Abstract
Introduction
Despite evidence that central nervous system (CNS) trauma, including traumatic brain injury and spinal cord injury, can cause sustained neurocognitive impairment, it remains unclear whether trauma-related variables are associated with incident dementia independently of other known risk factors.
Methods
All adults without dementia entering the health-care system with diagnoses of CNS trauma were examined for occurrence of dementia. All trauma-related variables were examined as predictors in sex-specific Cox regression models, controlling for other known risk factors.
Results
Over a median follow-up of 52 months, 32,834 of 712,708 patients (4.6%) developed dementia. Traumatic brain injury severity and spinal cord injury interacted with age to influence dementia onset; women were at a greater risk of developing dementia earlier than men, all other factors being equal.
Discussion
Risk stratification of patients with CNS trauma by sex is vital in identifying those most likely to develop dementia and in understanding the course and modifying factors.
Keywords: Traumatic brain injury, Spinal cord injury, Comorbidity, Risk factors, Dementia, Prognostic modeling
1. Background
Traumatic brain injury (TBI) is one of the most disabling injuries and results in a range of cognitive impairments, particularly in the domains of attention, memory, emotion, and behavior [1]. Between 50 and 60 million people are affected annually by TBI [2], and approximately 50% of the global population will sustain a TBI in their lifetime [2]. Traumatic spinal cord injury (SCI) occurs in 236–1298 people per million and has an annual rate of up to 246 cases per million people [3], often occurring in conjunction with TBI, and should be considered in the context of trauma to the central nervous system (CNS) [4].
Patients with a history of CNS trauma may develop proteinopathy [5], [6], a component of various neurodegenerative disorders including Alzheimer's disease (AD) and other dementias [7]. Tackling CNS trauma as a risk for dementia, however, requires systematic efforts to understand which inherent traumatic factors contribute to dementia development [8]. This is complicated by the fact that research has revealed mixed phenotypes of both CNS trauma and dementia in men and women [9], [10].
Five longitudinal studies (Supplementary Table 1, Supplement File 1) have focused on the causal relationship between TBI and incident dementia [11], [12], [13], [14], [15]. Each used varying definitions of exposure, outcome, covariates, and follow-up periods, and results were inconsistent. Some found a marked attenuation of AD or dementia risk in patients with TBI after multivariate adjustment, while others did not [11], [12], [13], [14], [15]. These studies either focused on certain age groups [11], [12] or occupations [13], [14], did not account for time to diagnosis in their analyses, or did not consider varying injury severity, combining penetrating and nonpenetrating [15], moderate and severe [12], [14], or all [15] TBI diagnoses into a single exposure definition. Furthermore, these studies did not consider the effects of SCI, which is important given the prevalence of comorbid SCI in TBI [16]. Additionally, although results were adjusted for sex, there was no risk stratification for this factor [11], [12], [13], [14], [15]. Because many neurodegenerative diseases often have different frequencies, presentations, and comorbidity risk factors across the lifespans of men and women, it is possible that they may be at different risk of developing dementia after CNS trauma [10].
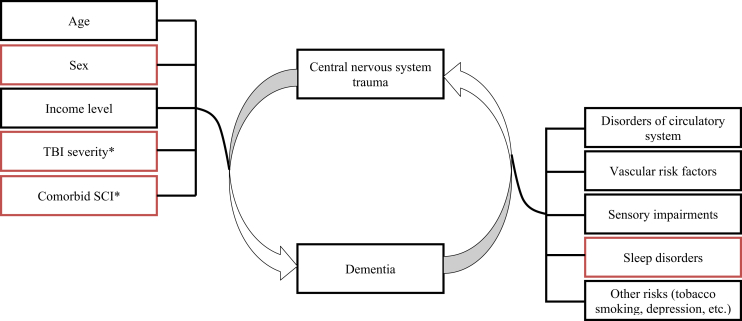
Here, we evaluated whether the risk of incident dementia was related to the severity and extent of CNS trauma in a large historical cohort of patients over more than a decade. Using methodologies that separated exposure and outcome, we hypothesized that incident dementia is related to TBI severity and extent of injury throughout adulthood. Furthermore, we hypothesized that several individual factors predict dementia onset and that the effects would be different between sexes (Fig. 1).
Fig. 1.
Hypothesized relationships related to dementia outcome after central nervous system trauma. Red color indicates previously unexplored hypothesis. Black color indicates other tested relationships, previously described. *The following variables were evaluated as predictors in our statistical models: (1) TBI severity, (2) presence of comorbid SCI, (3) the interaction of TBI severity and age, and (4) the interaction of comorbid SCI and age. Abbreviations: SCI, spinal cord injury; TBI, traumatic brain injury.
2. Methods
The study complied with the principle of the Declaration of Helsinki. The Toronto Rehabilitation Institute–University Health Network' Research Ethics Board approved this study. The findings were reported in compliance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines.
2.1. Data sources
Our data were obtained from the Institute for Clinical Evaluative Sciences [17], which stores information from emergency departments (EDs) and acute care units. Data pertained to residents of Ontario, Canada. Our sample was racially diverse and had access to universal health care. Diagnoses were indicated by entries under the the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision Canadian Enhancement classification system (ICD-10-CA) [17].
2.2. Study design and sample
Adult (≥18 years) Ontario residents who had received a diagnosis of CNS trauma (i.e., TBI or TBI with SCI, Supplementary Tables 1 and 2 in the Supplement File 1) in an ED or acute care unit between April 1, 2002 and March 31, 2013 were considered in this study. The dates of first TBI diagnosis were defined as an index date that marked the beginning of the study period for each patient. All individuals were followed up until the end of the study period or death, whichever occurred first. Details of inclusion and exclusion criteria are provided in Supplementary Fig. 1, Supplement File 2.
2.3. Variables
2.3.1. Predictors
The following variables were evaluated as predictors in our statistical models: (1) TBI severity, (2) presence of comorbid SCI, (3) the interaction of TBI severity and age [18], and (4) the interaction of comorbid SCI and age. TBI was defined using Centers for Disease Control and Prevention criteria [19], and injury severity was defined according to published severity classifications [20], [21]. Traumatic SCI was defined following published criteria [22] (Supplementary Materials 1 and 2, Supplement File 2). To minimize reverse causality and misdiagnoses, patients with ICD-10 codes of postconcussive syndrome (F07.2) were not included in TBI definition [23].
2.3.2. Outcome
Dementia was defined according to validated ICD-10 codes for the diagnosis of dementia in an inpatient setting [13], [24]. To minimize the chance of misdiagnoses, patients with delirium (F05-) were excluded from dementia definition [25] (Supplementary Table 3, Supplement File 2).
2.3.3. Potential confounders
Age, sex, disorders of the circulatory system, vascular risk factors, and income quantile (according to postal code) are important in predicting dementia [26], [27], [28]. Associative values between sleep disorder [29] and dementia were quantified to determine their effect on dementia onset.
2.4. Statistical analysis
Descriptive statistics were calculated for relevant data. In TBI populations, death may preclude the occurrence of dementia, resulting in overestimation of incidence in survivors [30]. Tests for differences based on injury severity, age, and time to death were performed (Supplementary Figs. 1 and 2, Supplement File 3), following which Standard Cox regression analyses were performed [31], ensuring that censoring was “independent” across injury severity and age (Supplement File 3).
Univariate and multivariable Cox regression models were used to investigate the relationship between CNS trauma-related predictors and incidence of dementia; results are expressed as hazard ratios (HRs) and 95% confidence intervals. For the purpose of sensitivity analysis, all calculations were repeated using subgroups of patients ≤65 years. To confirm findings from multivariable Cox regression model, the Fine and Gray competing risk regression model was used [32].
The following model diagnostic procedures were performed [33]: (1) tests of violations of the assumption of proportional hazards and identification of influential observations, (2) test of nonlinearity in the relationship between the log HR and covariates, (3) test of collinearity among predictors by the use of the variance inflation factor, and (4) evaluation of the effect of each predictor separately from the other predictors (Supplementary Material 3, Supplement File 3). Model performance was evaluated through quantitative summaries of predictors from the model, the likelihood χ2 for testing all covariates, the Akaike's information criterion, and discrimination C-index. Statistical significance of the predictors was assessed with the Wald test, with significance considered when P < .05.
2.5. Missing variables
We developed an algorithm that identified injury severity through a composite score that considers a patient's Glasgow Coma Scale score and the most severe injury at index date, regardless of anatomical location (Supplementary Material 2, Supplement File 2), based on an Abbreviated Injury Scale score, and has been shown to predict mortality in patients with head/neck trauma [34]. Patients with ICD-10-CA diagnostic codes of S06.0 (concussion) in whom severity of injury was not possible to establish due to the absence of Abbreviated Injury Scale and/or Glasgow Coma Scale data, were analyzed as a separate cohort, “unspecified injury severity.”
2.6. Sample size consideration
Between 18,200 and 24,500 dementia events were anticipated, based on an estimated sample of 700,000 with an average of 6 years of follow-up and the reported rate of incident dementia between 2.6% and 3.5% [12], [13] This would allow examination of at least 70 predictors in generic models and approximately 35 in separate models for each sex, using the rule of thumb of 10 events per predictor.
2.7. Clinical application
To demonstrate the effects of covariates and estimate the probability of developing postinjury dementia, we generated individualized predictions by converting results of Cox regressions into a single numerical estimate of the probability in each sex at 1, 5, and 10 years after the injury [35].
3. Results
3.1. Sample characteristics
During the study period, a total of 1,990,183 individuals entered EDs or acute care units with a diagnostic code indicating CNS trauma (Supplementary Fig. 1, Supplement File 1). The major causes of TBI included falls (n = 297,794, 41.78%), object strikes (n = 188,301, 26.42%), and assaults (n = 76,255, 10.70%). Object strikes and assaults were more frequent in men compared to women (31.45% vs. 19.05% and 14.26 % vs. 5.58%, respectively). Falls were more frequent in women compared to men (56.07 % vs. 32.04 %). Our final analyses included 712,708 adult patients (59% male, median age 44 years) without dementia at baseline. Table 1 shows baseline characteristics by TBI severity and SCI comorbidity.
Table 1.
Characteristics of patients who did develop dementia (n = 32,834) and those who did not (n = 679,874)
| Variables | Total |
With dementia |
Without dementia |
TBI severity |
Comorbid SCI |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
Severe |
Unspecified |
Present |
Absent |
||||
| n = 712,708 | n = 32,834 | n = 679,874 | n = 443,456 | n = 7365 | n = 17,390 | n = 244,497 | n = 29,695 | n = 683,013 | |
| Sociodemographic characteristics | |||||||||
| Male | 423,657 (59.44) | 13,342 (40.63) | 410,315 (60.35) | 293,264 (66.13) | 4920 (66.80) | 11,421 (65.68) | 114,052 (46.65) | 15,681 (52.81) | 407,976 (59.73) |
| Age at first TBI (years) | 44.00 (27.00–64.00) | 82.00 (76.00–87.00) | 42.00 (26.00–60.00) | 43.00 (27.00–63.00) | 49.00 (30.00–71.00) | 59.00 (38.00–77.00) | 44.00 (27.00–64.00) | 49.00 (31.00–73.00) | 44.00 (27.00–64.00) |
| Income Q1 (poorest) | 156,172 (21.91) | 7686 (23.41) | 148,486 (21.84) | 94,956 (21.41) | 1750 (23.76) | 3890 (22.37) | 55,576 (22.73) | 6878 (23.16) | 149,294 (21.86) |
| Income Q2 | 145,034 (20.35) | 7012 (21.36) | 138,022 (20.30) | 89,573 (20.20) | 1565 (21.25) | 3628 (20.86) | 50,268 (20.56) | 6257 (21.07) | 138,777 (20.32) |
| Income Q3 | 139,368 (19.55) | 6277 (19.12) | 133,091 (19.58) | 86,787 (19.57) | 1340 (18.19) | 3367 (19.36) | 47,874 (19.58) | 5931 (19.97) | 133,437 (19.54) |
| Income Q4 | 138,508 (19.43) | 5953 (18.13) | 132,555 (19.50) | 86,927 (19.60) | 1381 (18.75) | 3409 (19.60) | 46,791 (19.14) | 5556 (18.71) | 132,952 (19.47) |
| Income Q5 (wealthiest) | 133,626 (18.75) | 5906 (17.99) | 127,720 (18.79) | 85,213 (19.22) | 1329 (18.04) | 3096 (17.80) | 43,988 (17.99) | 5073 (17.08) | 128,553 (18.82) |
| Rural residence | 101,346 (14.22) | 4075 (12.41) | 97,271 (14.31) | 65,925 (14.87) | 982 (13.33) | 2395 (13.77) | 32,044 (13.11) | 5220 (17.58) | 96,126 (14.07) |
| Disorders of circulatory system | |||||||||
| Cerebrovascular disease | 19,207 (2.69) | 3386 (10.31) | 15,821 (2.33) | 8954 (2.02) | 624 (8.47) | 3157 (18.15) | 6472 (2.65) | 1230 (4.14) | 17,977 (2.63) |
| Ischemic heart disease | 36,312 (5.09) | 5212 (15.87) | 31,100 (4.57) | 20,512 (4.63) | 527 (7.16) | 1927 (11.08) | 13,346 (5.46) | 2364 (7.96) | 33,948 (4.97) |
| Atrial fibrillation | 22,014 (3.09) | 3623 (11.03) | 18,391 (2.71) | 12,009 (2.71) | 360 (4.89) | 1572 (9.04) | 8073 (3.30) | 1508 (5.08) | 20,506 (3.00) |
| Heart failure | 17,248 (2.42) | 2872 (8.75) | 14,376 (2.11) | 9582 (2.16) | 258 (3.50) | 1015 (5.84) | 6393 (2.61) | 1133 (3.82) | 16,115 (2.36) |
| Disorders of vascular system | |||||||||
| Obesity | 3903 (0.55) | 242 (0.74) | 3661 (0.54) | 1895 (0.43) | 36 (0.49) | 127 (0.73) | 1845 (0.75) | 274 (0.92) | 3629 (0.53) |
| Hyperlipidemia | 12,312 (1.73) | 1843 (5.61) | 10,469 (1.54) | 6718 (1.51) | 205 (2.78) | 693 (3.99) | 4696 (1.92) | 817 (2.75) | 11,495 (1.68) |
| Diabetes mellitus | 48,138 (6.75) | 6518 (19.85) | 41,620 (6.12) | 25,791 (5.82) | 701 (9.52) | 2312 (13.29) | 19,334 (7.91) | 2874 (9.68) | 45,264 (6.63) |
| Other disorders and signs | |||||||||
| Tobacco smoking | 870 (0.12) | 51 (0.16) | 819 (0.12) | 497 (0.11) | 15 (0.20) | 33 (0.19) | 325 (0.13) | 56 (0.19) | 814 (0.12) |
| Depression | 25,746 (3.61) | 1612 (4.91) | 24,134 (3.55) | 13,646 (3.08) | 343 (4.66) | 798 (4.59) | 10,959 (4.48) | 2010 (6.77) | 23,736 (3.48) |
| Visual impairments | 7630 (1.07) | 1121 (3.41) | 6509 (0.96) | 4373 (0.99) | 120 (1.63) | 344 (1.98) | 2793 (1.14) | 489 (1.65) | 7141 (1.05) |
| Hearing loss | 1689 (0.24) | 168 (0.51) | 1521 (0.22) | 901 (0.20) | 28 (0.38) | 111 (0.64) | 649 (0.27) | 125 (0.42) | 1564 (0.23) |
| Sleep disorder (any) | 6999 (0.98) | 537 (1.64) | 6462 (0.95) | 3818 (0.86) | 90 (1.22) | 230 (1.32) | 2861 (1.17) | 556 (1.87) | 6443 (0.94) |
| Incidents | |||||||||
| Dementia | 32,834 (4.61) | 32,834 (100.00) | - | 19,401 (4.37) | 405 (5.50) | 1117 (6.42) | 11,911 (4.87) | 2349 (7.91) | 30,485 (4.46) |
| Alzheimer's disease | 8451 (1.19) | 8451 (25.74) | - | 5078 (1.15) | 88 (1.19) | 252 (1.45) | 3033 (1.24) | 517 (1.74) | 7934 (1.16) |
| Follow-up time, in months | |||||||||
| To dementia | 25.00 (8.18–52.40) | 25.00 (8.18–52.40) | - | 25.53 (8.64–52.88) | 20.60 (3.52–51.84) | 32.82 (7.64–65.18) | 23.66 (7.59–50.18) | 28.32 (9.61–57.03) | 24.67 (8.08–52.01) |
NOTE. Data are given as median (interquartile range) or n (%).
Abbreviations: Q, quantile; SCI, spinal cord injury; TBI, traumatic brain injury.
3.2. Incidence of dementia
Over a median follow-up of 52 months (interquartile range 19.22–86.44), 32,864 patients (4.61%) were diagnosed with dementia (e.g. AD n = 5,983, 18.22%; vascular dementia n = 1,668, 5.08%; frontotemporal dementia n = 108, 0.33%; dementia of unspecified type n = 24,504, 74.63%). These diagnoses were distributed among patients with mild TBI (n = 19,401, 4.37%), moderate TBI (n = 405, 5.50%), severe TBI (n = 1,117, 6.42%), unspecified injury severity (n = 11,921, 4.87%) and comorbid SCI (n = 2,349, 7.91%). Two or more diagnoses of dementia were present in <2% of all cases (Table 1 and Supplementary Table 1, Supplement File 3).
Multivariable Cox regression analyses (Table 2) determined HR estimates and model fit statistics for 2 models we examined (generic and sex stratified) with any dementia, each fully adjusted with censoring at death, the competing event. In the fully adjusted simplest dementia model, TBI of moderate and unspecified injury severity was significantly associated with incident dementia, with such patients having a 12% and 10% higher hazard of developing dementia compared to those with mild TBI injury (Table 2). The hazard of unspecified injury severity on developing dementia was greater in men than in women (17% vs. 5%, respectively), and the hazard of moderate injury severity on dementia development was significant in women only (HR = 1.23 [95% CI, 1.09–1.40] in women; HR = 0.98 [95% CI, 0.84–1.15] in men). In sex-stratified models, the hazard of severe TBI for developing dementia was comparable between both sexes but nonsignificant (HR = 0.94 [95% CI, 0.86–1.02], and HR = 1.00 [95% CI, 0.92–1.09], respectively).
Table 2.
Model fitting and effect (HR and 95% CI), including regression coefficients (ß) and level of significance (ρ), of CNS trauma expressed by TBI severity and SCI comorbidity, controlling for potential confounders and risk factors for dementia
| Regression coefficients/predictors/P-value 1st row (all) 2nd row (men) 3rd row (women) |
Dementia model |
||
|---|---|---|---|
| Simplest; ß/HR (95% CI) ρ | Simplest + interaction of TBI severity with age; ß/HR (95% CI) ρ | Simplest + interaction of SCI with age; ß/HR (95% CI) ρ | |
| TBI severity | |||
| Mild (yes) | 1 | 1 | 1 |
| Unknown severity (concussion codes) versus mild | 0.09/1.10 (1.07–1.12)**** | 0.42/Fig. 2A**** | 0.09/1.10 (1.07–1.12)**** |
| 0.16/1.17 (1.13–1.22)**** | 0.78/Fig. 2B**** | 0.16/1.17 (1.13–1.22)**** | |
| 0.05/1.05 (1.02–1.08)*** | 0.02/Fig. 2B | 0.05/1.05 (1.02–1.08)*** | |
| Moderate versus mild | 0.11/1.12 (1.02–1.24)*** | 0.31/Fig. 2A | 0.11/1.12 (1.01–1.23)*** |
| −0.02/0.98 (0.84–1.15) | 0.16/Fig. 2B | −0.02/.98 (0.83–1.14) | |
| 0.21/1.23 (1.09–1.40)*** | 0.82/Fig. 2B | 0.21/1.23 (1.08–1.40)*** | |
| Severe versus mild | −0.04/0.97 (0.91–1.03) | 1.65/Fig. 2A**** | −0.04/0.96 (0.91–1.02) |
| −0.06/0.94 (0.86–1.02) | 1.96/Fig. 2B**** | −0.07/0.94 (0.86–1.02) | |
| 0.00/1.00 (0.92–1.09) | 1.47/Fig. 2B*** | 0.00/1.00 (0.91–1.09) | |
| Mild (yes) | 1 | 1 | 1 |
| SCI comorbidity | |||
| SCI (yes vs. no) | 0.08/1.08 (1.03–1.12)*** | 0.07/1.08 (1.03–1.12)*** | 1.35/(Fig. 3A)**** |
| 0.12/1.13 (1.06–1.21)*** | 0.12/1.13 (1.06–1.21)*** | 1.79/(Fig. 3B)**** | |
| 0.05/1.05 (1.00–1.11) | 0.05/1.05 (1.00–1.12) | 0.91/(Fig. 3B)*** | |
| Sex | |||
| Women | −0.13/0.88 (0.86–0.90)**** | −0.13/0.88 (0.86–0.90)**** | −0.13/0.88 (0.86–0.90)**** |
| Men | 1 | 1 | 1 |
| Age† | −0.05/0.96 (0.90–1.01) | −0.05/Supplementary Fig. 3, Suppl. File 2 | −0.04/Supplementary Fig. 4, Suppl. File 2 |
| −0.11/0.90 (0.86–0.97)*** | −0.11/Supplementary Fig. 3, Suppl. File 2*** | −0.10/Supplementary Fig. 4, Suppl. File 2*** | |
| 0.02/1.02 (0.93–1.13) | 0.02/Supplementary Fig. 3, Suppl. File 2 | 0.02/Supplementary Fig. 4, Suppl. File 2 | |
| Age2 | 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.01)**** | 0.00/1.00 (1.00–1.01)**** |
| 0.01/1.01 (1.00–1.01)**** | 0.01/1.01 (1.00–1.01)**** | 0.01/1.01 (1.00–1.01)**** | |
| 0.00/1.00 (1.00–1.01)**** | 0.00/1.00 (1.00–1.01)**** | 0.00/1.00 (1.00–1.01)**** | |
| Age3 | 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** |
| 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** | |
| 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** | 0.00/1.00 (1.00–1.00)**** | |
| Controlling variables | |||
| Neighborhood income 2nd quantile versus 1st quantile (poorest) | −0.07/0.94 (0.91–0.97)**** | −0.07/0.94 (0.91–0.97)**** | −0.07/0.94 (0.91–0.97)**** |
| −0.10/0.90 (0.89–0.96)*** | −0.10/0.9105 (0.86–0.95)*** | −0.10/0.90 (0.86–0.95)*** | |
| −0.05/0.96 (0.92–0.99)*** | −0.05/0.96 (0.92–0.99)*** | −0.05/0.96 (0.92–0.99)*** | |
| Neighborhood income 3rd quantile versus 1st quantile | −0.08/0.92 (0.89–0.95)**** | −0.08/0.92 (0.89–0.95)**** | −0.08/0.92 (0.90–0.95)**** |
| −0.12/0.89 (0.84–0.94)**** | −0.12/0.89 (0.84–0.94)**** | −0.12/0.89 (0.84–0.94)**** | |
| −0.06/0.94 (0.90–0.98)*** | −0.07/0.94 (0.90–0.98)*** | −0.07/0.94 (0.90–0.98)*** | |
| Neighborhood income 4th quantile versus 1st quantile | −0.11/0.90 (0.87–0.93)**** | −0.11/0.90 (0.87–0.93)**** | −0.11/0.90 (0.87–0.93)**** |
| −0.19/0.83 (0.79–0.87)**** | −0.19/0.83 (0.76–0.87)**** | −0.19/0.83 (0.79–0.88)**** | |
| −0.05/0.95 (0.91–0.99)*** | −0.05/0.95 (0.91–0.99)*** | −0.05/0.95 (0.91–0.99)*** | |
| Neighborhood income 5th quantile versus 1st quantile | −0.15/0.86 (0.83–0.89)**** | −0.15/0.86 (0.83–0.89)**** | −0.15/0.86 (0.83–0.89)**** |
| −0.23/0.80(0.750–0.84)**** | −0.23/0.80 (0.76–0.84)**** | −0.23/0.80 (0.76–0.84)**** | |
| −0.10/0.90 (0.86–0.94)**** | −0.10/0.90 (0.86–0.94)**** | −0.10/0.90 (0.86–0.94)**** | |
| Cerebrovascular disease (yes vs. no) | 0.36/1.43 (1.37–1.48)**** | 0.36/1.43(1.38–1.48)**** | 0.36/1.43 (1.38–1.481)**** |
| 0.37/1.45 (1.37–1.53)**** | 0.37/1.45 (1.38–1.53)**** | 0.37/1.45 (1.37–1.528)**** | |
| 0.34/1.40 (1.33–1.48)**** | 0.34/1.40 (1.33–1.48)**** | 0.34/1.40 (1.33–1.478)**** | |
| Ischemic heart disease (yes vs. no) | −0.11/0.90 (0.87–0.93)**** | −0.11/0.90 (0.87–0.93)**** | −0.11/0.90 (0.87–0.93)**** |
| −0.16/0.85 (0.81–0.90)**** | −0.16/0.85 (0.81–0.90)**** | −0.16/0.85 (0.81–0.90)**** | |
| −0.06/0.94 (0.90–0.98)*** | −0.07/0.94 (0.90–0.98)*** | −0.06/0.94 (0.90–0.98)*** | |
| Diseases of arteries, arterioles and capillaries (yes vs. no) | 0.11/1.12 (1.05–1.19)*** | 0.11/1.12 (1.05–1.19)*** | 0.11/1.12 (1.05–1.19)*** |
| 0.16/1.18 (1.08–1.28)*** | 0.16/1.18 (1.08–1.28)*** | 0.16/1.18 (1.08–1.28)*** | |
| 0.03/1.03 (0.94–1.14) | 0.03/1.03 (0.94–1.14) | 0.03/1.03 (0.94–1.14) | |
| Atrial fibrillation (yes vs. no) | −0.06/0.94 (0.91–0.98)*** | −0.06/0.95 (0.91–0.98)*** | −0.06/0.95 (0.91–0.98)*** |
| −0.07/0.93 (0.88–0.99)*** | −0.07/0.94 (0.884–0.99)*** | −0.07/0.94 (0.88–0.99)*** | |
| −0.05/0.95 (0.91–1.00) | −0.05/0.95 (0.91–1.00) | −0.05/0.95 (0.91–1.00) | |
| Heart failure (yes vs. no) | 0.15/1.16 (1.110–1.21)**** | 0.15/1.16 (1.11–1.21)**** | 0.15/1.16 (1.11–1.21)**** |
| 0.18/1.20 (1.13–1.28)**** | 0.18/1.20 (1.13–1.28)**** | 0.18/1.20 (1.13–1.28)**** | |
| 0.121.13 (1.06–1.19)**** | 0.12/1.13 (1.06–1.19)**** | 0.12/1.13 (1.06–1.19)**** | |
| Obesity (yes vs. no) | −0.05/0.95 (0.84–1.08) | −0.05/0.95 (0.834–1.08) | −0.05/0.95 (0.83–1.08) |
| −0.12/0.89 (0.70–1.11) | −0.13/0.88 (0.70–1.11) | −0.13/0.88 (0.70–1.11) | |
| −0.01/0.99 (0.85–1.16) | −0.01/0.99 (0.85–1.16) | −0.01/0.99 (0.85–1.15) | |
| Tobacco smoking (yes vs. no) | 0.67/1.95 (1.45–2.56)**** | 0.66/1.94 (1.48–2.56)**** | 0.67/1.95 (1.48–2.56)**** |
| 0.42/1.52 (1.02–2.28)*** | 0.42/1.520 (1.02–2.27)*** | 0.42/1.53 (1.02–2.28)*** | |
| 0.91/2.49 (1.71–3.64)**** | 0.91/2.491 (1.71–3.64)**** | 0.91/2.49 (1.70–3.63)**** | |
| Hyperlipidemia (yes vs. no) | 0.04/1.04 (0.99–1.099) | 0.04/1.04 (0.99–1.09) | 0.04/1.04 (0.99–1.09) |
| 0.09/1.10 (1.02–1.18)*** | 0.09/1.09 (1.02–1.18)*** | 0.09/1.09 (1.02–1.18)*** | |
| −0.00/1.00 (0.93–1.07) | −0.01/0.99 (0.93–1.07) | −0.00/1.00 (0.93–1.07) | |
| Diabetes mellitus (yes vs. no) | 0.38/1.46 (1.42–1.50)**** | 0.38/1.457 (1.42–1.50)**** | 0.38/1.46 (1.42–1.50)**** |
| 0.40/1.49 (1.42–1.55)**** | 0.39/1.484 (1.42–1.55)**** | 0.40/1.49 (1.42–1.55)**** | |
| 0.36/1.44(1.38–1.49)**** | 0.36/1.435 (1.38–1.49)**** | 0.36/1.44 (1.38–1.49)**** | |
| Depression (yes vs. no) | 0.60/1.82(1.73–1.91)**** | 0.60/1.82 (1.73–1.91)**** | 0.59/1.811 (1.72–1.91)**** |
| 0.70/2.01 (1.85–2.18)**** | 0.69/2.00 (1.85–2.18)**** | 0.70/2.004 (1.85–2.18)**** | |
| 0.54/1.71 (1.61–1.83)**** | 0.54/1.71 (1.61–1.83)**** | 0.54/1.709 (1.60–1.82)**** | |
| Vision impairments (yes vs. no) | −0.06/0.95 (0.89–1.00) | −0.06/0.946 (0.89–1.01) | −0.06/0.95 (0.89–1.00) |
| −0.01/0.99 (0.91–1.09) | −0.01/0.994 (0.91–1.09) | −0.01/1.00 (0.91–1.09) | |
| −0.09/0.92 (0.85–0.99)*** | −0.09/0.918 (0.85–0.99)*** | −0.09/0.92 (0.85–0.99)*** | |
| Hearing loss (yes vs. no) | 0.16/1.18 (1.01–1.37)*** | 0.16/1.18 (1.01–1.37)*** | 0.16/1.18 (1.01–1.37)*** |
| 0.21/1.24 (0.99–1.55) | 0.21/1.24 (0.99–1.55) | 0.21/1.24 (0.99–1.55) | |
| 0.12/1.13 (0.91–1.38) | 0.12/1.13 (0.92–1.39) | 0.12/1.13 (0.92–1.39) | |
| Sleep disorder (yes vs. no) | 0.22/1.25 (1.15–1.36)**** | 0.22/1.25 (1.15–1.36)**** | 0.22/1.25 (1.14–1.36)**** |
| 0.23/1.26 (1.11–1.42)*** | 0.22/1.25 (1.11–1.41)*** | 0.23/1.25 (1.11–1.41)*** | |
| 0.21/1.23 (1.09–1.40)*** | 0.21/1.24 (1.09–1.40)*** | 0.21/1.23 (1.09–1.40)*** | |
| Model fitting | |||
| LRχ2 | 111134.13**** | 111172.20**** | 111172.94**** |
| 52,654.88**** | 52,692.07**** | 52,686.80**** | |
| 54,048.52**** | 54,059.47**** | 54,057.45**** | |
| AIC | 735762.68 | 735688.60 | 735687.87 |
| 277549.06 | 277517.88 | 277519.15 | |
| 413829.46 | 413824.51 | 413822.52 | |
| Degree of freedom | 18 | 21 | 19 |
| 17 | 20 | 18 | |
| 17 | 20 | 18 | |
| Corrected C-index | 0.93 | 0.93 | 0.93 |
| 0.95 | 0.95 | 0.95 | |
| 0.90 | 0.90 | 0.90 | |
NOTE. Corrected C-index for all models ranged from 0.90 to 0.95.
ρ = level of significance: ***P < .001; ****P < .0001.
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; CNS, central nervous system; HR, hazard ratio; LR, likelihood ratio; TBI, traumatic brain injury; SCI, spinal cord injury; Suppl., Supplement.
Significant nonlinearity was observed: a restricted cubic spline transformation was used.
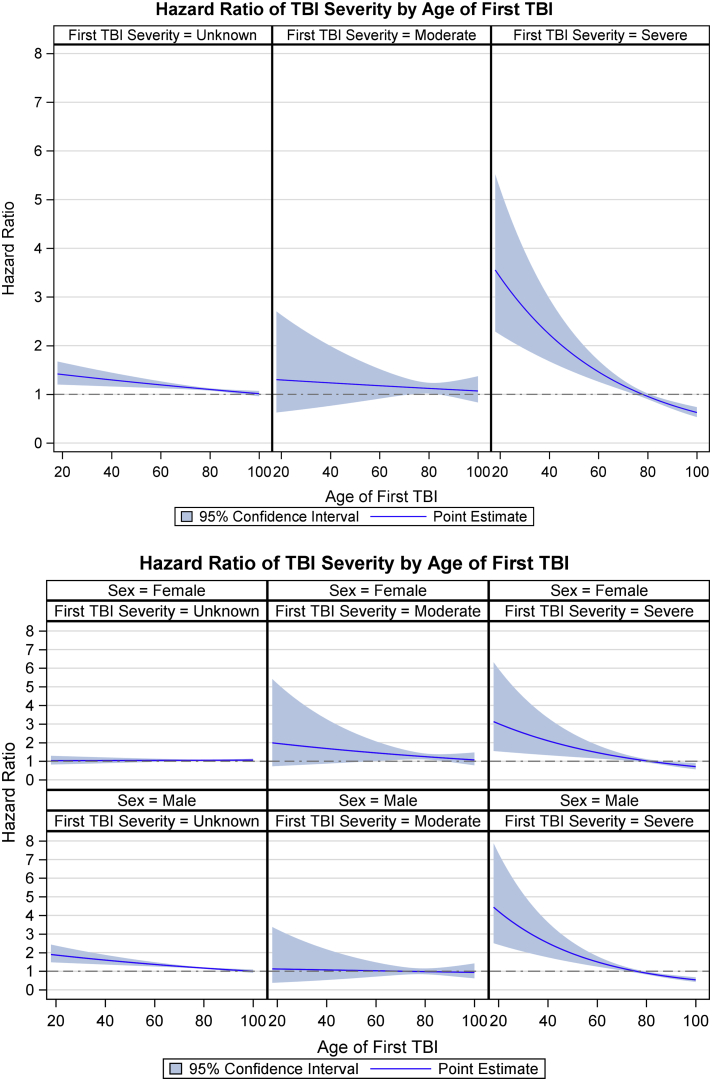
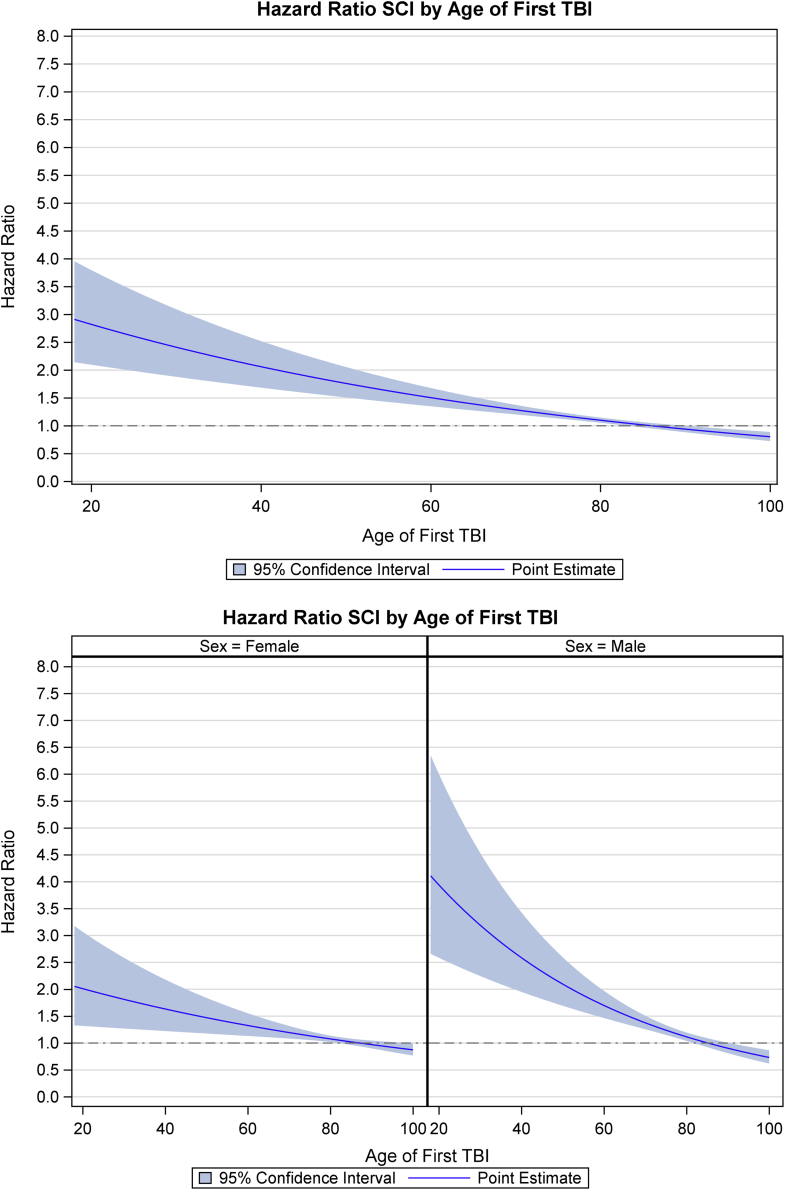
Since interactions between TBI severity and age, and comorbid SCI and age, were evident (Supplementary Figs. 2 and 3, Supplementary File 3), we calculated adjusted HRs for dementia for each. The plot based on the models with interactions (Fig. 2A and 2B and Fig. 3A and 3B) shows the effect of severe TBI is greater than that of other injury severities (mild, unspecified, and moderate), more in men than in women, and it reduced with age. Similarly, Fig. 3A and 3B highlight the effect of age at the time of event and comorbid SCI on developing dementia.
Fig. 2.
(A) Proportional hazard ratio plots of the estimated risk of dementia versus patient age at first TBI event, and by TBI severity, after adjustment for sex and known risk factors. Mild TBI severity was used as a reference. (B) Proportional hazard ratio plots of the estimated risk of dementia versus patient age at first TBI event, and by TBI severity and by sex, after adjustment for known risk factors. Mild TBI severity was used as a reference. Abbreviations: F, female; M, male; TBI, traumatic brain injury.
Fig. 3.
(A) Proportional hazard ratio plots of the estimated risk of dementia versus patient age at first TBI event in the presence of comorbid SCI, after adjustment for sex and known risk factors. No SCI comorbidity was used as a reference. (B) Proportional hazard ratio plots of the estimated risk of dementia versus patient age at first TBI event in the presence of comorbid SCI, by sex, after adjustment for known risk factors. No SCI comorbidity was used as a reference. Abbreviations: F, female; M, male; TBI, traumatic brain injury; SCI, spinal cord injury.
Socioeconomic status, disorders of the circulatory system, sleep disorders, and vascular risk factors were associated with incident dementia, and HRs varied between sexes (Table 2). All models were well calibrated and highly discriminative. The fit for models with only known predictors of dementia was significantly worse than final models of dementia, indicating that variables independently contribute to outcome.
3.3. Additivity in CNS trauma
To test additivity of SCI and TBI in CNS trauma, we investigated HRs for developing dementia in TBI and TBI with SCI separately, in different groups (men vs. women; with and without age interaction). Presence of SCI had a significant effect on the fully adjusted simplest model on the risk of developing dementia in men (HR = 1.13 [95% CI, 1.06–1.21]) but not women (HR = 1.05 [95% CI, 1.00–1.12]) (Table 2).
3.4. Interactions
Nonlinearity in the relationship between age and outcome was observed (Supplementary Fig. 3, Supplement File 3). To offset incorrect interpretation of the HR, restricted quadratic and cubic transformation was performed for age (Table 2).
We investigated interactions between TBI severity and age, and SCI and age at baseline. Visual and quantitative approaches were used to investigate the effect of modifiers. Main-effect–only models were compared to full models with interactions (Table 2). The effect of severe TBI on incident dementia became apparent in younger men and women and decreased with age; it was not observable in either sex aged >80 years (Fig. 2A and 2B).
3.5. Competing risk analysis
In the Fine and Gray regression, the effects of TBI severity and SCI comorbidity on incident dementia had similar HRs to those from the Cox regression in the overall and sex-stratified models (Supplementary Tables 2–4 in Supplement File 4).
3.6. Nomogram
A nomogram point system was used to assign each predictor with a value ranging from 0 to 100 in a graphic interface. Based on the estimated regression coefficients, we ranked the estimated effects, disregarding statistical significance (absolute beta values). Resulting nomogram plots for both sexes are presented in Supplementary Figs. 1 and 2 (Supplement File 5). The points assigned to each predictor are presented in Supplementary Table 1 (Supplement File 4). To estimate the role of CNS trauma-related variables using nomograms, we compared 2 cases that were similar except for sex. First, the total number of points was calculated for a man with mild TBI, aged 20 years without comorbid SCI or other known risk factors of dementia except smoking. The total points were 50, indicating a probability of developing dementia within the next 10 years of <0.1. The total number of points for a woman of similar description was 33.2, indicating a probability of developing dementia within the next 10 years of 0.15.
3.7. Sensitivity analyses
We performed subgroup analysis, testing the effect of injury severity and SCI comorbidity on early onset dementia. Our fully adjusted simplest dementia model confirmed associations between severe TBI and comorbid SCI with incident dementia (HR 1.45 [95% CI, 1.05–2.01] and HR 1.33 [95% CI, 1.03–1.71], respectively). When results were stratified by sex, the association remained significant in men (HR 1.52 [95% CI, 1.04–2.20] and HR 1.57 [95% CI, 1.15–2.13, respectively]) but not in women (HR 1.25 [95% CI, 0.65–2.38] and 0.96 [95% CI, 0.60–1.52]) (Supplementary Table 2, Supplement File 4).
4. Discussion
In this large representative cohort of racially diverse persons with universal health-care insurance (N = 712,708 adult patients, 59% men) who sustained CNS trauma (i.e., TBI alone or with comorbid SCI) over a decade-long period, almost 5% developed dementia. In the fully adjusted models for sex and known risk factors of dementia, severe TBI and concussive injury severity (unspecified injury severity S06 codes under the ICD-10 classification system) were significantly associated with incident dementia. SCI comorbidity in TBI has an additive effect on risk. Age interacts with trauma-related variables (injury severity and comorbid SCI) to lead to the development of dementia; the risk is greater in younger people and it diminishes with age. This study highlighted a novel risk of dementia onset in persons free of dementia at the date of injury; this novel risk is the presence of a sleep disorder. Although the effects attributed to injury severity, comorbid SCI, and sleep disorder are small compared to other risk factors (i.e., depression, smoking, diabetes mellitus, etc.), even a small increase in dementia risk can bring a patient to a considerably higher risk group (Supplementary Figs. 1 and 2, Supplement File 5). This was also the first study to present data on risk of dementia onset for men and women separately. Societal advances such as improved living standards [36], lifestyle modifications [37], and quaternary preventions [38], [39] could prove vital in reducing risk in both men and women.
Nearly all previous studies of TBI and dementia risk have compared TBI patients within themselves or to other trauma patients [11], [12], [13], [14], [15]. Some found a marked attenuation of dementia risk with age [12], [15], while others did not (Supplementary Table 1, Supplement File 1). We attribute these differences to study limitations including reverse causality; not accounting for injury severity or treating unspecified injury severity codes as mild; and selected groups of older age. In our study, we addressed these limitations by applying a 1-year look-back window to exclude previous dementia, excluding delirium and substance-related dementia and postconcussive syndrome, and treating unspecified injury severity as a separate cohort. For the first time, we considered comorbid SCI, the interaction of age, and indices of social deprivation, separately in men and women. Our results show that TBI alone, or with SCI, differentially affects men and women of varying ages, with effect of severe TBI and concussions (e.g., unspecified injury severity S06.0 codes) decreasing with increasing age. Our results support the notion that the CNS is not a static entity and that its structure and function at the time of injury matters when it comes to reorganization of neural pathways in response to the damage [40].
It is well known in the field of cognitive aging that older adults compared with younger adults often have poorer episodic memory but better semantic memory, and the accumulation of knowledge overall is increased in older adults compared with younger adults, highlighting that not all aspects of cognition are adversely affected by older age [41], [42], [43]. With damage, compensation for the damaged tissue occurs in numerous ways including but not limited to reorganization of existing neuronal networks and development of new networks [44]. Greater connectivity between brain regions, and spinal cord regions in the older person compared to those younger, may serve a beneficial role in preserving cognitive functioning in those who sustain injury later in life, as opposed to those who have sustain injury earlier in life. Likewise, while development of new networks is superior in younger individuals as compared to those who are older, they are not always beneficial networks [45]. It is also possible that after TBI in younger people, microglia exhibits altered morphology and an immune-reactive phenotype, which can compound their risk, to a greater extent [46]. Given the complexity of brain function, future studies of the impact of brain injury on cognitive functioning will have to include examination of dynamic interactions among brain regions, in conjunction with underlying structural changes in response to injuries of similar severities across the lifespan.
Our findings are consistent with proposed pathophysiological mechanisms of dementia. Diffuse axonal injury in concussion (e.g., S06.0 codes) predominately affects dense white matter tracts in the corpus callosum, the midbrain, and the reticular system [47], [48]. This alters baseline neurotransmitter levels and interferes with major dopaminergic fiber tracts connecting to the prefrontal cortex, involved in cognitive processes [49]. In most severe injury, the hippocampus undergoes excitotoxic damage caused by the release of neurotransmitters [50], producing impairments in executive function and attention [51]. The observed change in the effect of severe TBI and SCI on dementia onset in both sexes, in both main and sensitivity analyses, is important. This observation supports the hypothesis that the effects of TBI severity and extent of CNS trauma on dementia onset are not constant across the lifespan and that underlying dysfunction at the neuronal, cellular, and molecular levels following trauma (e.g., the loss of dendrites and synapses, neuronal death, formation of abnormal capillaries, plaques, and neurofibrillary tangles [52]) varies throughout life [53], and thus, they should be considered in future studies.
Numerous researchers have studied the relationship between TBI and sleep in clinical settings, with coinciding findings and conclusions: sleep disorders are prevalent in this population and are an integral component in postinjury physical and mental health [54]. This study highlighted the association between sleep disorders and dementia onset, in both men and women, across the lifespan. It is crucial that this evidence is acknowledged in research and practice. Sleep has been shown to play a protective role in warding off toxic protein accumulation [55], and scientific evidence is rapidly developing, suggesting that the “glymphatic” system of the CNS, the interstitial spaces surrounding glia, and neurons, has substantially higher clearance of amyloid beta during sleep as compared with wakefulness, potentially reducing evolution of neurodegeneration in those with healthy sleep [56]. Future research on this topic is greatly needed.
Our study has several limitations. Although our models with dementia risk factors had high predictive ability, reflecting that the majority of important predictors were included, some predictors (e.g., toxic environmental exposures, family history of dementia or AD) [57], [58] were not available. Additionally, we were not positioned to investigate the effect of any intervention for TBI, SCI, and other comorbidities on the development of dementia. Furthermore, it is likely that several included predictors, including socioeconomic status, had changed during the course of the study. Therefore, nomograms demonstrating the relationships between baseline predictors and temporal outcomes should not be used clinically until further external validation. Although validated algorithms were used to define dementia, misclassification of patients is possible, resulting in CNS trauma–related predictors to be calculated below their true values. With respect to defining incident dementia using ED or acute care data sets, a proportion of prevalent cases might be misclassified as incident cases, when dementia was not captured before the index date within our observational period. Similarly, preexisting dementia could have developed any time before the recorded date of diagnosis, while the time of dementia diagnosis after TBI event could be positively skewed. Furthermore, patients with more severe TBI may have been in more contact with health-care providers and thus are more likely to be diagnosed with dementia. To address this, we removed delirium cases from our analysis, and adjusted our models for baseline sociodemographic, economic, and clinical comorbidities and known predictors of health-care use in patients with TBI. Furthermore, we stratified our results by sex, accounting for differential help-seeking behaviors [10]. All analyses were based on a single cohort, as we could not further split this sample into training, validation, and testing cohorts [59] due to few events per SCI predictor in sex-specific models. Furthermore, the observed interactions between TBI severity and age, and SCI and age, are based on statistical grounds, making further research essential to confirm our findings and address some of these limitations using larger data sets with longer follow-up times. Future research should include further stratification of male and female patients using proposed risk-stratification models. Finally, our results highlight the potential need to operationalize CNS trauma in dementia risk models to concurrently reflect injury severity based on acute injury markers as well as physiological consequences occurring within the brain and spinal cord at different life stages. The failure to account for an interaction between trauma-related predictors and age could lead to bias and misinterpretation of the results in cases of TBI and SCI comorbidity. Studies must include identification of factor interactions, consider interaction effects when assessing dementia risks in CNS trauma, and be sex-specific. This could lead to significant implications for public health. While our results should be treated as a foray into studying link between CNS trauma-related variables and dementia onset in men and women across the lifespan, we believe that we provide a solid methodological framework that others may find useful in understanding the relationship and which can be used as a foundation in further research undertakings.
To conclude, TBI alone, or with SCI, differentially affects men and women of varying ages, and this should be considered when assessing postinjury dementia risk. Sex-specific regression models improve risk stratification [60].
Research in Context.
-
1
Systematic review: Among published reports on the causal relationship between traumatic brain injury and incident dementia, all found a significant association between exposure to severe injury and incident dementia. However, there was no risk stratification by sex, extent of injury, or age at time of injury to predict incident dementia—all significant oversights.
-
2
Interpretation: This study followed up 712,708 adult patients admitted to a publicly funded health-care system with a diagnosis of traumatic brain injury alone or with comorbid spinal cord injury over more than a decade. We observed that injury-related factors interact with age to influence new dementia onset and that women are at a greater risk of developing dementia earlier than men, all other risk factors being equal. This study also highlighted the association between sleep disorders and dementia onset, in both men and women, across the lifespan.
-
3
Future directions: Our results suggest that risk stratification of patients with central nervous system trauma by sex is vital in identifying those most likely to develop dementia and in understanding the course and modifying factors.
Acknowledgments
Author Contributions: T.M. contributed to literature search, study conception and design, ethics boards' application, obtaining administrative data, study design, defining data analyses steps, interpretation of results, and drafting of the manuscript. M.H. contributed to data cleaning, cohort creation, data analyses, interpretation of results, and visual presentation of results. M.E. contributed to data analyses and interpretation of results and provided statistical expertize on all stages of the project; A.C. contributed to study design, supervision of ethics boards' application, and obtaining administrative data, data interpretation, and critical revision of data analyses. All authors critically reviewed the final version of the manuscript and gave final approval of the submitted manuscript.
Funding: This work was supported by the postdoctoral research grant from the Alzheimer's Association (AARF-16-442937) to TM. The authors were also supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD089106 and the Canadian Institutes for Health Research Grant–Institute for Gender and Health (#CGW-126580). The funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
Disclaimers: This study made use of deidentified data from the Institute for Clinical Evaluative Sciences Data Repository, which is managed by the Institute for Clinical Evaluative Sciences with support from its funders and partners: Canada's Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research, and the Government of Ontario. The opinions, results, and conclusions reported are those of the authors. No endorsement by The Institute for Clinical Evaluative Sciences or any of its funders or partners is intended or should be inferred.
Footnotes
Conflict of Interest: The authors declare no financial and nonfinancial competing interests.
Supplementary data related to this article can be found at https://dx.doi.org/10.1016/j.dadm.2019.03.003.
Supplementary Data
References
- 1.Julien J., Joubert S., Ferland M.C., Frenette L.C., Boudreau-Duhaime M.M., Malo-Véronneau L. Association of traumatic brain injury and Alzheimer disease onset: A systematic review. Ann Phys Rehabil Med. 2017;60:347–356. doi: 10.1016/j.rehab.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., InTBIR Participants and Investigators Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 3.Furlan J.C., Sakakibara B.M., Miller W.C., Krassioukov A.V. Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci. 2013;40:456–464. doi: 10.1017/s0317167100014530. [DOI] [PubMed] [Google Scholar]
- 4.Bombardier C.H., Lee D.C., Tan D.L., Barber J.K., Hoffman J.M. Comorbid Traumatic Brain Injury and Spinal Cord Injury: Screening Validity and Effect on Outcomes. Arch Phys Med Rehabil. 2016;97:1628–1634. doi: 10.1016/j.apmr.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Tan X.L., Sun M., Brady R.D., Liu S., Llanos R., Cheung S. Transactive Response DNA-Binding Protein 43 Abnormalities after Traumatic Brain Injury. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5491. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Yokobori S., Zhang Z., Moghieb A., Mondello S., Gajavelli S., Dietrich W.D. Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg. 2015;83:867–878. doi: 10.1016/j.wneu.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 7.James B.D., Wilson R.S., Boyle P.A., Trojanowski J.Q., Bennett D.A., Schneider J.A. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016;139:2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollayeva T., Pacheco N., D'Souza A., Colantonio A. The course and prognostic factors of cognitive status after central nervous system trauma: a systematic review protocol. BMJ Open. 2017;7:e017165. doi: 10.1136/bmjopen-2017-017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groot C., Sudre C.H., Barkhof F., Teunissen C.E., van Berckel B.N.M., Seo S.W. Clinical phenotype, atrophy, and small vessel disease in APOEε2 carriers with Alzheimer disease. Neurology. 2018;91:e1851–e1859. doi: 10.1212/WNL.0000000000006503. [DOI] [PubMed] [Google Scholar]
- 10.Mollayeva T., Mollayeva S., Colantonio A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol. 2018;14:711–722. doi: 10.1038/s41582-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 11.Nordström P., Michaëlsson K., Gustafson Y., Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014;75:374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- 12.Gardner R.C., Burke J.F., Nettiksimmons J., Kaup A., Barnes D.E., Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes D.E., Kaup A., Kirby K.A., Byers A.L., Diaz-Arrastia R., Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj R., Kaprio J., Korja M., Mikkonen E.D., Jousilahti P., Siironen J. Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries. PLos Med. 2017;14:e1002316. doi: 10.1371/journal.pmed.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H.K., Lin S.H., Sung P.S., Wu M.H., Hung K.W., Wang L.C. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83:1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 16.Budisin B., Bradbury C.C., Sharma B., Hitzig S.L., Mikulis D., Craven C. Traumatic Brain Injury in Spinal Cord Injury: Frequency and Risk Factors. J Head Trauma Rehabil. 2016;31:E33–E42. doi: 10.1097/HTR.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Clinical Evaluation Sciences Privacy Code – Protecting Personal Health Information at ICES. Toronto: Institute for Clinical Evaluation Sciences. https://www.ices.on.ca/Data-and-Privacy/Privacy-at-ICES Available at:
- 18.Li W., Risacher S.L., McAllister T.W., Saykin A.J. Alzheimer's Disease Neuroimaging Initiative. Age at injury is associated with the long-term cognitive outcome of traumatic brain injuries. Alzheimers Dement (Amst) 2017;6:196–200. doi: 10.1016/j.dadm.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coronado V.G., Xu L., Basavaraju S.V. Centers for disease control and prevention. Surveillance for Traumatic Brain Injury-Related Deaths - United States, 1997-2007/60(SS05) https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6005a1.htm 1-32. Available at: [PubMed]
- 20.Corrigan J.D., Kreider S., Cuthbert J., Whyte J., Dams-O'Connor K., Faul M. Components of traumatic brain injury severity indices. J Neurotrauma. 2014;31:1000–1007. doi: 10.1089/neu.2013.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagné M., Moore L., Sirois M.J., Simard M., Beaudoin C., Kuimi B.L. Performance of International Classification of Diseases-based injury severity measures used to predict in-hospital mortality and intensive care admission among traumatic brain-injured patients. J Trauma Acute Care Surg. 2017;82:374–382. doi: 10.1097/TA.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 22.Munce S.E., Wodchis W.P., Guilcher S.J., Couris C.M., Verrier M., Fung K. Direct costs of adult traumatic spinal cord injury in Ontario. Spinal Cord. 2013;51:64–69. doi: 10.1038/sc.2012.81. [DOI] [PubMed] [Google Scholar]
- 23.Voormolen D.C., Cnossen M.C., Polinder S., von Steinbuechel N., Vos P.E., Haagsma J.A. Divergent classification methods of post-concussion syndrome after mild traumatic brain injury: Prevalence rates, risk factors, and functional outcome. J Neurotrauma. 2018;35:1233–1241. doi: 10.1089/neu.2017.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breitner J.C. Dementia - epidemiological considerations, nomenclature, and a tacit consensus definition. J Geriatr Psychiatry Neurol. 2006;19:129–136. doi: 10.1177/0891988706291081. [DOI] [PubMed] [Google Scholar]
- 25.Sepulveda E., Franco J.G., Trzepacz P.T., Gaviria A.M., Meagher D.J., Palma J. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry. 2016;16:167. doi: 10.1186/s12888-016-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deckers K., van Boxtel M.P., Schiepers O.J., de Vugt M., Muñoz Sánchez J.L., Anstey K.J. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 27.Kalkonde Y.V., Jawaid A., Qureshi S.U., Shirani P., Wheaton M., Pinto-Patarroyo G.P. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement. 2012;8:204–210. doi: 10.1016/j.jalz.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 28.METACOHORTS Consortium METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: An initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimers Dement. 2016;12:1235–1249. doi: 10.1016/j.jalz.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim M.M., Mysliwiec V. Overlooked implications of disturbed sleep in traumatic brain injury. JAMA Neurol. 2019;76:114–115. doi: 10.1001/jamaneurol.2018.3738. [DOI] [PubMed] [Google Scholar]
- 30.Calvo R.Y., Lindsay S.P., Edland S.D., Macera C.A., Wingard D.L., Ohno-Machado L. The differential associations of preexisting conditions with trauma-related outcomes in the presence of competing risks. Injury. 2016;47:677–684. doi: 10.1016/j.injury.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 31.Rulli E., Ghilotti F., Biagioli E., Porcu L., Marabese M., D'Incalci M. Assessment of proportional hazard assumption in aggregate data: a systematic review on statistical methodology in clinical trials using time-to-event endpoint. Br J Cancer. 2018;119:1456–1463. doi: 10.1038/s41416-018-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 33.Harrell F.E. Springer-Verlag; New York: 2001. Regression modelling strategies: With applications to linear models, logistic regression, and survivor analysis. [Google Scholar]
- 34.Lavoie A., Moore L., LeSage N., Liberman M., Sampalis J.S. The New Injury Severity Score: a more accurate predictor of in-hospital mortality than the Injury Severity Score. J Trauma. 2004;56:1312–1320. doi: 10.1097/01.ta.0000075342.36072.ef. [DOI] [PubMed] [Google Scholar]
- 35.Yang D. Build Prognostic Nomograms for Risk Assessment Using SAS®. SAS Glob Forum. 2013 support.sas.com/resources/papers/proceedings13/264P-2013.pdf Available at: [Google Scholar]
- 36.Marešová P., Mohelská H., Dolejš J., Kuča K. Socio-economic Aspects of Alzheimer's Disease. Curr Alzheimer Res. 2015;12:903–911. doi: 10.2174/156720501209151019111448. [DOI] [PubMed] [Google Scholar]
- 37.Larsson S.C., Wolk A. The role of lifestyle factors and sleep duration for late-onset dementia: A cohort study. J Alzheimers Dis. 2018;66:579–586. doi: 10.3233/JAD-180529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangin D., Heath I., Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. Br Med J. 2012;344:e3526. doi: 10.1136/bmj.e3526. [DOI] [PubMed] [Google Scholar]
- 39.Chan V., Mollayeva T., Ottenbacher K.J., Colantonio A. Clinical profile and comorbidity of traumatic brain injury among younger and older men and women: a brief research notes. BMC Res Notes. 2017;10:371. doi: 10.1186/s13104-017-2682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mollayeva T., Mollayeva S., Pacheco N., D'Souza A., Colantonio A. The course and prognostic factors of cognitive outcomes after traumatic brain injury: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;99:198–250. doi: 10.1016/j.neubiorev.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Metcalfe J., Casal-Roscum L., Radin A., Friedman D. On Teaching Old Dogs New Tricks. Psychol Sci. 2015;26:1833–1842. doi: 10.1177/0956797615597912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen P.A., Sliwinski M., Bowie T., Madden D.J. Differential age effects in semantic and episodic memory. J Gerontol B Psychol Sci Soc Sci. 2002;57:P173–P186. doi: 10.1093/geronb/57.2.p173. [DOI] [PubMed] [Google Scholar]
- 43.Cook I.A., Bookheimer S.Y., Mickes L., Leuchter A.F., Kumar A. Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int J Geriatr Psychiatry. 2007;22:332–342. doi: 10.1002/gps.1678. [DOI] [PubMed] [Google Scholar]
- 44.Nardone R., Höller Y., Brigo F., Seidl M., Christova M., Bergmann J. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73. doi: 10.1016/j.brainres.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 45.Elbert T., Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist. 2004;10:129–141. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- 46.Norden D.M., Muccigrosso M.M., Godbout J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremblay S., Henry L.C., Bedetti C., Larson-Dupuis C., Gagnon J.F., Evans A.C. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137:2997–3011. doi: 10.1093/brain/awu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson V.E., Stewart W., Smith D.H. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong Q.Z., Phillips L.L., Lyeth B.G. Metabotropic glutamate receptor protein alterations after traumatic brain injury in rats. J Neurotrauma. 1999;16:893–902. doi: 10.1089/neu.1999.16.893. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang Z., Shen Z., Chen Y., Dai Z., Zhang X., Mao Y. Mapping the changes of glutamate using glutamate chemical exchange saturation transfer (GluCEST) technique in a traumatic brain injury model: A longitudinal pilot study. ACS Chem Neurosci. 2018 doi: 10.1021/acschemneuro.8b00482. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Bigler E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. 2013;7:395. doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killin L.O., Starr J.M., Shiue I.J., Russ T.C. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16:175. doi: 10.1186/s12877-016-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alladi S., Hachinski V. World dementia: One approach does not fit all. Neurology. 2018;91:264–270. doi: 10.1212/WNL.0000000000005941. [DOI] [PubMed] [Google Scholar]
- 54.Mollayeva T., Colantonio A., Mollayeva S., Shapiro C.M. Screening for sleep dysfunction after traumatic brain injury. Sleep Med. 2013;14:1235–1246. doi: 10.1016/j.sleep.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Cedernaes J., Osorio R.S., Varga A.W., Kam K., Schiöth H.B., Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer's disease. Sleep Med Rev. 2017 Feb;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hladky S.B., Barrand M.A. Metabolite clearance during wakefulness and sleep. Handb Exp Pharmacol. 2017 doi: 10.1007/164_2017_37. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Hastie T., Tibshirani R., Friedman J. Springer; New York, NY: 2009. Model assessment and selection. The elements of statistical learning: Data mining, inference, and prediction; pp. 219–223. [Google Scholar]
- 58.Vatcheva K.P., Lee M., McCormick J.B., Rahbar M.H. The effect of ignoring statistical interactions in regression analyses conducted in epidemiologic studies: an example with survival analysis using cox proportional hazards regression model. Epidemiology (Sunnyvale) 2015;6 doi: 10.4172/2161-1165.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millen J.E., Glauser F.L., Zimmerman M. Physiological effects of controlled concussive brain trauma. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:856–862. doi: 10.1152/jappl.1980.49.5.856. [DOI] [PubMed] [Google Scholar]
- 60.Khachaturian Z.S., Mesulam M.M., Khachaturian A.S., Mohs R.C. The special topics section of Alzheimer's & dementia. Alzheimers Dement. 2015;11:1261–1264. doi: 10.1016/j.jalz.2015.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.