Abstract
Background
PTEN gene triggers cells to undergo apoptosis and promotes myocardial dysfunction. Several TNF family cytokines are elevated during acute myocardial infarction (AMI). Their role in predicting subsequent prognosis in these setting remains poorly understood. We assessed serum levels of PTEN gene activity & TNF-α in acute ST elevation myocardial infarction and determined the impact of their levels on both left ventricular function and the clinical outcome in these patients.
Methods and results
Seventy patients with AMI and seventy persons as control group were subjected to: ECG, echocardiography, serum TNF-α and PTEN gene assessment. Patients were classified into: Group I (n = 32): All had left ventricular systolic failure. Group II (n = 38): without left ventricular systolic failure. Group I had a statistically significant higher serum levels of both TNF-α & PTEN gene activity as compared to group II. EF% at presentation was weakly correlated with serum levels of both markers in both groups. However at follow up, EF% in group I showed a significant negative correlations with both serum levels of TNF-α and PTEN gene activity (r = 0.77 & r = 0.67, respectively). During one year follow, 5 patients died of cardiovascular causes and 6 patients had recurrent hospitalization with heart failure. These patients had statistically significant increased serum levels of TNF-α & PTEN gene activity levels as compared by other patients.
Conclusions
Patients with acute myocardial infarction had statistically significant increased serum levels of PTEN & TNF-α gene activity. Both markers predict worsening of left ventricular systolic functions, development of heart failure and death.
Keywords: PTEN gene, TNF-α, Acute myocardial infarction
1. Introduction
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) gene acts as a tumor suppressor gene. It regulates the cycle of cell division and modifies proteins and fats by removing phosphate groups. It acts as part of a chemical pathway that signals cells to stop dividing, triggers cells to self-destruct (undergo apoptosis) [1] and promotes myocardial dysfunction. Moreover, cardiac-specific PTEN inactivation protects the heart from functional failure and fibrosis in a mouse model [2].
Tumor necrosis factor-alpha (TNF-α) is a master cytokine that is produced in significant quantities within the infarcted myocardium very soon after myocardial infarction (MI). It activates other cytokines such as interleukin-1 (IL-1) and IL-6 and orchestrates the host tissue response to acute injury [3]. Despite several TNF family cytokines are elevated during acute MI, their role in predicting subsequent prognosis in these setting remains poorly understood [4].
We assessed serum levels of TNF-α & PTEN gene activity in acute ST elevation myocardial infarction and determined the impact of their levels on both left ventricular function and the clinical outcome in these patients.
2. Methods
2.1. Study population
This is an observational comparative study that included seventy patients with acute ST elevation myocardial infarction as defined in the 3rd universal definition of myocardial infarction [5] and seventy persons (age & sex matched) as a control group.
Patients with any of the following criteria had been excluded from the study: old myocardial infarction, atrial flutter, atrial fibrillation, valve prosthetic, any systemic disease can elevate TNF-α, rheumatic valvular affection, left ventricular aneurysm, previous history of heart failure, poor echocardiographic view. Informed consent was obtained from each patient. All included patients were medically managed at time of this study including thrombolytic therapy, aspirin, clopidogrel, enoxaparine, nitroglycerine, ACE-I, B-blockers and statins.
After obtaining Minia University review board approval, all patients and controls were subjected to: (A) Assessment of typical chest pain. (B) Assessment of Killip class [6], Class I: No evidence of congestive heart failure. Class II: Rales, increased jugular venous pressure or S3 gallop. Class III: acute pulmonary edema. Class IV: cardiogenic shock. (C) Assessment of coronary risk factor.
2.2. Twelve leads ECG
To confirm the diagnosis of AMI, to determine the extent of AMI according to Aldrich score [7] by quantitating initial ST-segment changes on the presenting ECG.
2.3. Laboratory investigation
Venous blood samples were obtained from all controls and included patients, 24 hour post admission. Serum TNF-α samples were frozen for use within 6 month at −80 °C [8], were not run in duplicate and had been determined at one session by human TNF-alpha kit supplied by Koma Biotech Incorporation using sandwich ELISA protocol. PTEN gene activity was determined by its phosphatase activity using P-Nitrophenyl phosphate as a colorimetric substrate exactly as described [9].
2.4. Echocardiography
All patients underwent echocardiographic examination within 2 days of admission. The measurements represent a mean of at least three consecutive cardiac cycles. Diastolic parameters as trans-mitral early and late diastolic velocities and E/A ratio were assessed. Left Ventricular ejection fraction % was calculated from apical 4-chamber view with modified Simpson's method [10]. Left ventricular systolic dysfunction was defined as a decreased LV (<50%) effective ejection fraction [[11], [12], [13], [14]]. Left ventricular diastolic dysfunction was defined as impaired left ventricular filling or impaired left ventricular distensibility [[11], [12], [13], [14]]. Wall motion score index (WMSI) was assessed according to the standard 16 segment model of the American society of echocardiography [15]. Wall motion score index equals the sum of these scores divided by number of scored segments.
2.5. Follow up
All the patients were discharged after optimizing their medical treatment. Follow up visit was performed after one month and included assessment of recurrent anginal pain, heart failure (either new onset or deterioration of the functional class), ECG, physical examination and echocardiography. Then, one year clinical follow up for any cardiovascular events (Death was considered as cardiac related unless proved otherwise, recurrent hospitalization with heart failure).
2.6. Statistical analysis
Discrete values were presented as counts and percentages and compared using qui square test. Continuous values were expressed as mean ± SD. Significance was determined using t-test. A probability level of P < 0.05 was considered significant. Changes of parameters between baseline and subsequent measurements were evaluated by a paired t-test. A student unpaired t-test was used to analyze differences between patients groups and subgroups. Data were analyzed by SPSS version 16 software for windows (SPSS Inc.; Chicago; Illinois; USA).
3. Results
Out of 70 patients, 43 (61.4%) were males. Their mean age was (55 ± 10) years ranged from 35 to 75 years. Patients were classified into two groups: Group I (n = 32): All had acute STEMI with left ventricular systolic failure. They had been sub-classified into sub–group IA (n = 20): in whom EF% had been deteriorated at follow up and sub–group IB (n = 12): in whom EF% had stable at follow up. Group II (n = 38): All had acute STEMI without left ventricular systolic failure. Group III (n = 70): Age and sex matched normal persons as a control group.
There was statistically nonsignificant difference between groups or subgroups regarding the major risk factors for CAD as diabetes mellitus, hypertension, smoking, dyslipidemia or positive family history for premature CAD (Table 1). Out of 32 patients in group I, 22 (27%) were in Killip class II, 8 (10%) were in Killip class III and 2 (2%) were in Killip class IV. All patients in group II had Killip class I.
Table 1.
Comparison between Group I & Group II regarding their age, sex, major risk factors for CAD.
| Group I (n = 32) |
Group II (n = 38) |
P value | |
|---|---|---|---|
| Age (mean ± SD), y | 59 ± 8 | 54 ± 9 | 0.02 |
| Men, n (%) | 20 (62.5%) | 23 (60.5%) | 0.94 |
| Diabetes mellitus, n (%) | 14 (43%) | 10 (26%) | 0.21 |
| Hypertension, n (%) | 11 (34%) | 6 (15%) | 0.11 |
| Smoker, n (%) | 24 (75%) | 27 (71%) | 0.91 |
| Dyslipidemia, n (%) | 14 (44%) | 20 (52%) | 0.72 |
| Hyperuricemia, n (%) | 4 (13%) | 6 (16%) | 0.83 |
| + ve family history for premature CAD, n (%) | 1 (1.25%) | 1 (1.25%) | 0.28 |
3.1. ECG data
Group I had statistically significant increased percentage of myocardium at risk as compared to group II (22.8 ± 4.8 vs 13.9 ± 3.6, P = 0.001). However, there was no significant correlation between the extent of myocardium at risk on ECG and TNF-α or PTEN serum levels in either group I (r = 0.29 & r = 0.19 respectively) or group II (r = 0.23 & r = 0.13, respectively).
3.2. Echocardiographic data
Proper endocardial definition was feasible in all patients. Group I had a statistically significant lower EF% as compared to both group II (42 ± 7% vs 53 ± 3%, P = 0.001) and group III (42 ± 7% vs 63 ± 4%, P = 0.001). Group II had a statistically significant lower EF% as compared to group III (53 ± 3% vs 63 ± 4%, P = 0.001). During follow up period EF% was significantly deteriorated from 42 ± 7 to 39 ± 13%, P = 0.03 in group I. However, it was significantly improved from 53 ± 3% to 58 ± 4%, P = 0.001 in group II.
Out of 32 patients in group I, 27 (84%) had diastolic dysfunction and persists during follow up period. Out of 38 patients in group II, 21 (55%) had diastolic dysfunction, 2 patients had normalized diastolic function during follow up. Group I had a statistically significant increase number & percentage of patients with diastolic dysfunction as compared to group II [27(84%) vs 21(55%), P = 0.01] at presentation and [27 (84%) vs 19 (50%), P = 0.003] at follow up (Table 2).
Table 2.
Comparison between groups regarding their EF%, WMSI and diastolic dysfunction both at presentation and follow up.
| Group I n = 32 |
Group II n = 38 |
Group III n = 10 |
P value | |
|---|---|---|---|---|
| • Ejection fraction % At presentation At follow up |
42 ± 7% 39 ± 13% |
53 ± 3% 58 ± 4% |
63 ± 4% 62 ± 4% |
0.001⁎ 0.001⁎⁎ 0.001⁎⁎⁎ 0.001⁎ 0.004⁎⁎ 0.001⁎⁎⁎ |
| • WMSI At presentation At follow up |
1.5 ± 0.2 1.6 ± 0.5 |
1.3 ± 0.1 1.1 ± 0.1 |
1.0 ± 0.0 1.0 ± 0.0 |
0.001⁎ 0.001⁎⁎ 0.001⁎⁎⁎ 0.001⁎ 0.001⁎⁎ 0.003⁎⁎⁎ |
| • Diastolic dysfunction At presentation, n (%) At follow up, n (%) |
27 (84%) 27 (84%) |
21 (55%) 19 (50%) |
0 (0%) 0 (0%) |
0.01⁎ 0.003⁎ |
P (group I versus II).
P (group II versus III).
P (group I versus III).
3.3. Laboratory data
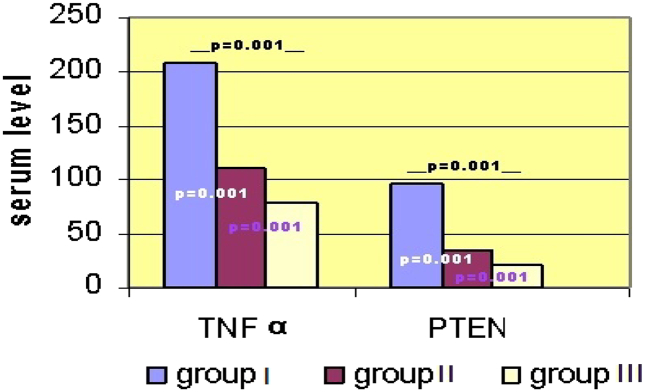
Group I had a statistically significant higher serum levels of both TNF-α & PTEN gene activity as compared to group II (207 ± 80 vs 111 ± 16, P = 0.001) & (96 ± 41 vs 35 ± 13, P = 0.001) respectively. Group I had a statistically significant higher serum levels of both TNF-α & PTEN gene activity as compared to group III (207 ± 80 vs 79 ± 16, P = 0.001) & (96 ± 41 vs 20 ± 5, P = 0.001) respectively. Group II had a statistically significant higher serum levels of both TNF-α & PTEN gene activity as compared to group III (111 ± 16 vs 79 ± 16, P = 0.001) & (35 ± 13 vs 20 ± 5, P = 0.001) respectively (Fig. 1).
Fig. 1.
Comparison between groups regarding their mean serum TNF-α and PTEN gene activity levels.
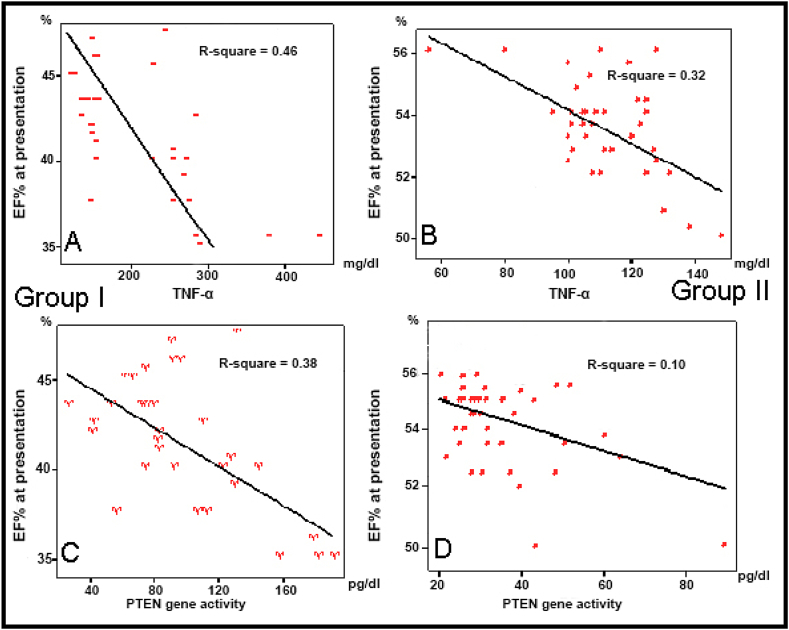
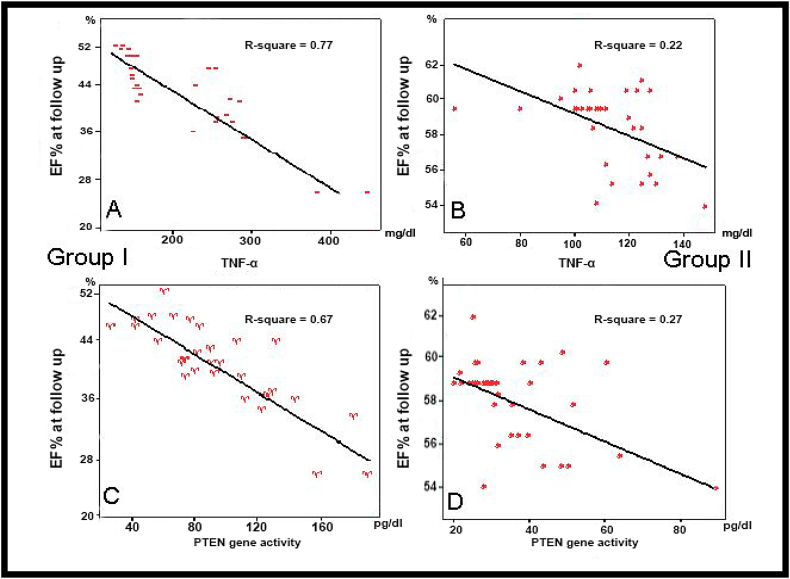
EF% at presentation was weakly correlated with serum levels of TNF-α, and PTEN gene activity in both group I (r = 0.46 & r = 0.38, respectively) and in group II (r = 0.32 & r = 0.10, respectively) (Fig. 2). However, at follow up EF% in group I showed a significant negative correlations with both serum levels of TNF-α and PTEN gene activity (r = 0.77 & r = 0.67, respectively). But it showed a weak correlations in group II (r = 0.22 & r = 0.27, respectively) (Fig. 3).
Fig. 2.
Correlation between EF% at presentation and serum levels of TNF-α [A & B] and PTEN gene activity [C & D] in Group I & II.
Fig. 3.
Correlation between EF% at Follow up and serum levels of TNF-α [A & B] and PTEN gene activity [C & D] in Group I & II.
Sub-group IA had statistically significant higher serum levels of TNF-α & PTEN gene activity [240 ± 82 and 115 ± 38 versus 151 ± 34 and 64 ± 22, P = 0.001 and P = 0.001, respectively] as compared to those in sub-group IB. There is no statistically significant difference in EF% at presentation between both sub-groups. However, at follow up, sub-group IA had a statistically significant deterioration in EF% from 42 ± 7% to 32 ± 6%, (P = 0.001) and sub-group IB had a statistically significant improvement in EF% from 44 ± 5% to 50 ± 2% (P = 0.001) (Table 3). 19 out of 20 patients in sub-group IA had serum level of TNF-α ≥151 mg/dl &10 out of 12 patients in sub-group IB had serum level of TNF-α <151 mg/dl (Table 4). TNF-α ≥151 mg/dl had a sensitivity, specificity, positive predictive value and negative predictive value of 90.8%, 90.9%, 83.3% and 90.6% respectively.
Table 3.
Comparison between sub-group IA, and sub-group IB regarding their serum TNF-α, PTEN levels and EF%.
| Sub-group 1A n = 20 |
Sub-group 1B n = 12 |
P value | |
|---|---|---|---|
| TNF-α | 240 ± 82 | 151 ± 34 | 0.001 |
| PTEN | 115 ± 38 | 64 ± 22 | 0.001 |
| EF% *At presentation *At follow-up |
42 ± 7% 32 ± 6% |
44 ± 5% 50 ± 2% |
0.47 0.001 |
Table 4.
Agreement between TNF-α levels in both subgroups.
| TNF-α ≥151 mg/dl |
TNF-α <151 mg/dl |
|
|---|---|---|
| Subgroup IA, n = 20 | 19 | 1 |
| Subgroup IB, n = 12 | 2 | 10 |
15 out of 20 patients in sub-group IA had serum level of PTEN gene activity ≥83 pg/dl &11 out of 12 patients in sub-group IB had serum level of PTEN gene activity <83 pg/dl (Table 5). PTEN gene activity ≥83 pg/dl had a sensitivity, specificity, positive predictive value and negative predictive value of 93.7%, 68.7%, 75% and 91.6% respectively.
Table 5.
Agreement between PTEN levels in both subgroups.
| PTEN ≥83 pg/dl |
PTEN <83 pg/dl |
|
|---|---|---|
| Sub-group IA, n = 20 | 15 | 5 |
| Sub-group IB, n = 12 | 1 | 11 |
During one year follow up (ranged from 331 to 389 days), 5 patients died, all were of cardiovascular causes and 6 patients had recurrent hospitalization with heart failure. These patients had a statistically significant increased serum levels of TNF-α &PTEN gene activity levels as compared by other patients (266 ± 95 vs 134 ± 44, P = 0.001), (123 ± 46 vs 52 ± 30, P = 0.001), respectively. Also, all these patients had serum TNF-α ≥ 151 mg/dl and PTEN gene activity levels ≥83 pg/dl.
4. Discussion
4.1. Principal findings
Our study revealed that patients with acute STEMI had statistically significant increased serum levels of TNF-α &PTEN gene activity as compared to normal controls. Patients with acute STEMI and left ventricular systolic failure in whom EF% deteriorated at follow up had a statistically significant increased serum levels of TNF-α & PTEN gene activity as compared to those, in whom EF% not deteriorated at follow up, in spite of nonstatistically significant difference between their EF% at time of presentation. Also, patients with worse clinical outcome (death & recurrent hospitalization by heart failure) had significantly increased serum levels of TNF-α & PTEN gene activity as compared to others.
Shimoda et al. [16] assessed tissue ACE and TNF-α levels in acute MI patients. Their levels were higher in acute MI patients than in controls (P = 0.001). In acute MI patients with in-hospital complications spontaneous and stimulated levels of tissue ACE and TNF-alpha were higher than in patients without complications (P = 0.01). Also, Fahim et al. [17] conducted a study on 20 acute MI patients and 10 healthy age and sex matched controls. Sequential estimation of plasma TNF-α level was carried out on admission, at 24 and at 48 h post admission using ELISA. Acute MI patients showed a significant increase of plasma TNF-α level on admission and 24 h post admission but not after 48 h. However, a significant increase was still seen at 48 h post admission in patients with signs of heart failure compared to those without signs of heart failure. They concluded that TNF-α might be an early marker of myocardial damage because of the early increase of its level after ischemic injury and TNF-α level may be an important indicator for the occurrence of heart failure in acute MI.
Debrunner et al. [18] revealed that the inflammation-associated cytokines TNF-α, IL-6 and IL-1Ra were significantly elevated in patients with MI complicated by cardiogenic shock when compared to patients with uncomplicated MI. Also, Gonzálvez et al. [19] found that TNF-α concentrations were greater in patients with ischemic events or heart failure both at admission and at 48 h. Specifically, TNF-α concentrations were analyzed in the group of 9 patients who died. At admission, these values were not significantly greater in this group compared to those in the surviving patients. Nevertheless, at 48 h, TNF-α concentrations were significantly greater in the patients who died than in surviving patients. TNF-α concentrations recorded at 48 h were significantly negatively correlated with LVEF (r = 0.416; P < 0.001); this correlation was maintained at 6 months among the 65 surviving patients (r = 0.256; P = 0.039). In addition, there was a positive correlation between TNF-α concentrations and IL-6, sICAM-1, and CRP concentrations at admission (r = 0.269, P = 0.02; r = 0.444, P < 0.001; and r = 0.286, P = 0.01, respectively), and after 48 h (r = 0.309, P = 0.007; r = 0.163, P = 0.1; and r = 0.418, P = 0.001, respectively). It had also been directly associated with myocardial damage after ischemia/reperfusion, oxidative stress in patients with STEMI, myocardial rupture and chronic ventricular dysfunction, apoptosis, and peripheral endothelial dysfunction.
On the other hand, Puhakka et al. [20] studied the interrelationships of IL-6, TNF-alpha, tissue injury, infarct size, cardiac function, and collagen formation in humans. They revealed that patients with left ventricular dysfunction (EF < 40%) had similar TNF-α levels as those with preserved left ventricular function and concluded that TNF-α measurement is useless in the assessment of infarct size or left ventricular function during the immediate post-infarction period. The regulatory role of PTEN in post-MI remodeling was further supported by Nirmal et al. [21] findings in mice over expressing PTEN, which exhibited increased cardiac dysfunction and mortality. Heart failure and LV rupture might result from massive cell death and degradation of extracellular matrix [22]. Moreover, it has been reported that PI3K/Akt signaling is required for angiogenesis and cardiac repair after MI, and that this signaling pathway was impaired in the infarcted heart [23]. Also, Nirmal et al. [21] demonstrated that PTEN expression is upregulated in WT mice after MI, suggesting that increased PTEN activity may inhibit the activation of the PI3K/Akt signaling pathway in the infarcted heart. Furthermore, PTEN promotes post-MI remodeling by regulating inflammation, cytokine production, and MMP expression. Cardiac-specific PTEN deletion or pharmacological inhibition of PTEN has been shown to inhibit apoptosis and limit infarct size after ischemia-reperfusion. Decreased PTEN activity was associated with subsequent reduced extracellular matrix remodeling and improved LV function [24].
4.2. Limitations of study
PTEN gene is very difficult target in experimental studies and lack of highly specific commercially available activator or inhibitors led to limited available data. Also, small number of patients included in this study is another limitation.
5. Conclusions
Patients with acute STEMI had statistically significant increased serum levels of TNF-α & PTEN gene activity. Both markers reflect the extent of myocardial damage and hence predict worsening of EF%, development of heart failure and death.
5.1. Clinical implication
Serum levels of TNF-α ≥151 mg/dl and PTEN gene activity ≥83 pg/dl were recommended as markers in acute STEMI for predicting heart failure and death during the next year.
5.2. Further directions
Targeting PTEN gene & TNF-α may be an effective approach to enhance cardiac repair following acute MI.
Conflict of interest
None of the authors has any conflict of interest to report.
References
- 1.Gil A., Andres-Pons A., Fernandez E. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol. Biol. Cell. 2006;(9):4002–4013. doi: 10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oudit G.Y., Kassiri Z., Zhou J. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc. Res. 2008;78:505–514. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari R., Bachetti T., Confortini R. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92(6):1479–1486. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A., Paladugu B., Mensing J., Kumar A., Parrillo J.E. Nitric oxide-dependent and -independent mechanisms are involved in TNF-alpha -induced depression of cardiac myocyte contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(5):R1900–R1906. doi: 10.1152/ajpregu.00146.2006. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Joseph S, Alpert Allan S, Jaffe Maarten L, Simoons Bernard R, Chaitman and Harvey D. Third Universal Definition of Myocardial Infarction, on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. JACC 2012 Vol. 60.
- 6.Lee K.L., Woodlief L.H., Topol E.G. Predictor of 30-day mortality in the era of reperfusion for acute myocardial infarction. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 7.Schroder R., Dissmonn R., Bruggemann T. Extent of early ST segment elevation resolution: a simple but strong prediction of outcome in patients with AMI. J. Am. Coll. Cardiol. 1994;24:384–391. doi: 10.1016/0735-1097(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 8.Friebe A1, Volk HD. Stability of tumor necrosis factor alpha, interleukin 6, and interleukin 8 in blood samples of patients with systemic immune activation. Arch Pathol Lab Med. 2008 Nov; 132(11):1802–6. doi: 10.1043/1543-2165-132.11.1802. [DOI] [PubMed]
- 9.Takai A., Murata M., Torigoe K., Isobe M., Mieskes G., Yasumoto T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992;284:539–544. doi: 10.1042/bj2840539. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanolla L., Marino P., Golia G., Anselmi M., Zardini P., Borghi C., Ambrosioni E. The extent of regional wall motion abnormalities identifies patients at risk of extensive left ventricular remodeling: implications for the design of post myocardial infarction trials. J. Ital. Cardiol. 1999;29(1):20–26. [PubMed] [Google Scholar]
- 11.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:1810. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129. [DOI] [PubMed]
- 13.Bonow R.O., Bennett S., Casey D.E., Jr. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association task force on performance measures (writing committee to develop heart failure clinical performance measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112:1853. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 14.Little WC1 and Applegate RJ. Congestive heart failure: systolic and diastolic function. J Cardiothorac Vasc Anesth. 1993 Aug; 7(4 Suppl 2): 2–5. [DOI] [PubMed]
- 15.Braunwald E., Antman E.M., Beasley J.W. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda Y., Satoh M., Nakamura M., Akatsu T., Hiramori K. Activated tumour necrosis factor-alpha shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin. Sci. (Lond.) 2005;108(4):339–347. doi: 10.1042/CS20040229. [DOI] [PubMed] [Google Scholar]
- 17.Fahim M.R., Halim S.M., Kamel I. Tumor necrosis factor alpha in patients with acute myocardial infarction. Egypt. J. Immunol. 2004;11(1):31–37. [PubMed] [Google Scholar]
- 18.Debrunner M., Schuiki E., Minder E., Straumann E., Naegeli B., Mury R., Bertel O., Frielingsdorf J. Proinflammatory cytokines in acute myocardial infarction with and without cardiogenic shock. Clin. Res. Cardiol. 2008;97(5):298–305. doi: 10.1007/s00392-007-0626-5. [DOI] [PubMed] [Google Scholar]
- 19.Gonzálvez Manuel, José A Ruiz-Ros, Matías Pérez-Paredes, María L Lozano, Francisco J García-Almagro, Francisco Martínez-Corbalán, Diego M Giménez, Andrés Carrillo, Andrés Carnero, Tomás Cubero, Juan J Gonzálvez, Isabel Ureña y and Vicente Vicente. Prognostic value of tumor necrosis factor-alpha in patients with ST-segment elevation acute myocardial infarction. Rev. Esp. Cardiol. 2007; 60: 1233–1241. [DOI] [PubMed]
- 20.Puhakka M., Magga J., Hietakorpi S., Penttila I., Uusimaa P., Risteli J., Peuhkurinen K. Interleukin-6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. J. Card. Fail. 2003;9(4):325–332. doi: 10.1054/jcaf.2003.38. [DOI] [PubMed] [Google Scholar]
- 21.Nirmal P., Yuan Y., Xiaoxu Z., Djahida B., Zheqing P.C. Phosphatase PTEN is critically involved in post-myocardial infarction remodeling through the Akt/Interleukin-10 signaling pathway. Basic Res. Cardiol. 2012;107(2):248. doi: 10.1007/s00395-012-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinbongard P., Heusch G., Schulz R. TNF alpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Miki T., Miura T., Tanno M., Nishihara M., Naitoh K., Sato T., Takahashi A., Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res. Cardiol. 2007;102:163–170. doi: 10.1007/s00395-006-0622-3. [DOI] [PubMed] [Google Scholar]
- 24.Keyes K.T., Xu J., Long B., Zhang C., Hu Z., Ye Y. Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function post infarction. Am. J. Phys. 2010;298:H1198–H1208. doi: 10.1152/ajpheart.00915.2009. [DOI] [PubMed] [Google Scholar]