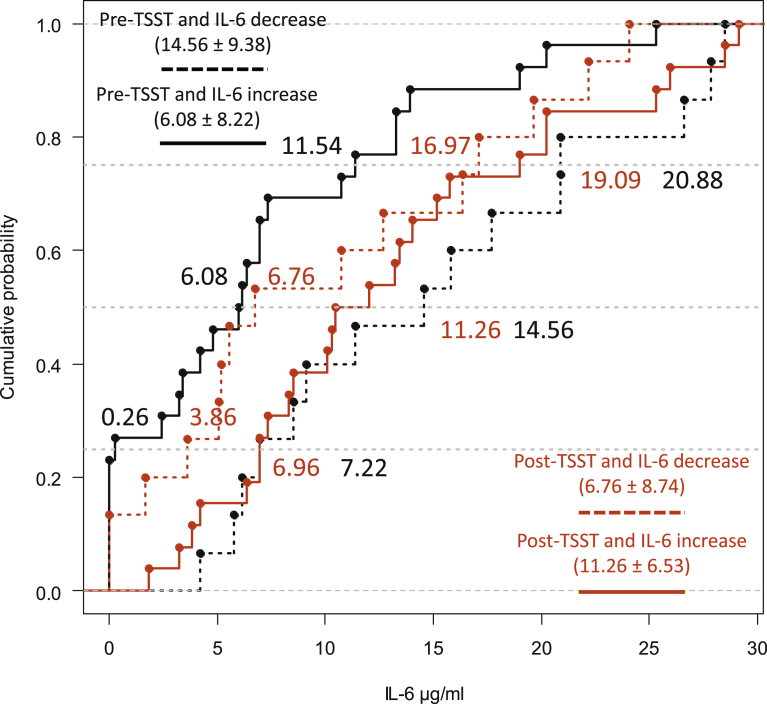
While in 37% of the participants, i.e. 15 participants, the IL-6 levels changed from high to low before and after the TSST, in the remaining 63% of the participants, i.e. 26 participants, these levels changed from low to high. The comparison of quartiles showed (see Fig. 2) that the pre- and post-TSST IL-6 distributions of participants whose IL-6 values changed from high to low differed (Q.25: diff = 4.24, 95%CI [2.17, 8.28], p < .0001; Q.50: diff = 5.42, 95%CI [2.98, 8.32], p < .0001; Q.75: diff = 5.19, 95%CI [2.98, 7.75], p < .0001). A complete rightward distributional shift was also found in participants whose IL-6 values went from low to high during the pre- and post-TSST (Q.25: diff = -5.78, 95%CI [-7.54, -3.64], p < .0001; Q.50: diff = -5.85, 95%CI [-8.27, -3.71], p < .0001; Q.75: diff = -7.21, 95%CI [-10.26, -4.94], p < .0001).
Fig. 2.
ECDF plots of the IL-6 levels that changed from high to low (decreased) and from low to high (increased) before and after the TSST. The pairwise comparisons of interest were between ‘pre-TSST and IL-6 increase’ vs ‘post-TSST and IL-6 increase’ (i.e. solid lines) and ‘pre-TSST and IL-6 decrease’ vs ‘post-TSST and IL-6 decrease’ (i.e. dotted lines). The horizontal dotted grey lines cut through the distributions' quartiles (i.e. .25, .5, and .75).
4. Discussion
The data showed a significant increase in mean arterial pressure only after the experimental TSST test. This increase had a differential influence on the inflammatory activity indexed by IL-6. In those participants whose level of IL-6 concentration was low before the test, such levels increased after TSST; the opposite effect happened in those who had an elevated level of IL-6 in the pre-TSST. It has been documented that TSST can induce an increase in cortisol for both men and women. In turn, cortisol can regulate inflammatory activity to maintain homeostasis. Note though that there is research suggesting that the relationship between the changes of cortisol and IL-6 (increments vs decrements) should be examined further (Koelsch et al., 2016; Pulopulos et al., 2018).
The results presented suggest a differential behaviour depending on the level of IL-6 pre-stress test. This change may be related to the psychological vulnerability of presenting chronic stress in those participants whose IL-6 levels decreased as this change indicates a high level of IL-6 prior to a stress test in clinically healthy persons (Carpenter et al., 2010). This would allow identifying participants with acute stress and chronic stress (Lasselin et al., 2018; Rodríguez-Medina et al., 2018). However, we still do not know the biological function of this difference between those who increase and those who decrease IL-6. We were able to describe this biological difference, but the lack of other cytokine that accompanies IL-6 does not allow us to know the pro-inflammatory or anti-inflammatory nature.
For future studies, it is suggested to implement a psychological intervention to decrease sympathetic activity and evaluate its effect on inflammatory activity (Koelsch et al., 2016; Lasselin et al., 2016).
Methodologically speaking, the quantile analysis renders unnecessary transforming the data and instead enables dealing with entire distributions. Furthermore, the design of the study ensures some level of ecological validity.
Declarations
Author contribution statement
David A. Rodríguez-Medina: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Gerardo Leija-Alva: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Benjamín Domínguez-Trejo; Luis A. Morales-Hernández: Contributed reagents, materials, analysis tools or data.
María del Rocío Hernández-Pozo: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Irving A. Cruz-Albarrán: Conceived and designed the experiments.
Fernando Marmolejo-Ramos: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was funded by the grant PAPIIT-DGAPA-UNAM: IT300519 (i.e. Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica de la Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México, ref. no. IT300519). The authors thank Beatríz Gómez, Javier Moctezuma, Omar Manjarrez, Gabriela Jael Pérez García, and Leonardo Rodríguez Sosa.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
David A. Rodríguez-Medina, Email: psic.d.rodriguez@comunidad.unam.mx.
Fernando Marmolejo-Ramos, Email: fernando.marmolejoramos@adelaide.edu.au.
References
- Allen A., Kennedy P., Cryan J., Dinan T., Clarke G. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Carpenter L., Gawuga C., Tyrka A., Lee J., Anderson G., Price L. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T., McKinley P., Seeman T., Choo T., Lee S., Sloan R. Heart rate variability predicts levels of inflammatory markers: evidence for the vagal anti-inflammatory pathway. Brain Behav. Immun. 2015;49:94–100. doi: 10.1016/j.bbi.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G., Ménard C., Russo S. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress. 2016;4:15–22. doi: 10.1016/j.ynstr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Boehlig A., Hohenadel M., Nitsche I., Bauer K., Sack U. The impact of acute stress on hormones and cytokines and how their recovery is affected by music-evoked positive mood. Sci. Rep. 2016;6(1) doi: 10.1038/srep23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J., Kemani M., Kanstrup M., Olsson G., Axelsson J., Andreasson A. Low-grade inflammation may moderate the effect of behavioral treatment for chronic pain in adults. J. Behav. Med. 2016;39(5):916–924. doi: 10.1007/s10865-016-9769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J., Lekander M., Axelsson J., Karshikoff B. Sex differences in how inflammation affects behavior: what we can learn from experimental inflammatory models in humans. Front. Neuroendocrinol. 2018;50:91–106. doi: 10.1016/j.yfrne.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Lockwood K., Jennings J., Matthews K. Psychophysiological correlates of systemic inflammation in black and white men. Brain Behav. Immun. 2017;59:93–102. doi: 10.1016/j.bbi.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Boylan J.M., Coe C.L., Curhan K., Levine C.S., Markus H.R., Park J., Kitayama S., Kawakami N., Karasawa M., Love G., Ryff C. Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain Behav. Immun. 2013;34:79–85. doi: 10.1016/j.bbi.2013.07.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulopulos M., Hidalgo V., Puig-Pérez S., Salvador A. Psychophysiological response to social stressors: relevance of sex and age. Psicothema. 2018;30(2):171–176. doi: 10.7334/psicothema2017.200. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Medina D., Domínguez-Trejo B., Cortés-Esteban P., Cruz-Albarrán I., Morales-Hernández L., Leija-Alva G. Biopsychosocial assessment of pain with thermal imaging of emotional facial expression in breast cancer survivors. Medicines (Basel) 2018;5(2):30. doi: 10.3390/medicines5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology. 2018;57(Suppl_2):ii43–ii50. doi: 10.1093/rheumatology/kex513. [DOI] [PubMed] [Google Scholar]
- Wilcox R.R. fourth ed. Academic Press; San Diego, CA: 2017. Introduction to Robust Estimation and Hypothesis Testing. [Google Scholar]