Pancreatic adenocarcinoma is one of the most lethal and therapeutically resistant malignancies.1 There is no uniform consensus regarding standard of care for treatment of unresectable, locally advanced pancreatic cancer (LAPC). Treatment options include chemotherapy alone, chemotherapy followed by chemoradiotherapy, or stereotactic body radiation therapy.2, 3
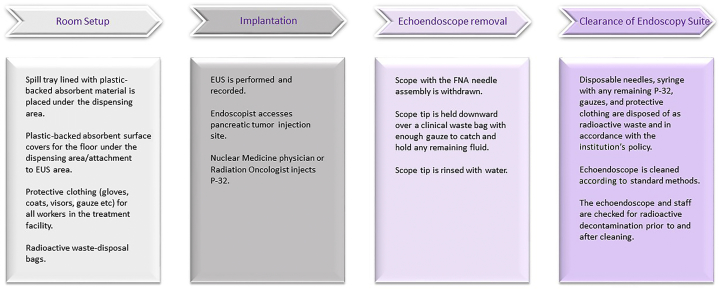
We describe here our first experience with an EUS-guided implantation of a novel brachytherapy device, phosphorus-32 microparticles (P-32 OncoSil, in conjunction with standard chemotherapy in a patient with LAPC) (Video 1, available online at www.VideoGIE.org). The P-32 was administered as part of the OncoPaC-1 clinical trial (ClinicalTrials.gov number, NCT03076216) approved by the institutional review board at our institution. P-32 is an experimental medical device intended for use in brachytherapy that carries the radioactive β-emitter P-32 inside inactive silicon particles. It is implanted into pancreatic tumors by EUS during the 4th or 5th week of the first cycle of chemotherapy (Fig. 1).
Figure 1.
Overview of phosphorus-32 microparticles P-32 implantation.
Case report
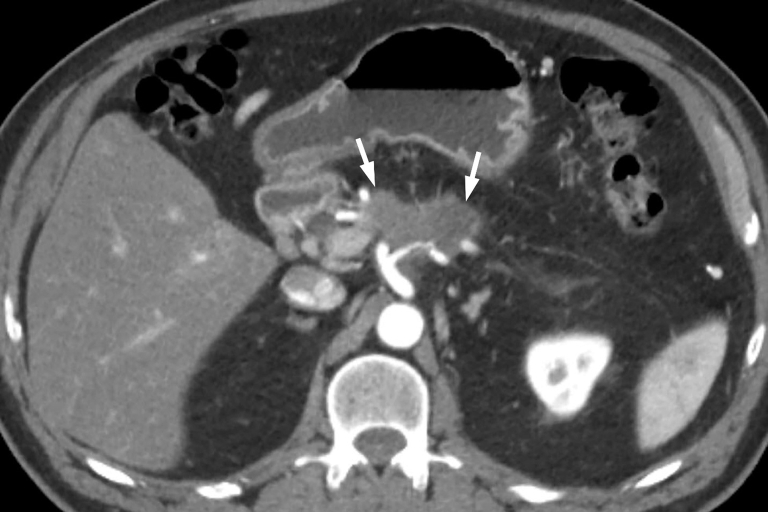
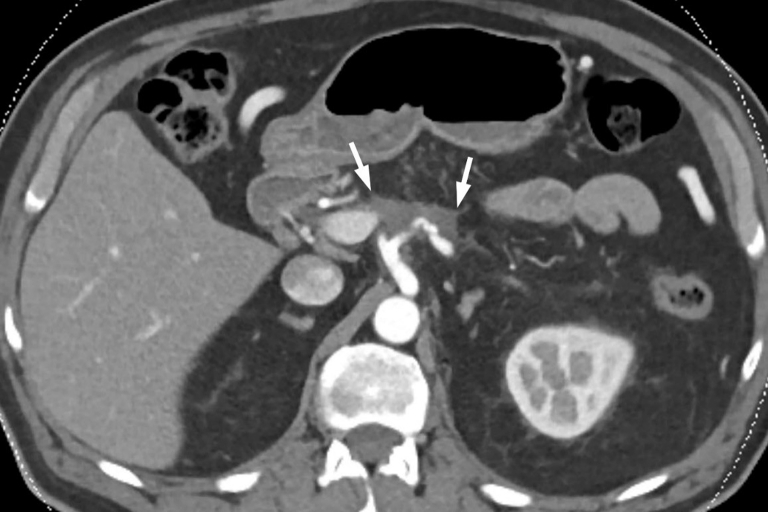
A 72-year-old man presented with epigastric pain radiating to the midback. CT of the abdomen showed a 5.0- × 3.6-cm hypodense mass in the pancreatic body, encasing the splenic vein, splenic artery, celiac axis, and portal vein confluence (Fig. 2). Enlarged intra-abdominal lymph nodes were also identified. Cytologic examination of an EUS-guided FNA specimen revealed adenocarcinoma. The CA 19-9 marker was markedly increased: 635 U/mL (normal, 0-35 U/mL). The patient was informed about the OncoPaC-1 clinical trial, and he opted to be treated on protocol.
Figure 2.
CT view of hypodense tumor, 5.0 × 3.6 cm (white arrows), in the pancreatic body before therapy. It encases the splenic vein and splenic artery, and it partially encases the celiac axis and the portal vein confluence.
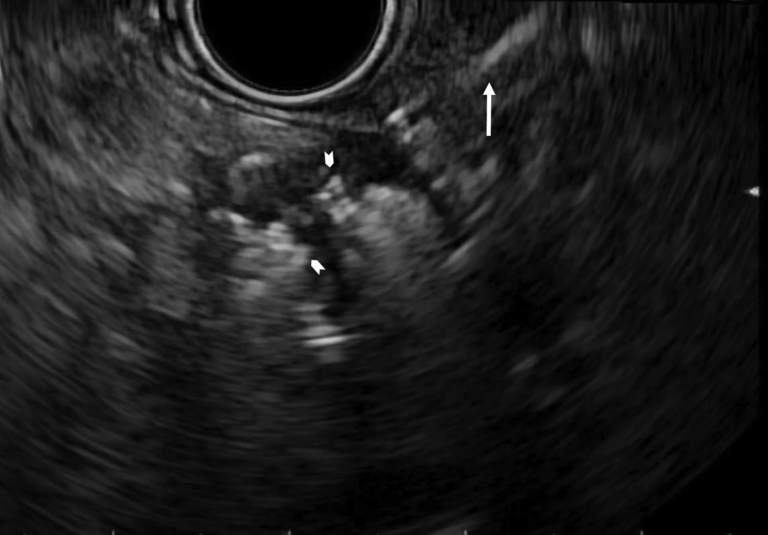
The patient started chemotherapy with gemcitabine + nab-paclitaxel. The predefined suspension preparation protocol of P-32 microparticles stated that the final radioactive concentration of P-32 required to deliver 100 Gy to the tumor should be 6.6 MBq/mL. As per protocol, the microparticles should be suspended in 8% of the tumor volume. In this case, the tumor volume was 23.3 mL, and the calculated dose of P-32 microparticles was determined to be 1.87 mL, corresponding to a radioactivity dose of 12.3 MBq. EUS was performed to deliver the P-32 into the pancreatic tumor during the 4th week of the first chemotherapy cycle. A 22-gauge needle was used to puncture the tumor by use of a transgastric approach through an avascular angle. The P-32 was injected into the center of the tumor with a needle tracking technique by pulling the needle back when the injection became difficult. One mL of saline solution was used to flush the P-32 remaining in the needle into the tumor, and then the needle was withdrawn back into the sheath. The injection of P-32 was seen as an echogenic blush within the tumor (Fig. 3). Radiation safety is an important consideration in the use of P-32. After the procedure, all materials were sterilized and disposed of according to radiation safety guidelines and under the supervision of the institutional radiation safety department.
Figure 3.
EUS view showing the hypoechoic tumor after injection of phosphorus-32 microparticles P-32 injection mostly replaced by the hyperechoic “cloud” (arrowheads) and the catheter sheath at the edge of the tumor through which P-32 was delivered (arrow).
Follow-up
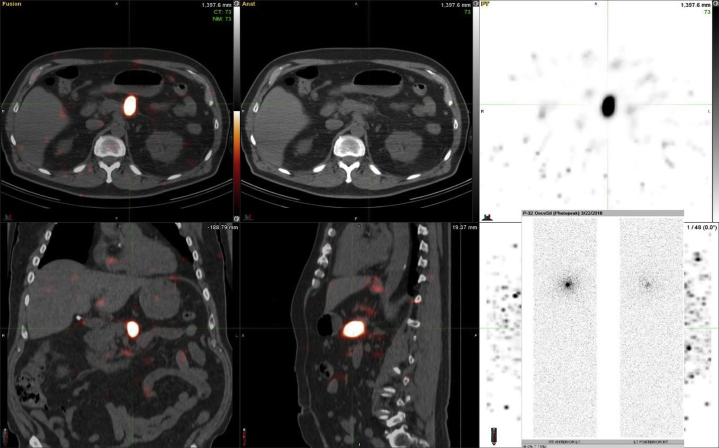
Whole-body planar and single photon emission computed tomography (SPECT)/CT scintigraphic imaging of P-32 bremsstrahlung radiation was performed within 4 hours after implantation and again at 7 days to assess the intratumoral and any potential extratumoral distribution of the P-32 microparticles. Both SPECT/CT scans confirmed local collection of the radioactivity in the known pancreatic tumor (Fig. 4).
Figure 4.
Single photon emission computed tomography/CT bremsstrahlung imaging showing excellent intratumoral retention of activity within the pancreatic body 4 hours after implantation.
The chemotherapy regimen was resumed after implantation. Follow-up CT scans were repeated every 8 weeks to assess tumor response. CT scan assessments showed a 58% decrease in tumor volume from baseline (23.2 mL) to week 16 (9.7 mL) (Fig. 5). There was improvement of the caliber of the splenoportal confluence, which would also be indicative of tumor response. The patient had the follow-up visit at week 22. His pre-existing abdominal pain completely resolved, and the serum CA 19-9 level (25 U/mL) has trended downward significantly. The patient is currently without side effects or toxicity from the implant procedure, and he has been able to successfully continue his chemotherapy regimen.
Figure 5.
Follow-up CT scan showing a decrease in tumor size (white arrows) from 5.0 × 3.6 cm at baseline to 4.2 × 2.8 cm at week 16, with improvement of the caliber of the splenoportal confluence.
Conclusion
Our first experience with P-32 brachytherapy in conjunction with chemotherapy in a patient with LAPC showed that this novel treatment modality is promising and technically feasible. Given the 58% decrease in tumor volume, we believe the response is beyond what would be expected from chemotherapy alone. The final results of this trial are expected to shed further light on the safety and efficacy of P-32 brachytherapy.
Disclosure
The OncoPaC-1 trial is sponsored by OncoSil Medical Ltd, Sydney, Australia. Dr Bhutani is the recipient of research support from Oncosil, Galera, and Augmenix. Dr Tamm is associated with General Electric. Dr Herman is the recipient of research support from Oncosil, Galera, Aduro, and Augmenix, and is a consultant for BTG, AbbVie, Celgene, AstraZeneca, BMS, Varian, and Boston Scientific. The other authors disclosed no financial relationships relevant to this publication.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Bhutani at manoop.bhutani@mdanderson.org or Dr Herman at JMHerman@mdanderson.org.
Supplementary data
EUS-guided implantation of phosphorus-32 microparticles brachytherapy in a patient with unresectable locally advanced pancreatic cancer.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Pancreatic Cancer. Volume 2018, 2018. https://seer.cancer.gov/statfacts/html/pancreas.html Available at: Accessed August 7, 2018.
- 2.Cardenes H.R., Chiorean E.G., DeWitt J. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11:612–623. doi: 10.1634/theoncologist.11-6-612. [DOI] [PubMed] [Google Scholar]
- 3.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EUS-guided implantation of phosphorus-32 microparticles brachytherapy in a patient with unresectable locally advanced pancreatic cancer.