Abstract
MicroRNAs have been proposed as novel biomarkers for the diagnosis and treatment of many types of cancer. The levels of five candidate microRNAs (miRNAs) (miR-99a-5p, miR-31-5p, miR-138-5p, miR-21-5p, and miR-375-3p) in sera from oral cancer patients and paired tumor and normal tissues were detected by real-time qPCR. The diagnostic power of these miRNAs was analyzed by receiver operating characteristic (ROC) curves. Patient-derived xenograft (PDX) models of oral cancer were established and utilized to verify the potential therapeutic effect of miR-31-5p. Candidate miRNAs were screened from our previous studies and verified in 11 paired oral cancer and adjacent normal tissues. Only serum miR-31-5p levels were significantly different between oral cancer patients and healthy controls and between pre- and postoperative patients. Based on the logistic regression model, this panel of five miRNAs distinguished oral cancer patients from healthy control, with an area under the ROC curve (AUC) of 0.776 (sensitivity = 76.8% and specificity = 73.6%). Furthermore, a miR-31-5p mimic enhanced the proliferation of normal epithelial cells, and antagomiR-31-5p inhibited the proliferation of oral cancer cells in vitro. In vivo, antagomiR-31-5p significantly inhibited tumor growth in oral cancer PDX models. Our findings suggest that circulating miR-31-5p might act as an independent biomarker for oral cancer diagnosis and could serve as a therapeutic target for oral cancer.
Keywords: oral cancer, biomarker, circulating miRNA, miR-31-5p, target therapy
Introduction
Oral cancer is a common malignant tumor of the oral cavity, with the sixth highest incidence of malignancy worldwide.1 In recent years, many studies have been devoted to identifying novel molecular biomarkers, such as circulating tumor DNA,2 exosomes,3 and circulating microRNAs (miRNAs),4 for the early diagnosis, prognostic prediction, and target therapy of cancers. miRNAs are a class of small, noncoding RNAs that can posttranscriptionally regulate mRNA expression and influence almost all cellular pathways.5 Previous studies have demonstrated the regulation of miRNAs in major biological processes of cancers, including proliferation, differentiation, migration, angiogenesis, and carcinogenesis.6, 7 Accumulating evidence indicates that numerous stable miRNAs exist in human serum and that the high stability of miRNAs in blood enables circulating miRNAs to be used as potential markers for malignancies.8 To date, some circulating miRNAs have been found to act as biomarkers for diagnosis, for monitoring the therapeutic effects of surgery or radiotherapy, for the evaluation of lymph node metastasis of cancer, and so on.9, 10, 11, 12, 13
Our previous studies demonstrated that the dysregulation of some miRNAs, such as miR-138, miR-99a, miR-21, miR-375, miR-181a, miR-24, miR-222, and miR-7, contributes to the progression and metastasis of head and neck cancer and oral cancer.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 For example, the miR-21- hydroxyprostaglandin dehydrogenase (HPGD) regulatory module might play an important role in tongue squamous cell carcinoma (TSCC) initiation and progression.25 Deregulation of the miR-99 family contributes to the tumorigenesis of head and neck squamous cell carcinoma (HNSCC), in part by targeting the IGF1R and mTOR signaling pathways.16 miR-138 plays an important role in cell migration, epithelial-mesenchymal transition (EMT), and cell cycle progression and may be involved in the DNA damage response and repair, senescence, and differentiation in oral cancer.17 To date, many studies have been conducted to discover possible miRNA biomarkers for oral cancer.29, 30 For example, Liborio-Kimura et al.30 found that miR-494 was an independent candidate tumor suppressor miRNA in oral cancer. Additionally, we revealed that miR-486-3p, miR-139-5p, and miR-21 could be used as biomarkers for the detection of TSCC.31 Moreover, miR-21 and miR-375 from oral cytology was found to be used as biomarkers for oral tongue cancer detection in our previous study.25
Although circulating miRNAs have emerged as promising noninvasive biomarkers for oral cancer diagnosis, the identification of a miRNA pattern as a biomarker for oral cancer is challenging because of the heterogeneity of the disease, different methodologies used, and sample size. Here, we first screened five candidate miRNAs based on our previous studies. Then, a large number of serum samples was used to perform multiple-stage validation to increase the statistical power and to investigate the potential diagnostic values of these miRNAs for oral cancer. Finally, we established oral cancer patient-derived xenograft (PDX) models and investigated the therapeutic effect of antagomiR-31-5p (a miR-31-5p inhibitor) on oral cancer PDX. We identified and successfully validated miR-31-5p as a useful circulating biomarker in the diagnosis of oral cancer, and we conclude that miR-31-5p is a promising therapeutic target for oral cancer.
Results
Dysregulated miRNA Signatures in Oral Cancer Patients
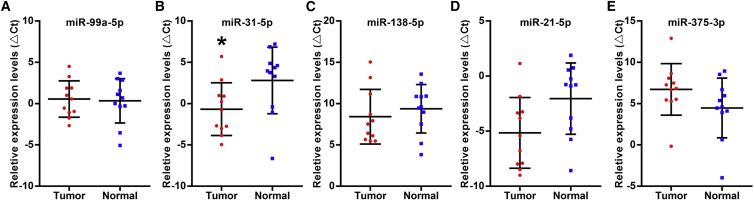
Four miRNA profiles from head and neck cancer or oral cancer based on our previous studies are shown in Table 1 and include (1) a TaqMan-array-based miRNA profiling study on cancer cells that were selectively harvested using laser capture microdissection (LCM) from snap-frozen TSCC specimens and matching normal tissues;31 (2) a meta-analysis of 13 published miRNA profiling studies on HNSCC;16 (3) a differential analysis on miRNA profiling data of 130 TSCC cases and 13 normal control tissues that were extracted from The Cancer Genome Atlas (TCGA) data portal;25 and (4) a microarray-based miRNA profiling study to identify miRNAs associated with enhanced metastatic potential using an in vitro model.20 By combining the results of these miRNA profiling studies, a master list of five differentially expressed miRNAs (miR-99a-5p, miR-31-5p, miR-138-5p, miR-21-5p, and miR-375-3p) was identified in at least two miRNA profiling studies. These five dysregulated miRNAs were further validated in 11 paired oral cancer tissue specimens and matched normal tissues (cohort 2). As described in Figure 1, the expression pattern was consistent with the miRNA variation trend in Table 1, but only the miR-31-5p expression level was significantly higher in oral cancer tissues.
Table 1.
Dysregulated miRNA Signatures in Oral Cancer Identified from MicroRNA Profiling Studies and the TCGA Dataset in Our Previous Studies
| OSCC Tissuea | Meta-Analysisb | TCGA Datac | OSCC Cell Lined |
|---|---|---|---|
| Upregulation miRNAs | |||
| miR-21 | miR-21 | miR-21 | miR-18 |
| miR-223 miR-196b | miR-155 | miR-31 | miR-27a |
| miR-31 | miR-130b | miR-130b miR-155 | miR-27b |
| miR-146b | miR-223 | miR-223 | miR-31 |
| miR-31 | miR-32 | ||
| miR-7 | miR-106b | ||
| miR-34b | miR-203 | ||
| miR-25 | miR-566-pre | ||
| miR-181b | miR-574 | ||
| miR-181a | let-7a | ||
| let-7i | let-7b | ||
| miR-146b | let-7c | ||
| miR-338 | miR-16 | ||
| miR-34c | miR-200a | ||
| Downregulation miRNAs | |||
| miR-486 | miR-100 | miR-99a | miR-7 |
| miR-138 | miR-99a | miR-100 | miR-21 |
| miR-139 | miR-375 | miR-125b | miR-98 |
| miR-204 | miR-125b | miR-375 | miR-99b |
| miR-489 miR-422a | miR-378 | miR-101 | |
| miR-26a | miR-125a | ||
| miR-195 | miR-138 | ||
| miR-26b | miR-193 | ||
| miR-224 | |||
| miR-594-pre | |||
A TaqMan array-based miRNA profiling study on cancer cells that were selectively harvested using LCM from snap-frozen TSCC specimens and matching normal tissues.
A meta-analysis of 13 published miRNA profiling studies on HNSCC. In at least three published studies.
A differential analysis on miRNA profiling data of 130 TSCC cases and 13 normal control tissues that were extracted from TCGA data portal
A microarray-based miRNA profiling study to identify miRNAs associated with enhanced metastatic potential using an in vitro model. In at least two pairs of cell lines with enhanced metastatic potential.
Figure 1.
Relative Expression Levels of Five Candidate miRNAs in Paired Oral Cancer Tissues and Matched Normal Tissues
Real-time qPCR was performed to assess the levels of miR-99a-5p (A), miR-31-5p (B), miR-138-5p (C), miR-21-5p (D), and miR-375-3p (E) in 11 paired oral cancer tissues and matched normal tissues. The vertical lines represent the 25th percentile value, the mean value, and the 75th percentile value of the observations. *p < 0.05.
Based on these studies, we further hypothesized that these miRNAs were differentially expressed in the sera of oral cancer patients.
Candidate Serum miRNAs Act as Biomarkers in Oral Cancer Patients
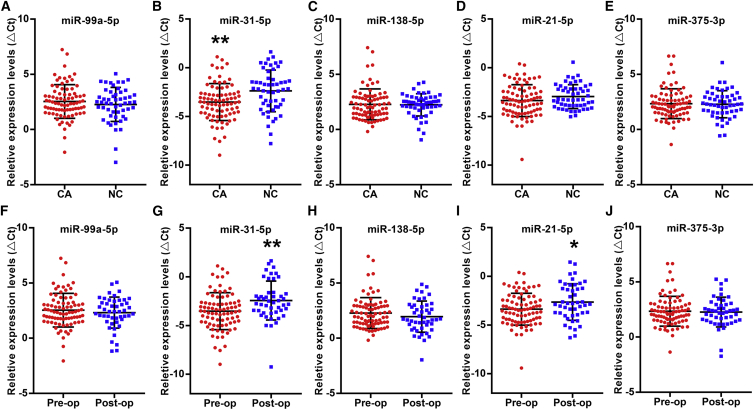
To evaluate the expression differences in these five miRNAs in the sera of oral cancer patients, the miRNA level was determined by real-time qPCR in the serum of cohort 1, which included oral cancer patients (n = 82) and normal controls (n = 53). As shown in Figures 2A–2E, we found that the miR-31-5p level (2−ΔCt) was significantly increased in the sera of oral cancer patients compared to normal controls, and the fold change (2−[ΔCt(OC)−ΔCt(NC)]) was 2.21. No statistically significant difference in the other four candidate miRNAs (miR-99a-5p, miR-138-5p, miR-21-5p, and miR-375-3p) was observed between oral cancer patients and normal controls. Among oral cancer patients (Table S1), the serum levels of miR-99a-5p, miR-138-5p, and miR-375-3p were related to clinical stage (p < 0.05). However, serum miRNA concentrations did not differ according to age, gender, or tumor size.
Figure 2.
Relative Serum Levels of Five Candidate miRNAs in Oral Cancer by Real-Time qPCR
Real-time qPCR was performed to assess the serum levels of miR-99a-5p (A), miR-31-5p (B), miR-138-5p (C), miR-21-5p (D), and miR-375-3p (E) in 82 oral cancer patients and 53 normal controls and the serum levels of miR-99a-5p (F), miR-31-5p (G), miR-138-5p (H), miR-21-5p (I), and miR-375-3p (J) in 82 oral cancer patients before the operation and 47 oral cancer patients after the operation. The vertical lines represent the 25th percentile value, the mean value, and the 75th percentile value of the observations. *p < 0.05; **p < 0.01.
Moreover, differences in these serum miRNA levels between pre- and postoperation patients (cohort 4) were also determined by Mann-Whitney tests. As shown in Figures 2F–2J, the serum levels of miR-31-5p and miR-21-5p (2−ΔCt) were significantly higher in oral cancer patients before the operation than after the operation, and the fold change (2−[ΔCt(pre)−ΔCt(post)]) was 2.13 and 1.63, respectively. No significant difference in the serum levels of miR-99a-5p, miR-138-5p, and miR-375-3p was observed in oral cancer patients before and after the operation. Furthermore, we analyzed the correlation of these miRNA levels between tissue samples and serum samples from oral cancer patients (cohort 3) and found no correlation of these miRNA levels between preoperative sera and tumor tissues in oral cancer patients (Table 2).
Table 2.
The Correlation of miRNA Expression Levels between Sera and Tumor Tissues of Oral Cancer Patients
| miRNA | Serum (ΔCt) | Tissue (ΔCt) | r | p Value |
|---|---|---|---|---|
| miR-99a-5p | 2.98 ± 1.68 | 1.34 ± 2.62 | 0.203 | 0.342 |
| miR-31-5p | −2.92 ± 2.13 | −0.28 ± 4.00 | −0.057 | 0.790 |
| miR-138-5p | 3.07 ± 1.56 | 7.54 ± 3.56 | 0.018 | 0.932 |
| miR-21-5p | −3.42 ± 1.75 | −3.62 ± 3.68 | 0.109 | 0.612 |
| miR-375-3p | 2.82 ± 1.64 | 6.05 ± 3.37 | 0.108 | 0.614 |
Evaluation of the Diagnostic Potential of miRNA Signatures for Oral Cancer
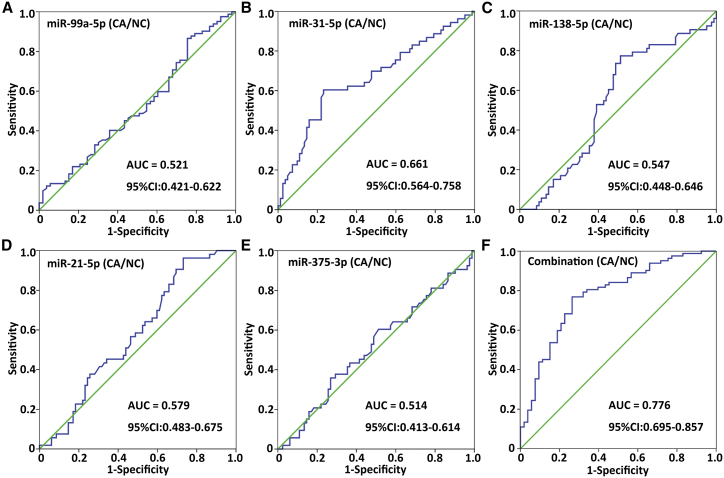
To assess the diagnostic value of the five circulating miRNAs in discriminating oral cancer patients from healthy subjects, receiver operating characteristic (ROC) curve analyses were conducted. As shown in Figure 3 and Table 3, of the five candidate miRNAs investigated, serum miR-31-5p exhibited the highest accuracy in diagnosing oral cancer, with an area under the ROC curve (AUC) of 0.661 (p = 0.002). The observed AUC of the five-miRNA panel was 0.776, with a sensitivity of 76.8% and a specificity of 73.6% (Figure 3F). Moreover, the predicted probability from the logistic regression model based on the five-miRNA panel was as follows: logit (p = oral cancer [cancer/normal]) = −5.816 + (0.608 × ΔCtmiR-99a-5p) − (0.659 × ΔCtmiR-31-5p) + (0.228 × ΔCtmiR-138-5p) − (0.475 × ΔCtmiR-21-5p) + (0.349 × ΔCtmiR-375-3p).
Figure 3.
Diagnostic Values of Five Serum miRNAs in Patients with Oral Cancer
ROC curves of serum miR-99a-5p (A), miR-31-5p (B), miR-138-5p (C), miR-21-5p (D), and miR-375-3p (E) levels and the five-miRNA panel (F) were used to differentiate oral cancer patients from normal controls in cohort 1.
Table 3.
ROC Curve Analyses of Serum miRNAs for Distinguishing Oral Cancer Patients from Normal Controls
| AUC | Sensitivity | Specificity | p Value | 95% CI | |
|---|---|---|---|---|---|
| miR-99a-5p | 0.521 | 86.6% | 24.5% | 0.680 | 0.421–0.622 |
| miR-31-5p | 0.661 | 69.8% | 52.4% | 0.002 | 0.564–0.758 |
| miR-138-5p | 0.547 | 67.9% | 51.2% | 0.360 | 0.448–0.646 |
| miR-21-5p | 0.579 | 64.2% | 46.3% | 0.123 | 0.483–0.675 |
| miR-375-3p | 0.514 | 60.4% | 50.0% | 0.787 | 0.413–0.614 |
| Combinationa | 0.776 | 76.8% | 73.6% | 0.000 | 0.695–0.857 |
CI, confidence interval
Combination of miR-99a-5p, miR-31-5p, miR-138-5p, miR-21-5p, and miR-375-3p
Next, we used a ROC curve to assess the diagnostic value of the five circulating miRNAs in discriminating preoperative oral cancer patients from postoperative patients. As shown in Figure S2 and Table S2, serum miR-31-5p showed the highest accuracy in discriminating preoperative oral cancer patients from postoperative patients. The observed AUC of the five-miRNA panel was 0.891, with a sensitivity of 81.7% and a specificity of 63.8% (Figure S2F). The predicted probability from the logistic regression model based on the five-miRNA panel to distinguish preoperative patients from postoperative patients was as follows: logit (p = oral cancer [pre/post]) = −4.704 + (0.419 × ΔCtmiR-99a-5p) − (0.59 × ΔCtmiR-31-5p) + (0.524 × ΔCtmiR-138-5p) − (0.392 × ΔCtmiR-21-5p) + (0.075 × ΔCtmiR-375-3p).
Collectively, the above results indicated that the upregulated serum miR-31-5p level was able to discriminate oral cancer patients from healthy subjects and monitor the recurrence of oral cancer. Circulating miR-31-5p might serve as a candidate biomarker for the diagnosis of oral cancer. Moreover, a circulating miRNA panel based on these five miRNAs might also have the potential to act as a biomarker for the detection and therapeutic monitoring of oral cancer with high accuracy.
Effect of miR-31-5p on the Proliferation of Oral Cancer Cells In Vitro
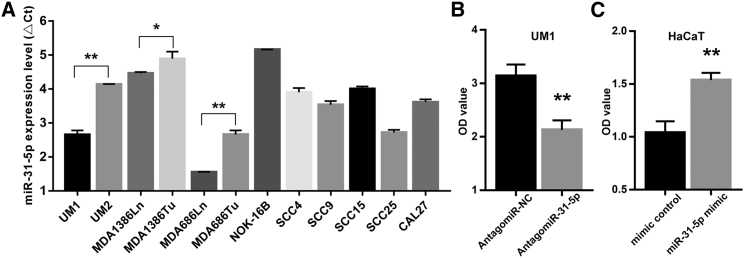
To further investigate the effect of miR-31-5p on the proliferation of oral cancer, we first measured the expression of miR-31-5p in oral cancer cell lines and normal oral epithelial cell lines. As shown in Figure 4A, miR-31-5p expression levels were significantly increased in oral cancer cell lines compared with normal epithelial cell lines (NOK-16B). Moreover, we found that the expression levels of miR-31-5p were significantly higher in cell lines with higher migratory and invasive potential (UM1/MDA1386Ln/MDA686Ln) than their paired cell lines (UM2/MDA1386Tu/MDA686Tu). A miR-31-5p inhibitor (antagomiR-31-5p) significantly inhibited the proliferation of UM1 cells (Figure 4B), and miR-31-5p mimics significantly enhanced the proliferation ability of normal epithelial HaCaT cells (Figure 4C). These data indicate that miR-31-5p has the ability to enhance the proliferation of oral cancer cells.
Figure 4.
The Expression Level of miR-31-5p in Oral Cancer Cell Lines and the Effect of miR-31-5p on the Proliferation of Oral Cancer Cells In Vitro
(A) Relative expression levels of miR-31-5p in oral cancer cell lines and normal epithelial cell lines were detected by real-time qPCR. (B) The proliferation ability of UM1 cells treated with antagomiR-NC or antagomiR-31-5p was measured by the CCK-8 assay. (C) The proliferation ability of HaCaT cells treated with control mimic or miR-31-5p mimic was measured by the CCK-8 assay. The results are shown as mean ± SD. *p < 0.05; **p < 0.01.
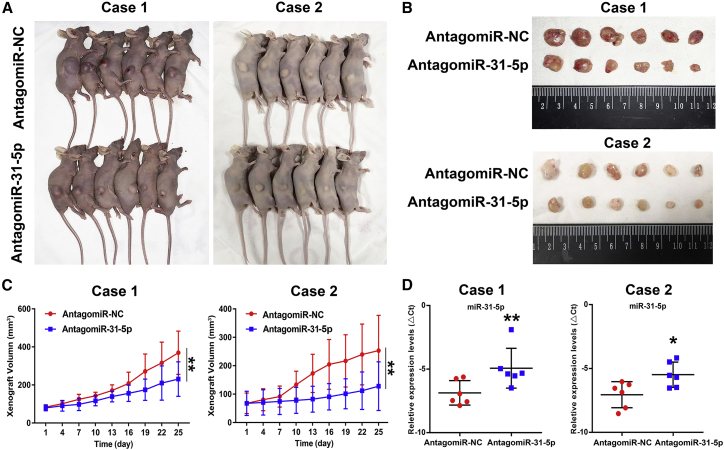
Effect of miR-31-5p on the Growth of Oral Cancer in PDX Models
To further validate the role of miR-31-5p in the tumorigenesis of oral cancer, we constructed patient-derived xenograft models of oral cancer. Two PDX models used in the present study were established using small biopsied tumor specimens from patients with advanced-stage oral cancer. These PDX model mice (2 cases) were intratumorally injected with antagomiR-31-5p or antagomiR-NC two times a week. As shown in Figure 5, antagomiR-31-5p significantly delayed the growth of PDX in two primary oral cancer patients. The inhibition rate of tumor growth was 37.5% (case 1) and 49.5% (case 2). Further, real-time qPCR was performed to detect the expression levels of miR-31-5p in the excised PDX tumors and revealed decreased miR-31-5p expression in the antagomiR-31-5p-treated groups compared to the antagomiR-NC-treated groups (Figure 5D). In addition, the immunohistochemical staining of tumor tissue derived from the xenografts indicated a marked increase in the expression of phosphatase and tensin homolog (PTEN) and a reduction in p-AKT, both of which are target genes of miR-31-5p, in the antagomiR-31-5p-treated groups (Figure S3). Taken together, these results verified that the intratumoral injection of antagomiR-31-5p could effectively inhibit tumor growth in oral cancer PDX models.
Figure 5.
Effect of miR-31 on the Growth of Oral Cancer PDX In Vivo
(A) Representative images of oral cancer PDX model mice. Mice were intratumorally injected with antagomiR-31-5p (n = 6), and antagomiR-NC served as a control (n = 6) in every case. (B) Representative images of tumor tissues from PDX model mice at 28 days after treatment. (C) Tumor growth curves of oral cancer PDX. AntagomiR-31-5p significantly inhibited PDX growth in two patients. **p < 0.01. (D) Relative expression levels of miR-31-5p in PDX tumors were assessed by real-time qPCR after normalizing to U6. The vertical lines represent the 25th percentile value, the mean value, and the 75th percentile value of the observations. *p < 0.05; **p < 0.01.
Discussion
miRNAs have shown great potential as diagnostic, prognostic, and therapeutic biomarkers because they modulate tumorigenesis and the progression of various cancers.28 A tremendous number of studies, including ours,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 have been carried out to investigate the role of miRNA dysregulation in the development and prognosis of oral cancer.4, 9, 32 Among these studies, the upregulation of miR-31 and miR-21 and the downregulation of miR-99a, miR-138, and miR-375 were often found in oral cancer.15, 16, 17, 18, 19, 20, 23, 24, 25, 28, 33, 34, 35, 36, 37, 38 Some miRNAs or miRNA signatures have become promising biomarkers in oral cancer.10, 31 For example, Chen et al.10 found a five-miRNA signature, including miR-99a, miR-31, miR-410, miR-424, and miR-495, in predicting radiotherapy response in oral cancer. We also revealed miRNA-21 and miRNA-375 from oral cytology as biomarkers for oral tongue cancer detection.25 Maclellan et al.39 revealed that miRNAs showing disease-associated expression changes in blood are not necessarily the same ones that are differentially expressed in cancer tissues, and miRNA expression patterns in tumor and serum samples from the same patient might not correlate. To further identify powerful circulating miRNA biomarkers for oral cancer, we first screened miRNAs that are dysregulated in oral cancer based on our previous studies16, 20, 25, 31 and selected five potential miRNA biomarkers, namely, miR-21-5p, miR-31-5p, miR-138-5p, miR-99a-5p, and miR-375-3p. Although the pattern of these five circulating miRNA levels was consistent with the expression pattern in the tissue of oral cancer patients, the expression of these five miRNAs did not correlate between serum and tissue.
An increasing number of studies have revealed the potential of circulating miRNAs as novel, noninvasive biomarkers for detecting, monitoring, and predicting the prognosis of patients with oral cancer.9, 12, 13 Pedersen et al.9 found that the proposed combination of miR-30a-5p and miR-769-5p in plasma from oral cancer patients could serve as a minimally invasive biomarker for the diagnosis and control of T-site recurrences. Emerging evidence has indicated that miR-31-5p is upregulated and acts as an oncogenic miRNA in a wide variety of neoplasms,40, 41, 42 including oral cancer.41 Meta-analyses indicated that high miR-31-5p expression is associated with a poor overall survival (OS) in patients with general cancers and that miR-31-5p may be a useful clinical prognostic biomarker.43, 44 Moreover, in the 4-nitroquinoline-1-oxide (4NQO)-induced mouse tongue carcinogenesis model, Kao et al.45 found that an increase in miR-31-5p staining paralleled the severity of 4NQO-induced epithelial pathogenesis in the tongue epithelium. A progressive increase in miR-31-5p in both saliva and plasma samples was also noted. In the current study, we found that the miR-31-5p level, but not the levels of the other four miRNAs (miR-99a-5p, miR-138-5p, miR-21-5p, and miR-375-3p), was significantly increased in the sera of oral cancer patients compared to those in normal controls. Of the five candidate miRNAs investigated, serum miR-31-5p exhibited the highest accuracy in diagnosing oral cancer. Moreover, the combination of these five miRNA signatures also provided a high AUC for the diagnosis of oral cancer. Thus, circulating miR-31-5p might be useful as a systemic biomarker that distinguishes oral cancer from a noncancerous status, and circulating miRNA panels based on these five miRNAs might also have the potential to act as biomarkers for the detection of oral cancer with high accuracy.
Because treatment (surgery and radiation) can alter the appearance of normal tissue surrounding a lesion, which can delay recurrence detection, circulating miRNAs may be applicable as an alternative means of posttreatment monitoring.38 Maclellan et al.39 demonstrated that many circulating miRNAs are tumor specific, as the circulating miRNA levels correspondently decrease or increase after tumor resection. In the present study, we also observed that circulating miR-31-5p levels in oral cancer patients declined after surgery. Therefore, circulating miR-31-5p may be useful to monitor the recurrence and curative effect of oral cancer.
As a biomarker, many studies have implemented a miRNA mimic, including miR-31-5p,40, 46 or an inhibitor as a therapeutic target of cancer.47 For example, Liu et al.40 found that the engineered knockdown of miR-31-5p substantially repressed lung cancer cell growth and tumorigenicity in a dose-dependent manner. Lai et al.48 revealed that miR-31-5p had a functional impact on oral cancer cell migration and invasion and direct regulation of the rate-limiting enzyme in peroxisomal β-oxidation, ACOX1. Liu et al.41 also found that the ectopic expression of miR-31 increased the oncogenic potential of HNSCC cells under normoxic conditions in cell culture or in tumor xenografts. In the present study, miR-31-5p was significantly upregulated in a panel of oral cancer cell lines. antagomiR-31-5p inhibited the proliferation of oral cancer cells, and miR-31-5p mimic promoted the proliferation ability of normal epithelial HaCaT cells. We also used oral cancer PDX models to investigate the target effect of miR-31-5p and found that antagomiR-31-5p significantly delayed the growth of patient-derived xenografts in two primary oral cancer patients. It has been reported that PTEN, a representative tumor suppressor in the phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (AKT) pathway, was negatively related to the expression of miR-31 in HNSCC.49 Zheng et al.50 found that the miR-31-SOX10 axis regulates tumor growth and the chemotherapy resistance of melanoma via the PI3K/AKT pathway. In our study, the expression level of p-AKT was significantly reduced in antagomiR-31-5p-treated xenografts, and the PTEN expression level was increased. These results suggest that miR-31-5p might function as a therapeutic target miRNA in oral cancer via the PTEN/AKT pathway.
In conclusion, we defined distinctive circulating miRNA signatures for oral cancer diagnosis and revealed that circulating miR-31-5p might be a potential diagnostic biomarker. Circulating miR-31-5p may be useful for monitoring recurrence and the curative effect of oral cancer. miR-31-5p might be a powerful therapeutic target miRNA in oral cancer via the PTEN/AKT pathway.
Materials and Methods
Written informed consent for the biological studies was obtained from each patient involved in the study, and the study was approved by the ethical committee of First Affiliated Hospital, Sun Yat-Sen University (no. 2017173). All animal studies were approved by the ethical committee of First Affiliated Hospital, Sun Yat-Sen University (no. 2017113).
Study Design and Patient Cohort
The study design is shown in Figure S1. First, five differentially expressed miRNAs (miR-99a-5p, miR-31-5p, miR-138-5p, miR-21-5p, and miR-375-3p) were screened from our previous miRNA profiling studies.16, 20, 25, 31 Then, these differentially expressed miRNAs were verified in 11 paired match tumor tissues and adjacent tissues. Next, an independent cohort (cohort 1), which included 82 oral cancer patients and 53 normal subjects, was used to determine the dysregulated miRNAs in the sera of oral cancer patients. Samples for cohort 2 (11 paired match tumor tissues and adjacent normal tissues), cohort 3 (24 paired match tumor tissues and preoperative sera), and cohort 4 (82 cases of preoperation sera and 47 cases of postoperation sera) were obtained from cohort 1. All patients provided written informed consent and were operated between November 2016 to August 2017. The demographics and clinical data of this patient cohort are presented in Table S3. All tumors underwent stage classification according to the American Joint Committee on Cancer system. None of the patients had received adjuvant chemotherapy or radiotherapy before surgery. The study was approved by the Ethical Committee of the First Affiliated Hospital, Sun Yat-Sen University (no. 2017173).
Preparation of Serum and Tissue Samples
Serum samples were collected from oral cancer patients 1 week prior to the operation and 1 week after the operation. The 5 mL of venous blood from each participant was centrifuged at 4,000 rpm for 10 min at 4°C within 2 h of collection, followed by a second centrifugation at 12,000 rpm for 15 min at 4°C to eliminate any residual cell debris. Supernatant serum was then stored at −80°C until further processing. Twenty-four fresh sets of primary oral cancer tumor tissues and 11 adjacent normal tissues obtained during surgery were immediately immersed in RNA later (QIAGEN, Valencia, CA) and stored at −20°C until RNA extraction.
miRNA Extraction and Real-Time qPCR
Total RNA used to determine the miRNA level was isolated with the miRcute miRNA Isolation Kit (Tiangen Biotech, China) according to the manufacturer’s protocol. miRNAs were reverse transcribed into cDNA using a miRcute miRNA First-Strand cDNA Synthesis Kit (Tiangen Biotech), and a miRcute miRNA qPCR Detection Kit (SYBR Green; Tiangen Biotech, China) was used for real-time qPCR analysis on an ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. All reactions were performed in triplicate, and Ct values were measured with default threshold settings. The specificity of the real-time qPCR product was confirmed using melting curve analysis, and miRNAs with a Ct value of more than 35 and a detection rate of less than 75% in each group were excluded from further analyses. The relative expression levels of miR-99a-5p, miR-31-5p, miR-138-5p, miR-21-5p, and miR-375-3p were normalized to that of the internal control U6 using the delta Ct (δCt) method. Primer sequences of the miRNAs used for real-time qPCR are shown in Table S4.
Cell Culture and Treatment
Normal epithelial cell lines (HaCaT and NOK-16B) and human oral cancer cell lines (SCC4, SCC9, SCC15, SCC25 and CAL27, UM1/UM2, 1386Tu/1386Ln, and 686Tu/686Ln) were obtained from Dr. Xiaofeng Zhou.25 UM1 and UM2 are paired cell lines with different metastatic potential that were generated from a single patient with SCC of the tongue.18 1386Tu/1386Ln and 686Tu/686Ln are paired cell lines that were generated from primary tumors and lymph node metastatic diseases from HNSCC patients.20 These cell lines were cultured in DMEM/F12 medium (for UM1, UM2, 1386Tu, 1386Ln, 686Tu, 686Ln, SCC4, SCC9, SCC15, and SCC25 cells), high-glucose DMEM (for NOK-16B and CAL27 cells), or RPMI-1640 (for HaCaT cells) supplemented with 10% fetal bovine serum (Gibco, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin in a standard humidified incubator with 5% CO2 at 37°C.
miR-31-5p mimic, control mimic (miR1N0000001-1-5; RiboBio, Guangzhou, China), antagomiR-31-5p (miR-31 inhibitor), and antagomiR-NC (miR3N0000001-4-5; RiboBio) were designed and synthesized by RiboBio. The sequences of miR-31-5p mimic and antagomiR-31-5p are presented in Table S5. In brief, the indicated cells were plated into 6-well plates to 50% confluence. For each well, 5 μL of mimic (miR-31-5p mimic or control mimic; concentration 20 μM) or 10 μL of antagomiR (antagomiR-31-5p or antagomiR-NC; concentration 20 μM) and 12 μL of riboFECT Transfection Reagent (RiboBio) were added into 2 mL of antibiotic-free opti-MEM (Invitrogen, CA, USA) and then mixed together to form the transfection complex (50 nM for mimic; 100 nM for antagomiR). The transfection complex was added to the indicated cells and incubated for 24 h before replacing the medium.
Cell Proliferation
Cell proliferation assays were performed using cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer’s instructions as described previously.51 The indicated cells were seeded into 96-well plates at a density of 3 × 103 cells per well, and the optical density (OD value) of each well at a wavelength of 450 nm at 72 h was detected using an EL×800 plate reader (BioTek Instruments, Winooski, VT, USA).
PDX Model of Oral Cancer
To establish the PDX models, tumor tissues from two oral cancer patients were processed after tumor resection. The tumor specimen was cut into pieces with diameters of approximately 3–5 mm and implanted into the flanks of female nude mice (aged 4 or 5 weeks and weighing 18–22 g). When the tumors reached approximately 1,000–1,500 mm3, mice were euthanized, and xenografts were collected and subsequently implanted into another set of mice using the same procedure. The third generation of xenografts was treated intratumorally twice daily with either antagomiR-31-5p (10 nmol/50 μL) or antagomiR-NC four times when tumors reached a volume of 70 mm3. Tumor volumes were calculated as ½ × (length × width2), and the tumor growth curve (y = Aekday) was determined as previously described.32 Animals were then sacrificed 28 days after treatment, and real-time qPCR was performed to examine the expression of miR-31-5p in the PDX. Furthermore, immunohistochemical staining was used to detect the expression of miR-31 target genes (AKT and PTEN) in the xenograft tissues as previously described.23 The primary antibodies used were phosphorylated AKT (Cell Signaling Technology; 1:150; no. 4060) and PTEN (CST; 1:100; no. 9188).
The patients’ clinicopathologic characteristics for these two PDX models are listed in Table S6. All procedures for animal experiments were approved by the Ethical Committee of the First Affiliated Hospital, Sun Yat-Sen University (no. 2017113) and performed in accordance with institutional guidelines.
Statistical Analysis
All experiments were repeated at least three times, and the data are presented as the mean ± SD. Statistics were calculated using SPSS 19.0 software (SPSS, USA). The significance of differences between two groups was determined by the Mann-Whitney test or the Wilcoxon rank test and multiple groups by Kruskal-Wallis tests. Student’s t test was employed to compare differences in the OD value or tumor volume between two groups. ROC curve analysis was performed to evaluate the diagnostic accuracy (AUC, sensitivity, and specificity) of the miRNAs. Logistic regression was used to develop a combined miRNA panel to predict the probability of oral cancer. Correlations between variables were evaluated using Spearman’s rank correlation coefficient. Statistical significance was defined as a two-sided p value of less than 0.05.
Author Contributions
A.W. and K.L. conceived of, designed, and supervised the study. Z.L., Q.H., W.L., J.L., Q.W., X.W., Y.W., J.S., and L.L. collected the samples and performed the experiments. A.W., Z.L., Z.C., and X.Z. analyzed the data. J.H., Q.S., and L.C. provided technical assistance with the experiments. A.W. and Z.L. wrote the manuscript. All co-authors have reviewed and approved this version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by National Program on Key Research Project of China (2016YFC0902700; Precision Medicine), National Nature Science Foundation of China (NSFC81672659, NSFC81472523, and NSFC81272953), Guangzhou Science and Technology Program Project Collaborative Innovation Major Projects (201704020112), Guangdong Natural Science Foundation (2015A030313017 and 2017A030310197), The Science & Technology Planning Project of Guangdong Province (2017A030303015), and a Lilly USA Research Award in Cancer Prevention and Early Detection from Prevent Cancer Foundation.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.03.012.
Contributor Information
Keqian Lian, Email: liankeqian888@163.com.
Anxun Wang, Email: anxunwang@yahoo.com.
Supplemental Information
References
- 1.Tamura T., Ichikawa T., Nakahata S., Kondo Y., Tagawa Y., Yamamoto K., Nagai K., Baba T., Yamaguchi R., Futakuchi M. Loss of NDRG2 expression confers oral squamous cell carcinoma with enhanced metastatic potential. Cancer Res. 2017;77:2363–2374. doi: 10.1158/0008-5472.CAN-16-2114. [DOI] [PubMed] [Google Scholar]
- 2.Cabel L., Proudhon C., Romano E., Girard N., Lantz O., Stern M.H., Pierga J.Y., Bidard F.C. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2018;15:639–650. doi: 10.1038/s41571-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 3.Setterquist R.A., Rai A.J., Zeringer E., Li M., Barta T., Schageman J., Magdaleno S., Vlassov A.V. Development of a standard operating procedure for exosome isolation and analysis using clinical samples: application to cancer biomarker discovery. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research. 2014;74 Abstract nr 1870. [Google Scholar]
- 4.Domingues C.S.D.C., Serambeque B.P., Laranjo Cândido M.S., Marto C.M.M., Veiga F.J.B., Sarmento Antunes Cruz Ribeiro A.B., Figueiras A.R.R., Botelho M.F.R., Dourado M.A.R.F. Epithelial-mesenchymal transition and microRNAs: challenges and future perspectives in oral cancer. Head Neck. 2018;40:2304–2313. doi: 10.1002/hed.25381. [DOI] [PubMed] [Google Scholar]
- 5.Dallaire A., Frédérick P.M., Simard M.J. Somatic and germline microRNAs form distinct silencing complexes to regulate their target mRNAs differently. Dev. Cell. 2018;47:239–247.e4. doi: 10.1016/j.devcel.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Jinushi T., Shibayama Y., Kinoshita I., Oizumi S., Jinushi M., Aota T., Takahashi T., Horita S., Dosaka-Akita H., Iseki K. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3:1544–1552. doi: 10.1002/cam4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai R., Weng C., Dong H., Li S., Chen G., Xu Z. MicroRNA-409-3p suppresses colorectal cancer invasion and metastasis partly by targeting GAB1 expression. Int. J. Cancer. 2015;137:2310–2322. doi: 10.1002/ijc.29607. [DOI] [PubMed] [Google Scholar]
- 8.Liu C.J., Kao S.Y., Tu H.F., Tsai M.M., Chang K.W., Lin S.C. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010;16:360–364. doi: 10.1111/j.1601-0825.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen N.J., Jensen D.H., Lelkaitis G., Kiss K., Charabi B.W., Ullum H., Specht L., Schmidt A.Y., Nielsen F.C., von Buchwald C. MicroRNA-based classifiers for diagnosis of oral cavity squamous cell carcinoma in tissue and plasma. Oral Oncol. 2018;83:46–52. doi: 10.1016/j.oraloncology.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., Wen Y., Zhang J., Sun W., Lui V.W.Y., Wei Y., Chen F., Wen W. Prediction of radiotherapy response with a 5-microRNA signature-based nomogram in head and neck squamous cell carcinoma. Cancer Med. 2018;7:726–735. doi: 10.1002/cam4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X., Wang W., Yang Y., Du L., Yang X., Wang L., Zheng G., Duan W., Wang R., Zhang X. Identification of circulating microRNA signatures as potential noninvasive biomarkers for prediction and prognosis of lymph node metastasis in gastric cancer. Oncotarget. 2017;8:65132–65142. doi: 10.18632/oncotarget.17789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana H., Sho R., Takeda Y., Zhang X., Yoshida Y., Narimatsu H., Otani K., Ishikawa S., Fukao A., Asao H., Iino M. Circulating miR-223 in oral cancer: its potential as a novel diagnostic biomarker and therapeutic target. PLoS ONE. 2016;11:e0159693. doi: 10.1371/journal.pone.0159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes C.B., Magalhães L.L., Teófilo C.R., Alves A.P.N.N., Montenegro R.C., Negrini M., Ribeiro-Dos-Santos Â. Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC Cancer. 2018;18:721. doi: 10.1186/s12885-018-4631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q., Zhou X., Li S., Jin Y., Chen Z., Chen D., Cai Y., Liu Z., Zhao T., Wang A. MicroRNA-181a suppresses salivary adenoid cystic carcinoma metastasis by targeting MAPK-Snai2 pathway. Biochim. Biophys. Acta. 2013;1830:5258–5266. doi: 10.1016/j.bbagen.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y., Chen D., Cabay R.J., Wang A., Crowe D.L., Zhou X. Role of microRNA-138 as a potential tumor suppressor in head and neck squamous cell carcinoma. Int. Rev. Cell Mol. Biol. 2013;303:357–385. doi: 10.1016/B978-0-12-407697-6.00009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z., Jin Y., Yu D., Wang A., Mahjabeen I., Wang C., Liu X., Zhou X. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol. 2012;48:686–691. doi: 10.1016/j.oraloncology.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y., Wang C., Liu X., Mu W., Chen Z., Yu D., Wang A., Dai Y., Zhou X. Molecular characterization of the microRNA-138-Fos-like antigen 1 (FOSL1) regulatory module in squamous cell carcinoma. J. Biol. Chem. 2011;286:40104–40109. doi: 10.1074/jbc.C111.296707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L., Dai Y., Liu X., Wang C., Wang A., Chen Z., Heidbreder C.E., Kolokythas A., Zhou X. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum. Genet. 2011;129:189–197. doi: 10.1007/s00439-010-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L., Liu X., Kolokythas A., Yu J., Wang A., Heidbreder C.E., Shi F., Zhou X. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int. J. Cancer. 2010;127:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Jiang L., Wang A., Yu J., Shi F., Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang L., Liu X., Chen Z., Jin Y., Heidbreder C.E., Kolokythas A., Wang A., Dai Y., Zhou X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem. J. 2010;432:199–205. doi: 10.1042/BJ20100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Wang A., Heidbreder C.E., Jiang L., Yu J., Kolokythas A., Huang L., Dai Y., Zhou X. MicroRNA-24 targeting RNA-binding protein DND1 in tongue squamous cell carcinoma. FEBS Lett. 2010;584:4115–4120. doi: 10.1016/j.febslet.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji M., Wang W., Yan W., Chen D., Ding X., Wang A. Dysregulation of AKT1, a miR-138 target gene, is involved in the migration and invasion of tongue squamous cell carcinoma. J. Oral Pathol. Med. 2017;46:731–737. doi: 10.1111/jop.12551. [DOI] [PubMed] [Google Scholar]
- 24.He Q., Chen Z., Dong Q., Zhang L., Chen D., Patel A., Koya A., Luan X., Cabay R.J., Dai Y. MicroRNA-21 regulates prostaglandin E2 signaling pathway by targeting 15-hydroxyprostaglandin dehydrogenase in tongue squamous cell carcinoma. BMC Cancer. 2016;16:685. doi: 10.1186/s12885-016-2716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Q., Chen Z., Cabay R.J., Zhang L., Luan X., Chen D., Yu T., Wang A., Zhou X. microRNA-21 and microRNA-375 from oral cytology as biomarkers for oral tongue cancer detection. Oral Oncol. 2016;57:15–20. doi: 10.1016/j.oraloncology.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L., Ren Y., Tang H., Wang W., He Q., Sun J., Zhou X., Wang A. Deregulation of the miR-222-ABCG2 regulatory module in tongue squamous cell carcinoma contributes to chemoresistance and enhanced migratory/invasive potential. Oncotarget. 2015;6:44538–44550. doi: 10.18632/oncotarget.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D., Cabay R.J., Jin Y., Wang A., Lu Y., Shah-Khan M., Zhou X. MicroRNA deregulations in head and neck squamous cell carcinomas. J. Oral Maxillofac. Res. 2013;4:e2. doi: 10.5037/jomr.2013.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D., Chen Z., Jin Y., Dragas D., Zhang L., Adjei B.S., Wang A., Dai Y., Zhou X. MicroRNA-99 family members suppress Homeobox A1 expression in epithelial cells. PLoS ONE. 2013;8:e80625. doi: 10.1371/journal.pone.0080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C.N., Deng Y.T., Tang J.Y., Cheng S.J., Chen S.T., Li Y.J., Wu T.S., Yang M.H., Lin B.R., Kuo M.Y. MicroRNA-29b regulates migration in oral squamous cell carcinoma and its clinical significance. Oral Oncol. 2015;51:170–177. doi: 10.1016/j.oraloncology.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Libório-Kimura T.N., Jung H.M., Chan E.K. miR-494 represses HOXA10 expression and inhibits cell proliferation in oral cancer. Oral Oncol. 2015;51:151–157. doi: 10.1016/j.oraloncology.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z., Yu T., Cabay R.J., Jin Y., Mahjabeen I., Luan X., Huang L., Dai Y., Zhou X. miR-486-3p, miR-139-5p, and miR-21 as biomarkers for the detection of oral tongue squamous cell carcinoma. Biomark. Cancer. 2017;9:1–8. doi: 10.4137/BIC.S40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sakka H., Kujan O., Farah C.S. Assessing miRNAs profile expression as a risk stratification biomarker in oral potentially malignant disorders: a systematic review. Oral Oncol. 2018;77:57–82. doi: 10.1016/j.oraloncology.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Yap T., Koo K., Cheng L., Vella L.J., Hill A.F., Reynolds E., Nastri A., Cirillo N., Seers C., McCullough M. Predicting the presence of oral squamous cell carcinoma using commonly dysregulated microRNA in oral swirls. Cancer Prev. Res. (Phila.) 2018;11:491–502. doi: 10.1158/1940-6207.CAPR-17-0409. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z.Y., Luo Z.Q., Zhang L.J., Li J., Liu J.Q. Integrated analysis and microRNA expression profiling identified seven miRNAs associated with progression of oral squamous cell carcinoma. J. Cell. Physiol. 2017;232:2178–2185. doi: 10.1002/jcp.25728. [DOI] [PubMed] [Google Scholar]
- 35.Kao S.Y., Tsai M.M., Wu C.H., Chen J.J., Tseng S.H., Lin S.C., Chang K.W. Co-targeting of multiple microRNAs on factor-inhibiting hypoxia-Inducible factor gene for the pathogenesis of head and neck carcinomas. Head Neck. 2016;38:522–528. doi: 10.1002/hed.23912. [DOI] [PubMed] [Google Scholar]
- 36.Siow M.Y., Ng L.P., Vincent-Chong V.K., Jamaludin M., Abraham M.T., Abdul Rahman Z.A., Kallarakkal T.G., Yang Y.H., Cheong S.C., Zain R.B. Dysregulation of miR-31 and miR-375 expression is associated with clinical outcomes in oral carcinoma. Oral Dis. 2014;20:345–351. doi: 10.1111/odi.12118. [DOI] [PubMed] [Google Scholar]
- 37.Tu H.F., Lin S.C., Chang K.W. MicroRNA aberrances in head and neck cancer: pathogenetic and clinical significance. Curr. Opin. Otolaryngol. Head Neck Surg. 2013;21:104–111. doi: 10.1097/MOO.0b013e32835e1d6e. [DOI] [PubMed] [Google Scholar]
- 38.Zeljic K., Jovanovic I., Jovanovic J., Magic Z., Stankovic A., Supic G. MicroRNA meta-signature of oral cancer: evidence from a meta-analysis. Ups. J. Med. Sci. 2018;123:43–49. doi: 10.1080/03009734.2018.1439551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maclellan S.A., Lawson J., Baik J., Guillaud M., Poh C.F., Garnis C. Differential expression of miRNAs in the serum of patients with high-risk oral lesions. Cancer Med. 2012;1:268–274. doi: 10.1002/cam4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X., Sempere L.F., Ouyang H., Memoli V.A., Andrew A.S., Luo Y., Demidenko E., Korc M., Shi W., Preis M. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J. Clin. Invest. 2010;120:1298–1309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C.J., Tsai M.M., Hung P.S., Kao S.Y., Liu T.Y., Wu K.J., Chiou S.H., Lin S.C., Chang K.W. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–1644. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 42.Motoyama K., Inoue H., Takatsuno Y., Tanaka F., Mimori K., Uetake H., Sugihara K., Mori M. Over- and under-expressed microRNAs in human colorectal cancer. Int. J. Oncol. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 43.Wang S., Hu J., Zhang D., Li J., Fei Q., Sun Y. Prognostic role of microRNA-31 in various cancers: a meta-analysis. Tumour Biol. 2014;35:11639–11645. doi: 10.1007/s13277-014-2492-x. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y., Chen Y., Lin J., Liu Y., Luo K., Cao Y., Wang T., Jin H., Su Z., Wu H. Circulating miR-31 as an effective biomarker for detection and prognosis of human cancer: a meta-analysis. Oncotarget. 2017;8:28660–28671. doi: 10.18632/oncotarget.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao Y.Y., Tu H.F., Kao S.Y., Chang K.W., Lin S.C. The increase of oncogenic miRNA expression in tongue carcinogenesis of a mouse model. Oral Oncol. 2015;51:1103–1112. doi: 10.1016/j.oraloncology.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Tan X., Lin J., Yuan L., Chen J., Qiu L., Huang W. Minicircle-oriP-miR-31 as a novel EBNA1-specific miRNA therapy approach for nasopharyngeal carcinoma. Hum. Gene Ther. 2017;28:415–427. doi: 10.1089/hum.2016.136. [DOI] [PubMed] [Google Scholar]
- 47.Ahmadzada T., Reid G., McKenzie D.R. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 2018;10:69–86. doi: 10.1007/s12551-017-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai Y.H., Liu H., Chiang W.F., Chen T.W., Chu L.J., Yu J.S., Chen S.J., Chen H.C., Tan B.C. MiR-31-5p-ACOX1 axis enhances tumorigenic fitness in oral squamous cell carcinoma via the promigratory prostaglandin E2. Theranostics. 2018;8:486–504. doi: 10.7150/thno.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odar K., Boštjančič E., Gale N., Glavač D., Zidar N. Differential expression of microRNAs miR-21, miR-31, miR-203, miR-125a-5p and miR-125b and proteins PTEN and p63 in verrucous carcinoma of the head and neck. Histopathology. 2012;61:257–265. doi: 10.1111/j.1365-2559.2012.04242.x. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y., Sun Y., Liu Y., Zhang X., Li F., Li L., Wang J. The miR-31-SOX10 axis regulates tumor growth and chemotherapy resistance of melanoma via PI3K/AKT pathway. Biochem. Biophys. Res. Commun. 2018;503:2451–2458. doi: 10.1016/j.bbrc.2018.06.175. [DOI] [PubMed] [Google Scholar]
- 51.Sun J., Lu Z., Deng Y., Wang W., He Q., Yan W., Wang A. Up-regulation of INSR/IGF1R by C-myc promotes TSCC tumorigenesis and metastasis through the NF-κB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(5 Pt A):1873–1882. doi: 10.1016/j.bbadis.2018.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.