Abstract
The “Psychiatric Treatment Adverse Reactions” (PsyTAR) dataset contains patients’ expression of effectiveness and adverse drug events associated with psychiatric medications. The PsyTAR was generated in four phases. In the first phase, a sample of 891 drugs reviews posted by patients on an online healthcare forum, “askapatient.com”, was collected for four psychiatric drugs: Zoloft, Lexapro, Cymbalta, and Effexor XR. For each drug review, patient demographic information, duration of treatment, and satisfaction with the drugs were reported. In the second phase, sentence classification, drug reviews were split to 6009 sentences, and each sentence was labeled for the presence of Adverse Drug Reaction (ADR), Withdrawal Symptoms (WDs), Sign/Symptoms/Illness (SSIs), Drug Indications (DIs), Drug Effectiveness (EF), Drug Infectiveness (INF), and Others (not applicable). In the third phases, entities including ADRs (4813 mentions), WDs (590 mentions), SSIs (1219 mentions), and DIs (792 mentions) were identified and extracted from the sentences. In the four phases, all the identified entities were mapped to the corresponding UMLS Metathesaurus concepts (916) and SNOMED CT concepts (755). In this phase, qualifiers representing severity and persistency of ADRs, WDs, SSIs, and DIs (e.g., mild, short term) were identified. All sentences and identified entities were linked to the original post using IDs (e.g., Zoloft.1, Effexor.29, Cymbalta.31). The PsyTAR dataset can be accessed via Online Supplement #1 under the CC BY 4.0 Data license. The updated versions of the dataset would also be accessible in https://sites.google.com/view/pharmacovigilanceinpsychiatry/home.
Specifications table
| Subject area | Psychiatric medications, Consumer Health Informatics, Medical Standard Vocabularies |
| More specific subject area | Consumer health posts, Machine Learning Systems, Text mining, Adverse drug events, SNOMED CT, UMLS |
| Type of data | Categorical, string, numeric variables, analyzed |
| How data was acquired | Using an Application Program Interface (API) |
| Data format | Comma Separated Values (CSV) |
| Experimental factors | Sample consists of 891 of drug review posts collected randomly from a healthcare forum “askapatint.com” for four psychiatric medications including Zoloft, Cymbalta, Effexor XR, and Cymbalta. |
| Experimental features | Factors measure pharmacological aspects of psychiatric medications. |
| Data source location | Data collected from an online healthcare forum called “askapatint.com”, United States |
| Data accessibility Related research article |
Provided as online supplement Zolnoori, M., Fung, K. W., Patrick, T. B., Fontelo, et al. (2019). A systematic approach for developing a corpus of patient reported adverse drug events: A case study for SSRI and SNRI medications. Journal of biomedical informatics, 90, 103091. |
Value of the data
|
1. Data
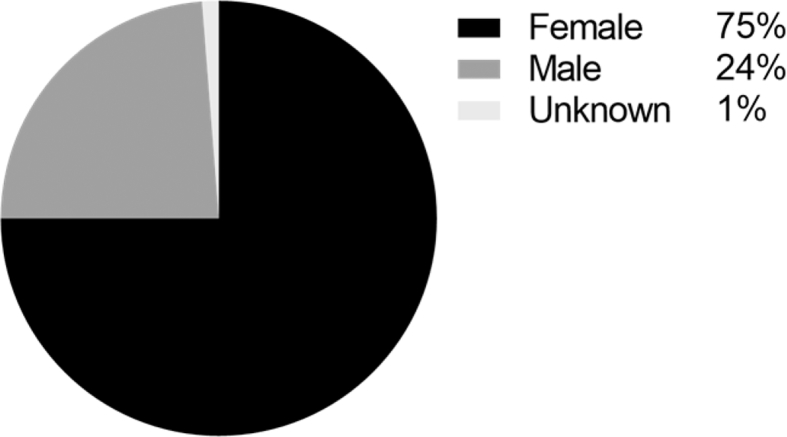
The sample of the PsyTAR contains 891 drug reviews collected randomly from an online healthcare forum “askapatient.com”. Fig. 1 shows the share of the sample for four drugs “Zoloft” and “Lexapro” from SSRIs (Selective Serotonin Reuptake Inhibitors) class and “Effexor XR” and “Cymbalta” from the SNRIs (Serotonin-Norepinephrine Reuptake Inhibitors) class. Fig. 2 shows the gender demographic distribution of the sample. The average of age and duration of usage were 37 and 18 months for the whole sample respectively.
Fig. 1.
Sample sizes for the four drugs of the dataset.
Fig. 2.
Gender distribution in the sample.
In the second phase, drug review posts were split into sentences, and then sentences were labeled for the presence of ADRs (Adverse drug reaction), WDs (Withdrawal Symptoms), SSIs (sign, symptom, illness), DIs (Drug Indications), EF (drug effectiveness), and INF (drug ineffectiveness). The total number of sentences in the sample is 6009. Fig. 3 shows frequency of sentences labeled for each of these items for the whole PsyTAR dataset and SSRI and SNRI classes separately.
Fig. 3.
Frequency of sentences labeled for each item in the dataset, and SSRIs and SNRIs class separately.
In the third phase, mentions of ADRs, WDs, SSIs, and DIs were identified and extracted from the sentences, and then classified as physiological, psychological, cognitive, or functional problem. Fig. 4 shows the total frequency of identified ADRs, WDs, DIs, and SSIs broken down by the type of entity including physiological, psychological, cognitive, and functional problems. Fig. 5 shows the percentage of identified ADRs, WDs, DIs, and SSIs for the entire PsyTAR dataset and type of entities separately.
Fig. 4.
Frequency of cognitive, physiological, psychological, and functional problems entity type by ADRs, WDs, DIs, and SSIs for the entire dataset.
Fig. 5.
Percentage of cognitive, physiological, psychological, and functional problems entity types by ADRs, WDs, DIs, and SSIs in the entire dataset.
In the fourth phase, all the identified entities were mapped to 918 unique UMLS concepts and 755 unique SNOMED CT concepts. Fig. 6 shows frequency of UMLS concepts for each ADRs, WDs, DIs, and SSIs. The 3180 unique identified ADRs in the third phase were mapped to 673 UMLS concepts, indicating the high semantic variabilities of patients expression of ADRs [1]. Fig. 7 shows the reduction of identified entities by mapping to the UMLS Metathesaurus concepts.
Fig. 6.
Frequency of UMLS concepts for each ADRs, WDs, DIs, SSIs after normalization.
Fig. 7.
Reduction of identified entities by mapping to the UMLS Metathesaurus concepts.
In this phase, we also identified qualifiers indicating severity and persistency of identified entities. Fig. 8 shows the frequency of identified qualifiers including “mild”, “moderate”, and “severe” indicating severity, and “persistent” and “not-persistent” indicating persistency of the identified entities (ADRs, WDs, DIs, SSIs).
Fig. 8.
Frequency of identified entities indicating severity and persistency of the identified entities (ADR, WD, DI, SSI).
2. Experimental design, materials and methods
The drug reviews were collected from a healthcare forum called “askapatient.com”. We developed an Application Programming Interface (API) to collect data from this forum. The sample size was calculated using the formula of sample size for qualitative studies [2]. In the next step, the drug reviews were processed for correcting grammatical errors and removing personal information (e.g., website, emails). Then, the reviews were split into sentences, and each sentence was double coded (labeled) for the presence of ADR, WD, DI, SSI, EF, and INF. The calculated inter-annotator agreement (IAA) using Kappa was 78% for the entire dataset. In the next phase, mentions of the ADR, WD, SSIs, and DIs were identified from the relevant sentences. Four annotators identified the boundary of the entities by strictly following guidelines developed for the entity identification phase. The calculated IAA for entity identification was 86% for the entire dataset. In the last phase, the identified entities were mapped to the corresponding UMLS Metathesaurus concepts and SNOMED CT concepts. All of the identified concepts were reviewed for consistency. The detailed methodology for developing this dataset is discussed in a separate manuscript [1].
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Library of Medicine (NLM) and Lister Hill National Center for Biomedical Communications, and Center of Excellence in Regulatory Science and Innovation (CERSI) grant to Yale University and Mayo Clinic from the US Food & Drug Administration (U01FD005938). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the HHS or FDA.
The PsyTAR dataset is under the CC BY 4.0 Data license. This license allows user to use the data with appropriate attribution to its origin. https://creativecommons.org/licenses/by/4.0/
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.103838.
Transparency document
The following is the supplementary data to this article:
References
- 1.Zolnoori M. A systematic approach for developing a corpus of patient reported adverse drug events: a case study for SSRI and SNRI medications. J. Biomed. Inform. 2019;90:103091. doi: 10.1016/j.jbi.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charan J., Biswas T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013;35(2):121–126. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.