Abstract
Myogenesis is a complex and precisely orchestrated process that is highly regulated by several non-coding RNAs and signal pathways. Circular RNAs (circRNAs) represent a novel subclass of endogenous non-coding RNAs that have been identified in multiple species and tissues and play a vital role in post-transcriptional regulation in eukaryotes, but the precise molecular mechanism of action remains largely unknown. Here, we screened a candidate circRNA derived from the SNX29 gene, termed circSNX29 from our previous circRNAs sequencing data of bovine skeletal muscle, and further characterized its regulation and function during muscle development. The overexpression of circSNX29 facilitated myoblasts differentiation and inhibited cell proliferation. Computational analysis using RNAhybrid showed the potential for circSNX29 to sponge to miR-744 with nine potential binding sites. We tested this via a luciferase screening assay and found that circSNX29 directly interacted with miR-744 and downregulation of miR-744 efficiently reversed the suppression of Wnt5a and CaMKIIδ. Importantly, through the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis, Fluo-4, AM, cell permeant-calcium ion fluorescent probing, and western blotting assays, we found that overexpression of Wnt5a and circSNX29 activated the non-canonical Wnt5a/Ca2+ pathway. Overall, the evidence generated by our study elucidates the regulatory mechanisms of circSNX29 to function as a sponge for miRNA-744 in bovine primary myoblasts.
Keywords: circRNA, non-canonical Wnt pathway, bovine, myogenesis
Introduction
Muscle development, growth, regeneration, and maintenance are crucial components of human and animal health, as muscles provide the necessary structural support that maintains movements and also stores energy required for overall body metabolism.1, 2 Thus, muscle growth plays a vital role in overall body metabolism. Core myogenic regulators, including myogenic differentiation (MyoD), myogenic factor 5 (Myf5), myogenin (MyoG), and myogenic regulatory factor 4 (Mrf4), are involved in the complex process of myogenesis,3, 4, 5, 6, 7 but other regulators that have been identified and suggested to regulate muscle development include microRNAs, long non-coding RNAs, and circular RNAs.8, 9, 10 However, the regulatory mechanisms underlying differences in muscle development and meat quality remain poorly understood.11
MicroRNAs (miRNAs) are small, highly conserved, non-coding RNA about 18∼24 nt in length that are involved in the regulation of diverse biological processes, such as cell proliferation, cell apoptosis,12 myoblast differentiation, and development.13, 14 Circular RNAs are another class of endogenously expressed non-coding RNAs formed as covalently closed loops through back-splicing, with greater stability and higher conservation in mammalian cells than microRNAs and long non-coding RNAs (lncRNAs).15 Circular molecules were previously observed in viral genomes,16 and the first circRNAs in eukaryotic cells were found in HeLa cells,17 but the potential roles of these molecules are just starting to be described.18 The development of RNA deep sequencing technology and bioinformatics has enabled in-depth characterization of circRNAs, including identification, abundance, measurements, and determination of potential function.19 However, we are at the early stage of identifying and characterizing circRNAs in livestock.20, 21 Previous study revealed that miR-744 was highly expressed in skeletal muscle tissue from rhesus monkeys.22 Other studies reported that miR-744 promotes cancer cell proliferation and migration;23 but a functional role of miR-744 during bovine primary myoblast development has not been established.
Several circRNAs have been described as competing endogenous RNAs (ceRNAs) that can function as a sponge for microRNAs.24 Although knowledge of circRNA function is limited,25 Sry and ciRS-7, two well-known circRNAs, serve as sponges for miR-138 and miR-7, respectively,15, 26 thereby suggesting that circRNAs can play a vital role in post-transcriptional regulation. Recently, a new subclass of circRNAs, termed exon-intron circRNAs (EIciRNAs), has been proven to serve as transcriptional regulators and interact with RNA polymerase II and U1 in the nuclear.27
Wnt5a protein influences cell homeostasis by regulating cell fate determination, cell proliferation, and differentiation.28, 29 It is reported that CaMKIIδ promotes muscle differentiation at multiple levels.30 In addition, McKinsey et al.31 and Backs et al.32 found that CaMKIIδ phosphorylates histone deacetylase 4 and 5 (HDAC4/5) to enhance nuclear exports and releases myocyte enhancer factor 2 (MEF2). Furthermore, the Wnt5a-calcium-CaMKIIδ pathway is also involved in basal cell carcinoma differentiation and retinoic-acid-induced myogenic abnormalities of tongue.33, 34
In this work, we generated and screened circRNAs sequencing data of Qinchuan cattle muscle tissue from NCBI: GSE87908 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=atglausktpsjlel&acc=GSE87908). We focused on endogenous circSNX29 and found that circSNX29 was differentially expressed in embryonic and adult muscle tissue. We then characterized this potential candidate circRNA and its function in bovine skeletal muscle development. The results revealed that circSNX29 reduced myoblast proliferation and promoted cell differentiation. Mechanistically, circSNX29 directly interacts with miR-744, positively upregulated the expression of Wnt5a and CaMKIIδ through improper activation of the Wnt5a/Ca2+ signaling pathway, and promoted myoblast differentiation. These results contribute to our current understanding of circRNA and microRNA function in myogenesis.
Results
Identification of miR-744 in Bovine Skeletal Muscle
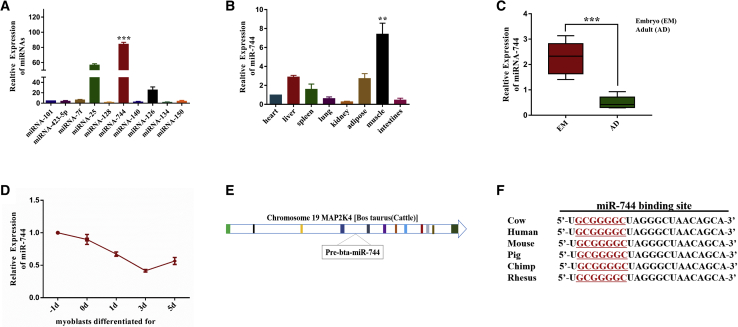
Our previous study found that several muscle-specific or mature microRNAs, including miR-744, exhibit drastically changed expression in Qinchuan bovine muscle tissue.35 Variations in microRNA expression revealed significant upregulation of miR-744 (Figure 1A), and these increased levels of miR-744 were confirmed via real-time PCR in muscle tissue (Figure 1B). We found miR-744 to be highly expressed at the embryonic stage compared with the adult stage (Figure 1C). Mature miR-744 is located in the fourth intron of mitogen-activated protein kinase kinase 4 (MAP2K4) in chromosome 19 (Figure 1D). Interestingly, the expression of miR-744 was downregulated during myoblast differentiation (Figure 1E). Based on these results, we conjecture that miR-744 may act as a negative regulator during muscle development. Noticeably, the sequence of miR-744 is highly conserved among cow, human, mouse, and rhesus monkeys (Figure 1F).
Figure 1.
Expression Profile of miR744 in Bovine Skeletal Muscle
(A) The expression of ten microRNAs (miRNAs) in skeletal muscle of Qinchuan cattle at embryonic stage. (B) miR-744 is expressed in different tissues of Qinchuan cattle at embryonic stage. (C) The expression of miR-744 in skeletal muscle of Qinchuan cattle between the embryonic stage and the adult stage. (D) miR-744 expression in different stages of differentiation (−1, 0, 1, 3, and 5 days). (E) The location of miR-744 in MAP2K4 gene. (F) miR-744 is highly conserved among different species. Values are mean ± SEM for three biological replicates; **p < 0.01; ***p < 0.001.
Overexpression of miR-744 Promotes Cell Proliferation while Suppressing Apoptosis
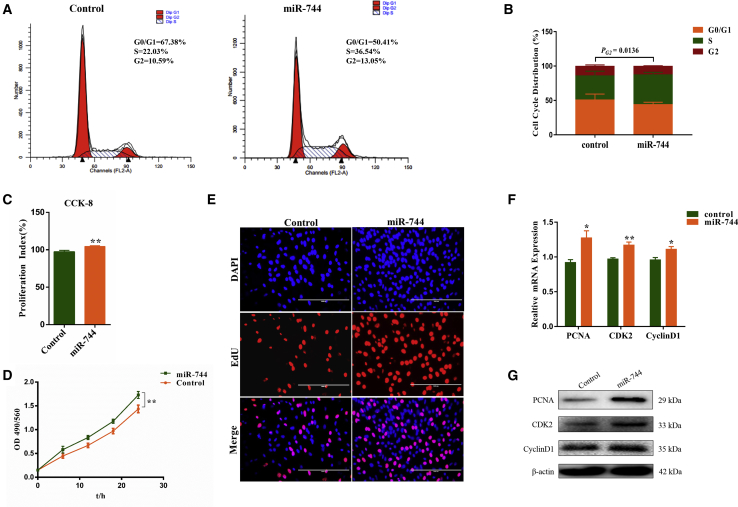
To investigate the functional role of miR-744 in the proliferation and apoptosis of bovine primary myoblasts, we performed flow cytometry assay, Cell Counting Kit-8 (CCK-8) assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, qRT-PCR analysis, 5-ethynyl-2′-deoxyuridine (EdU) assay, western blotting assay, and cell apoptosis assay. Flow cytometry assay showed that miR-744 notably increased the number of myoblasts in the S phase and decreased the proportion of cells at G0/G1 and G2 phases, suggesting an effect of miR-744 on cell proliferation (Figures 2A and 2B). Later, CCK-8 assay and MTT assay revealed that miR-744 significantly promoted cell viability (Figures 2C and 2D). We also detected cell proliferation using an EdU incorporation assay, and miR-744 increased the mitotic activity of myoblasts (Figure 2E). Similarly, we found that miR-744 significantly enhanced the expression of proliferation-related markers, including cyclin D1, CDK2, and proliferating cell nuclear antigen (PCNA) at both the mRNA and protein levels (Figures 2F and 2G).
Figure 2.
Effect of miR-744 Overexpression on the Cell Proliferation
(A–G) Myoblasts treated with pcDNA3.1 or pcDNA3.1-miR-744 were subsequently incubated with growth medium for 24 h and cell phase analyzed (A) and counted (B) by flow cytometry. (C) Cell proliferation index was measured by cell counting kit-8 (CCK-8) assay. (D) Cell proliferation was measured by MTT assay. (E) Cell proliferation was measured with 5-ethynyl-20-deoxyuridine (EdU); the scale bars represent 200 μm. (F and G) The expression of proliferation marker gene PCNA, CDK2, and cyclin D1 was measured by real-time qPCR (F) and western blotting (G). Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01.
We then asked whether miR-744 affects cell apoptosis. Hoechst 33342/propidium iodide (PI) dual staining assay was performed and revealed that miR-744 inhibited cell apoptosis (Figure S1A). Western blotting assay and qRT-PCR analysis similarly showed that miR-744 significantly increased protein and mRNA expression levels of three cell-survival-related genes, Bcl-2, p53, and Bax (Figures S1B and S1C). These results demonstrate that miR-744 promotes cell proliferation and suppresses myoblast apoptosis.
Overexpression of miR-744 Inhibits Cell Differentiation
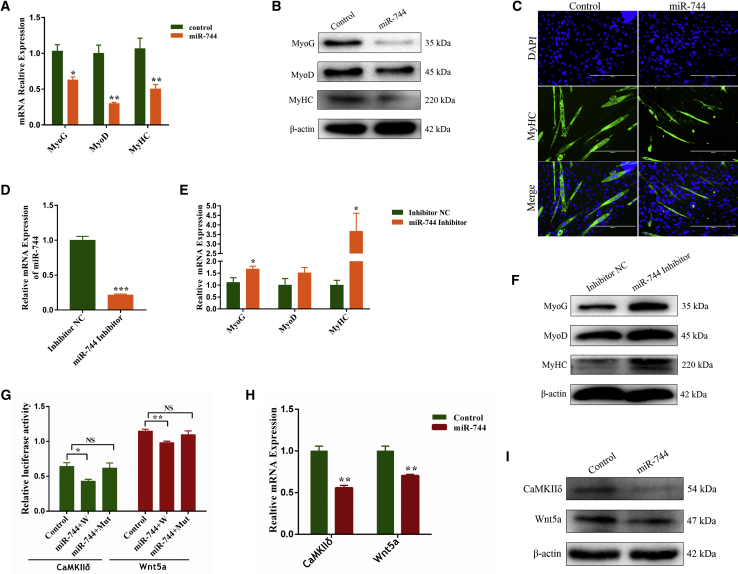
To explore whether miR-744 regulates myoblast differentiation, we measured the expression levels of miR-744 during the myoblast differentiation period and found that the expression of miR-744 decreased in a time-dependent manner (Figure 1E). Next, cells treated with pcDNA3.1-miR-744 or pcDNA3.1 were induced to differentiate for 4 days and then the expression of established myogenic markers, MyoD, MyoG, and myosin heavy chains (MyHCs), was detected at both the mRNA and protein levels (Figures 3A and 3B). Immunofluorescence assay revealed that miR-744 reduced MyHC expression and myotube formation (Figure 3C). Conversely, knockdown of miR-744 promoted cell differentiation (Figures 3D–3F). Together, these results imply that miR-744 suppresses myoblast differentiation.
Figure 3.
Overexpression of miR-744 Inhibits Differentiation of Bovine Primary Myoblasts
(A–C) Myoblasts were treated with pcDNA3.1 or pcDNA3.1-miR-744. (A and B) The mRNA and protein expression of cell differentiation markers, MyoG, MyoD, and MyHC, was measured by real-time qPCR (A) and western blotting (B). (C) Cell differentiation was also detected by immunofluorescence; the scale bars represent 200 μm. (D) The efficiency of miR-744 knockdown was evaluated by qPCR. (E) Real-time qPCR analysis of the differentiation-related genes. (F) Western blot analysis of MyOG, MyOD, and MyHC protein levels with β-actin treatment. (G) 293T cells were co-transfected with pcDNA3.1-miR-744 and psi-CaMKIIδ (or) psi-Wnt5a-3′ UTR-W/Mut. (H and I) The mRNA and protein expression of CaMKIIδ and Wnt5a was measured via real-time qPCR (H) and western blotting (I). Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01; ***p < 0.001; NS, no significant differences.
miR-744 Respectively Targets Wnt5a and CaMKIIδ
To elucidate the mechanism underlying miR-744-modulated cell proliferation and differentiation, we identified and screened potential targets of miR-744 by application of bioinformatics software TargetScan, miRTarBase, and DAVID and then focused on six candidate genes related to muscle development (Figure S1D). We found that the 3′ UTRs of Wnt5a and CaMKIIδ contained conserved target sites for miR-744 (Figure S1E). Luciferase reporter assay (Figure S1F) revealed that luciferase activity was significantly decreased when cells were co-transfected with pcDNA-miR-744 and psi-CaMKIIδ-3′ UTR or psi-Wnt5a-3′ UTR. Conversely, the luciferase activity showed a slight increase in the mutated treatment groups (Figure 3G). Consistently, we found that miR-744 dramatically inhibited the expression of CaMKIIδ and Wnt5a at both the mRNA and protein levels (Figures 3H and 3I).
Effects of Wnt5a and CaMKIIδ on Myoblast Proliferation, Apoptosis, and Differentiation
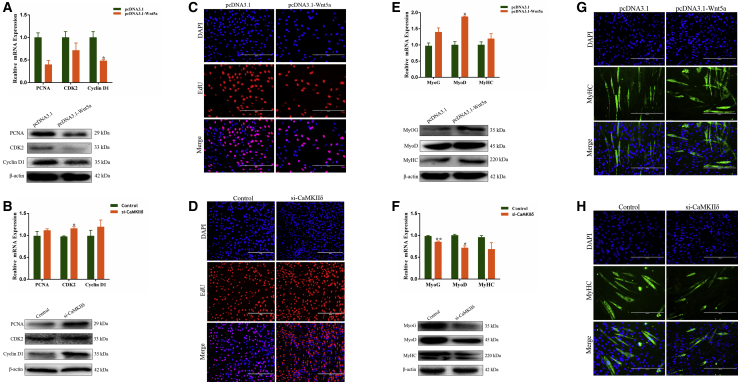
To characterize the roles of Wnt5a and CaMKIIδ in myoblast development, we overexpressed Wnt5a and knocked down CaMKIIδ by synthesizing small interfering RNA (siRNA) to target these genes (Figures S2A–S2D). We detected the expression of PCNA, CDK2, and cyclin D1 and found that Wnt5a overexpression decreased the expression levels of these genes (Figure 4A). Conversely, si-CaMKIIδ favored cell proliferation (Figure 4B). The results of the EdU assay were similar (Figures 4C and 4D). Also, we found that si-CaMKIIδ decreased the expression levels of three myogenic markers, MyoG, MyoD, and MyHC, whereas Wnt5a overexpression had the opposite effect on these markers (Figures 4E and 4F). Consistently, similar results were obtained by immunofluorescence assay (Figures 4G and 4H). For cell apoptosis, marker genes Bcl-2 and caspase-9 were detected using qPCR and western blotting assays. The results showed that si-CaMKIIδ inhibited expression of these marker genes, with an anti-apoptosis effect, whereas Wnt5a overexpression induced cell apoptosis (Figures S2E–S2G). Together, these results suggest that Wnt5a and CaMKIIδ may play vital roles in myoblast development.
Figure 4.
Effects of Wnt5a Overexpression and CaMKIIδ Silencing on the Cell Proliferation and Differentiation
(A and B) Bovine primary myocytes were treated with pcDNA3.1-Wnt5a (A) or si-CaMKIIδ (B), and the mRNA and protein levels of proliferation marker genes were measured by real-time qPCR and western blotting. (C and D) Bovine primary myocytes were treated with pcDNA3.1-Wnt5a (C) or si-CaMKIIδ (D), and cell proliferation was measured with 5-ethynyl-20-deoxyuridine (EdU); the scale bars represent 200 μm. (E and F) Bovine primary myocytes were treated with pcDNA3.1-Wnt5a (E) or si-CaMKIIδ (F), and the mRNA and protein expression of differentiation marker genes MyoG, MyoD, and MyHC was measured by real-time qPCR and western blotting. (G and H) C2C12 cells were treated with pcDNA3.1-Wnt5a (G) or si-CaMKIIδ (H), and cell differentiation was measured by immunofluorescence; the scale bar represents 200 μm. Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01.
Overexpression of circSNX29 Serves as a Competing Endogenous miRNA-744 Sponge to Attenuate Its Inhibitory of Wnt5a
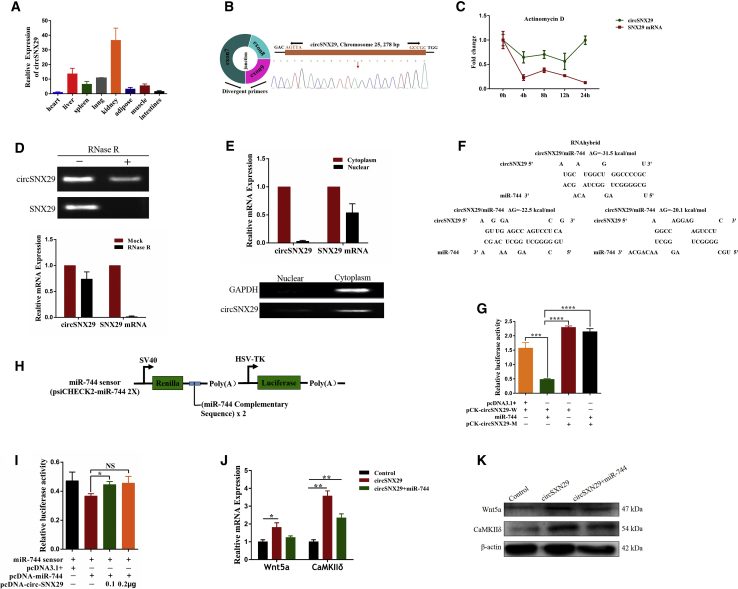
To identify specific circRNAs in bovine skeletal muscle, we randomly selected nine differentially expressed circRNAs from the circRNAs sequencing data and showed that the expression of circRNA243 was much higher at the embryonic stage compared with the adult stage (Figure S3A). Also, we found that circRNA243 is generally expressed in various tissues of fetal cattle (Figure 5A). As circRNA243 is transcribed from SNX29 gene, we termed it circSNX29. We confirmed the size and sequence of an amplified PCR product with specific circRNA junctions by Sanger sequencing (Figure 5B). To study the stability and localization of circSNX29 in myoblasts, we extracted total RNA of myoblasts treated with actinomycin D, a RNA synthesis inhibitor. The results showed that a half-life of circSNX29 was exceeding 12 h, whereas the linear SNX29 mRNA exhibited a half-life that was less than 4 h (Figure 5C). In addition, resistance to digestion with RNase R exonuclease further indicated that circSNX29 was more stable than linear SNX29 (Figure 5D). Finally, nucleoplasmic separation assay showed that circSNX29 was prevailingly expressed in the cytoplasm (Figure 5E). These findings demonstrate that circSNX29 is a candidate and stable circRNA.
Figure 5.
circSNX29 Acts as a ceRNA for miRNA-744
(A) The expression of circSNX29 in different tissues of Qinchuan cattle at embryonic stage. (B) Schematic view illustrating the design of primers for circSNX29 used in qPCR. (C) The levels of circSNX29 and linear SNX29 expression in myoblasts treated with actinomycin D (1 μg/mL) at the indicated time points were detected by qRT-PCR. (D) The mRNA levels of circSNX29 and linear SNX29 in myoblasts treated with RNase R were determined by qRT-PCR. (E) qRT-PCR data indicating the abundance of circSNX29 and SNX29 mRNA in either the cytoplasm or nucleus of myoblasts. (F) RNAhybrid predicted miR-744 binding sites at three distinct positions in circSNX29. (G) pcDNA3.1-miR-744 was co-transfected with psiCheck2-circSNX29-W (pCK-circSNX29-W) (or) psiCheck2-circSNX29-M (pCK-circSNX29-M) into 293T cells. (H) miR-744 sensor construct. (I) Indicated constructs were each transfected into HEK293T cells along with pcDNA3.1+ or pcDNA-miR-744. (J and K) The mRNA expression of direct targets of CaMKIIδ and Wnt5a was detected by qRT-PCR (J) and western blotting (K). Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Exonic circRNA primarily exists in the cytoplasm, where it functions as a microRNA sponge to mediate gene expression. Thus, we next assessed the ability of circSNX29 to bind to microRNAs. Online bioinformatics databases (TargetScan and RNAhybrid) were used to identify microRNA recognition sequences on bovine circSNX29 and revealed the presence of nine putative miR-744 binding sites, and three binding sites are shown in Figure 5F. circSNX29 overexpression significantly decreased the relative abundance of miR-744 (Figures S3B and S3C). In addition, our luciferase assay revealed that miR-744 suppressed Rluc expression of psiCHECK2-circSNX29W constructs (Figure 5G). Notably, the sensor assay further verified direct binding between circSNX29 and miR-744 (Figures 5H and 5I). To explore whether the underlying mechanism of circSNX29 on muscle development was attributed to miR-744-mediated regulation of Wnt5a and CaMKIIδ, we treated myoblasts with circSNX29 and (or) miR-744. As expected, circSNX29 significantly upregulated the expression of Wnt5a and CaMKIIδ, but miR-744 overexpression somewhat relieved this effect (Figures 5J and 5K). These results suggest that circSNX29 directly sequesters miR-744 and acts as a decoy to attenuate the suppressive effects of microRNA on Wnt5a and CaMKIIδ expression.
Effects of circSNX29 on Cell Differentiation, Proliferation, and Apoptosis
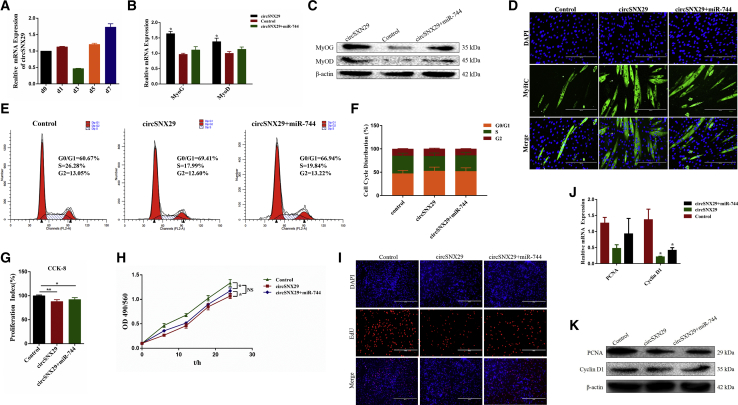
To study the involvement of circSNX29 in myoblast differentiation, the expression of circSNX29 was detected at different stages of differentiation. We found that the expression of circSNX29 exhibited an increasing and time-dependent trend (Figure 6A). We next measured the expression of MyoG and MyoD in myoblasts after 4 days of differentiation. The results showed that circSNX29 notably increased expression of MyoG and MyoD at the mRNA and protein levels, but when co-transfected with miR-744, the expression of MyoG and MyoD decreased dramatically (Figures 6B and 6C). Through the immunofluorescence assay, we found that circSNX29 favored MyHC expression and myotube formation, but this effect was somewhat reversed by miR-744 overexpression (Figure 6D). Our results reveal that circSNX29 facilitates differentiation of myoblasts by regulating concentrations of miR-744.
Figure 6.
circSNX29 Binding miR-744 to Promote Differentiation of Bovine Primary Myocytes
(A) circSNX29 expression in different stages of differentiation. (B–K) Myoblasts were treated with pcDNA2.1-circSNX29 (or) pcDNA3.1-miR-744 and pcDNA2.1-circSNX29. (B and C) The mRNA and protein expression of cell differentiation markers (MyoG and MyoD) were measured by real-time qPCR (B) and western blotting (C). (D) Cell differentiation was also measured by immunofluorescence assay; the scale bars represent 200 μm. Cell phase was analyzed (E) and counted (F) by flow cytometry. (G) Cell proliferation index was detected by CCK-8 assay. (H) Cell proliferation was detected via MTT assay. (I) Cell proliferation was detected with EdU; the scale bars represent 100 μm. (J and K) The expression of proliferation marker gene PCNA and cyclin D1 was measured by real-time qPCR (J) and western blotting (K). Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01.
Given the high abundance of circRNAs in muscle tissues, we next aimed to evaluate the biological roles of circSNX29 on cell proliferation. To do this, we first measured the proliferation of myoblasts transfected with pcDNA-circSNX29 and (or) miR-744 using cell cycle analysis, CCK-8 assays, MTT assays, EdU incorporation assays, qRT-PCR analysis, and western blotting assays. The results displayed that circSNX29 inhibited cell proliferation (Figures 6E–6K). Given that miR-744 can enhance the protection effect of myoblasts from apoptosis, we then asked whether circSNX29 could affect myoblast apoptosis by regulating miR-744. Hoechst 223342 and PI dual staining assay showed that circSNX29 induced myoblast apoptosis and eliminated the anti-apoptotic effect induced by co-transfection with miR-744 (Figure S3D). Notably, circSNX29 also increased expression of Bcl-2 and caspase-9 (Figure S3E). On the contrary, silencing of circSNX29 promoted cell proliferation and inhibited differentiation (Figure S4).Taken together, these results demonstrate that circSNX29 inhibits primary bovine myoblasts proliferation and induces cell apoptosis by sequestering miR-744.
Overexpression of circSNX29 Enhances Activation of the Wnt5a/Ca2+ Pathway by Regulating the miR-744-Wnt5a-CaMKIIδ Axis in Skeletal Muscle Development
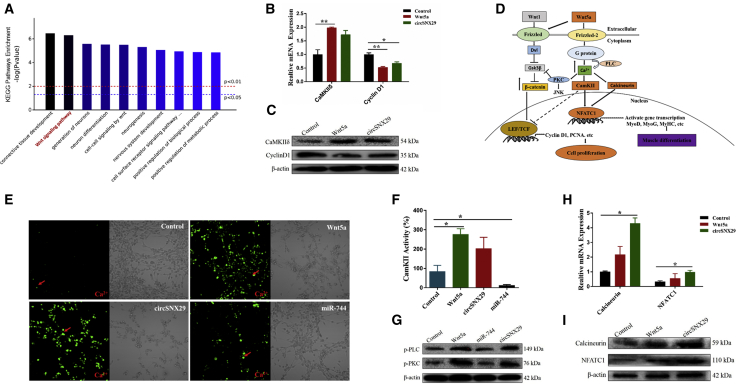
To further explain these findings, we then sought to explore the functional mechanism of circSNX29. Using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways database and the Omicsbean online software, we screened the top 10 enrichment pathways, such as Wnt signaling pathway (Figure 7A). Intriguingly, we found that overexpression of Wnt5a and circSNX29 in myoblasts significantly upregulated the expression levels of CaMKIIδ, a well-known positive regulator of Wnt5a/Ca2+ signaling,36 but repressed mRNA expression of cyclin D1 (Figures 7B and 7C). Based on these findings, Wnt5a/Ca2+ might be a crucial signaling pathway in skeletal muscle development.
Figure 7.
Overexpression of circSNX29 Promotes Activation of Wnt5a/Ca2+ Pathway by Regulating miR-744-Wnt5a/CaMKIIδ Axis
(A) KEGG enrichment pathways result. (B and C) The downstream mediators of Wnt5a were detected by qPCR (B) and western blotting (C). (D) Schematic presentation of Wnt5a/Ca2+/calcineurin/NFATC1 signaling pathways. (E) Analysis of the intracellular variation of calcium concentration by confocal microscopy. (F) CamKII activity assays. (G) Western blotting on myoblasts to detect the levels of phosphorylated PLC and PKC. (H and I) The expression of calcineurin and NFATC1 was detected by qRT-PCR (H) and western blotting (I). Values are mean ± SEM for three biological replicates; *p < 0.05; **p < 0.01.
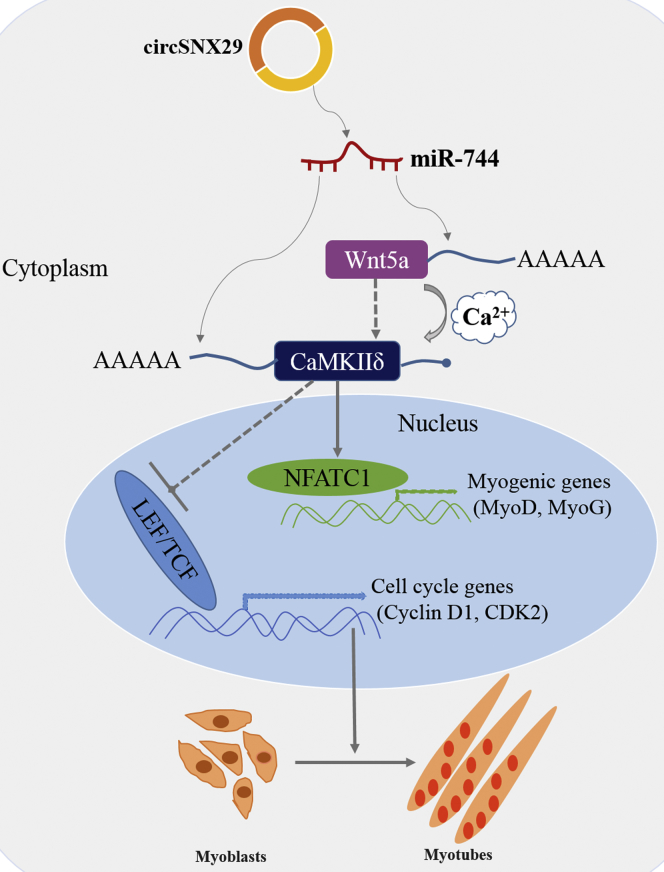
Previous studies have shown that Wnt5a activates both the Wnt5a/Ca2+ pathway (Figure 7D) and the canonical pathway.37, 38, 39, 40 In turn, activation of the Wnt5a/Ca2+ pathway may antagonize the canonical pathway.41 Thus, it is not known whether there is a change in β-catenin level; we found that Wnt5a and circSNX29 slightly increased β-catenin levels but miR-744 dramatically decreased it, implying that miR-744 may block the canonical Wnt pathway (Figure S3F). Subsequently, we focused on the Wnt5a/Ca2+ pathway. Confocal microscopy assay showed that overexpression of Wnt5a and circSNX29 obviously increased intracellular calcium concentration (Ca2+) compared with control cells, whereas miR-744 overexpression had no difference (Figure 7E). Congruously, CamKII activity assays revealed that Wnt5a and circSNX29 were higher than the miR-744 group (Figure 7F). Additionally, transfection of Wnt5a and circSNX29 into primary bovine myoblasts increased the levels of phosphorylated phospholipase C (PLC) and protein kinase C (PKC) (Figure 7G), thereby indirectly inhibiting the expression of PCNA and cyclin D1 (Figures 4A, 6J, and 6K), implying that Wnt5a and circSNX29 overexpression may activate the non-canonical signaling pathway and inhibit cell proliferation. Finally, we detected the effects of Wnt5a and circSNX29 on the expression of calcineurin and NFATC1, a well-known nuclear transcription factor of Wnt5a/Ca2+ signaling, and found that Wnt5a and circSNX29 significantly increase the mRNA and protein levels of both genes (Figures 7H and 7I). Downstream target genes of NFATC1 were also significantly increased, including MyoD, MyoG, and MyHC (Figures 4E and 6B–6D), suggesting that NFATC1 might regulate the transcription of myogenic marker genes to promote myoblast differentiation. Overall, these results indicate that Wnt5a/Ca2+ is a crucial pathway involved in muscle progression and is regulated by the cirSNX29-miR-744-Wnt5a-CaMKIIδ axis (Figure 8).
Figure 8.
Model of the Action circSNX29 Competitively Sponging miR-744-Mediating Myoblasts Differentiation
Discussion
In recent years, muscle growth and development mainly focus on protein-coding genes,42 microRNAs,43 and long non-coding RNAs.44 However, existing knowledge of circRNAs in myogenesis is largely limited. In the present comprehensive study, we identify a skeletal-muscle-related circSNX29 that promotes myogenesis in vitro. By functioning as a ceRNA, overexpression of circSNX29 sequesters miR-744 away from Wnt5a to increase the expression level of Wnt5a and phosphorylation level of PKC, thereby activating the Wnt5a/Ca2+ signaling pathway to regulate muscle development.
Based on the demonstrated properties of microRNAs, these molecules are of high interest for development as therapeutic strategies for the treatment of cancers and tumors.45, 46 Nevertheless, the specific function of microRNAs in bovine muscle development is largely unknown. In this study, miR-744 was identified from RNA sequencing analysis and was significantly upregulated in bovine embryonic muscle tissue compared with other microRNAs. Thus, we hypothesized that miR-744 might be involved in muscle development. As previous reports showed, miR-744 serves as either a tumor suppressor or oncogene in regulating various tumors.47 Intriguingly, Feng et al.48 indicated that miR-744 inhibited human hepatocellular carcinoma proliferation by downregulating c-Myc. Consistent with previous reports,47, 49 our current study showed that miR-744 can promote cell growth. These results provide new evidence for the role of miR-744 in the regulation of cell proliferation. To a large extent, muscle development is due to cellular differentiation. It is reported that many microRNAs may be involved in the progression of cell differentiation, such as miR-17-5p, miR-20a, and miR-106a.50 By modulating miR-744 activity, we found that miR-744 overexpression inhibited myoblast differentiation, whereas silencing of miR-744 promoted differentiation. To the best of our knowledge, we first demonstrated miR-744 as a potential and negative regulator of myogenesis. Notably, cell apoptosis plays a pivotal part in maintaining tissue homeostasis and the immune system. Previous study has shown that miR-744 negatively regulates Bcl-2, which results in the inactivation of apoptosis signaling of cervical cancer cells, ameliorating cervical cancer survival.51 Likewise, the results of our study also showed that miR-744 suppressed myoblast apoptosis, implying that miR-744 may act as an anti-apoptotic regulator of primary bovine myoblasts.
Our preliminary bioinformatics analysis revealed that Wnt5a and CaMKIIδ are potential targets of miR-744. Also, we further verified the results via luciferase reporter assay and explored the molecular mechanism by which miR-744 regulates myogenesis. To study the function of Wnt5a and CaMKIIδ in vitro, we performed Wnt5a overexpression or CaMKIIδ knockdown in primary bovine myoblasts. Overexpression of miR-744 inhibited Wnt5a and CaMKIIδ at the post-transcriptional level, whereas Wnt5a overexpression relieved miR-744-induced inhibition of cell differentiation and silencing of CaMKIIδ suppressed cell differentiation and apoptosis. It has been reported that Wnt5a and CaMKIIδ respectively played a positive role in osteogenic differentiation, vascular smooth muscle proliferation, and human failing hearts.30, 52, 53
As a new post-transcriptional regulator, it has been reported that circRNAs, same as endogenous RNAs, could participate in post-transcriptional regulation by binding microRNAs.24 For example, circRNAs interacted with many microRNAs related to skeletal muscle myogenesis, such as miR-378a-3p and miR-107, but so far only some bovine circRNAs have been confirmed to play a specific role in muscle development, such as circLM0720 and circFGFR4.54 In this study, based on the differentially expressed circRNAs, we found that the expression of circSNX29 was significantly downregulated in skeletal muscle. Here, with a combination of bioinformatic tools, we analyzed that circSNX29 might target with microRNAs. Luciferase reporter assays showed that circSNX29 is a direct target of miR-744 in bovine primary myoblasts. Additionally, we showed that circSNX29 acts as a modulator of cell proliferation and apoptosis by sponging miR-744 in myoblasts. Moreover, we found that circSNX29 overexpression (not linear SNX29 mRNA) significantly promoted cell differentiation, whereas this effect was reversed by miR-744 overexpression. Knockdown of circSNX29 in myoblasts upregulated the level of miR-744 and attenuated the expression of MyoG, MyoD, and MyHC, thereby inhibiting cell differentiation; we believe that the increased activity of miR-744 due to circSNX29 relieved its inhibition of miR-744. Therefore, circSNX29 promoted cell differentiation and indirectly promotes the transcription of MyoG, MyoD, and MyHC proteins. Because the mRNA level of circSNX29 is higher than that of SNX29 gene and the differentiation-promoting role is performed by circSNX29, the circRNA isoform of the SNX29 gene is likely an important functional product of pre-SNX29 mRNA back-splicing. Overall, these results further confirmed that circSNX29 functions as a moderator of cell differentiation, proliferation, and cell survival by binding miR-744.
The growth and development of skeletal muscle is a multistep process divided into four major stages: muscle precursor cells are originated from the somite; then myogenic precursors proliferate and differentiate into myoblasts; afterward, myoblasts fuse into myotubes; and finally, maturational myofibers give rise to the myotubes.55 These processes are regulated by a multitude of complex signaling pathways, including the Wnt protein (WNT) and transforming growth factor β (TGF-β) pathways.56, 57 In this work, we showed that circSNX29 could attenuate the inhibitory effects of miR-744 on Wnt5a and CaMKIIδ expression in bovine primary myoblasts. Wnt5a and CaMKIIδ are key regulators of non-canonical Wnt5a/Ca2+ pathways, and the binding of ectopic Wnt5a to Fz2 (Frizzled receptor 2) and Ror2 (tyrosine kinase-like orphan receptor 2) leads to the activation of the non-canonical Wnt pathways,58 which induces NFATC1 or β-catenin nuclear translocation, thereby regulating in the transcription of downstream genes, such as cyclin D1, c-Myc, and MyHC.38, 39 In addition, Liu et al.59 showed that downregulation of β-catenin can activate the non-canonical Wnt5a/Ca2+ pathway, thereby promoting osteogenic differentiation. However, our study demonstrated that downregulation of β-catenin blocked Wnt5a/Ca2+ signaling pathway during myoblast differentiation. Consistent with these findings, Wnt5a/Ca2+ signaling also occurred downstream of circSNX29-miR-744-Wnt5a in primary bovine myoblasts. More importantly, we demonstrated that overexpression of circSNX29 sponged miR-744 to activate the Wnt5a/Ca2+ pathway by upregulating Wnt5a and CaMKIIδ expression, which promoted myoblast differentiation progression. However, it is not known that transcription factor NFATC1 directly or indirectly binds to MyoG, MyoD, and MyHC, and further study is necessary to clarify how NFATC1 may regulate them.
Notably, the abnormal development of skeletal muscle is also accompanied by muscle diseases, such as atrophy, hypertrophy, and primary muscle disorders. Emerging evidences demonstrate a potential role of microRNAs in muscle development, diseases, and regeneration.60, 61 Williams et al.62 reported that the levels of miR-1, -206, and -133a promote regeneration and delay disease progression of neuromuscular synapses. Similar to miR-133a, we have also observed that miR-744 inhibits myoblasts differentiation, suggesting microRNA’s potency to treat muscular diseases. In addition, the dysregulation of microRNAs may result in diseases associated with skeletal muscle.60 To date, the regulatory studies of circRNAs in muscle diseases are still in the early stage. Several studies indicate that circRNAs may play key roles in Duchenne muscular dystrophy (DMD) and cardiovascular and heart diseases.63, 64, 65 In agreement with downregulated circZNF609 in differentiated DMD myoblasts,66 we have identified a downregulated circSNX29 in bovine primary myoblasts, implying that the impacts of circSNX29 may be also essential for muscular diseases. Overall, these observations of microRNAs and circRNAs will open up the possibility of developing therapies for muscle disorders in the future.
In summary, we identified a novel circSNX29 serves as an endogenous miR-744 sponge to activate Wnt5a/Ca2+/CaMKIIδ pathway, resulting in promoting bovine myoblast differentiation, which may be used as a potential target for regulation of myogenesis.
Materials and Methods
Sample Preparation
Total RNAs of eight tissues at embryonic (heart, liver, spleen, lung, kidney, subcutaneous adipose, muscle, and intestine) were obtained and snap-frozen in liquid nitrogen and stored at −80°C freezer until use. In addition, we collected six musculus longissimus tissue samples of Qinchuan cattle from two developmental states (embryonic, 90 days; adult, 24 months) from a local slaughterhouse in Xi’an, PRC. The animal materials were obtained with informed consent, and all experimental procedures were approved by the Animal Care and Use Committee of Northwest A&F University.
Cell Lines
C2C12 and 293T cell lines were provided by our laboratory. Growth medium (GM) for cell culture was high-glucose DMEM (HyClone, Logan, UT, USA) with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). To induce myogenic differentiation, GM was replaced by differentiation medium (DM) containing high-glucose DMEM supplemented with 2% horse serum (HyClone, Logan, UT, USA) after C2C12 cells reached 70%∼90% confluence. Medium was changed every 2 days.
qRT-PCR
The nuclear and cytoplasmic fractions were extracted using a Nucleoprotein Extraction kit (Sangon Biotech). Total RNA was extracted by TRIzol reagent according to the manufacturer (TaKaRa, Dalian, China). For RNase R treatment, 1 μg of total RNA was incubated for 20 min at 37°C with or without 2 units μg−1 of RNase R and purified following the RNeasy MinElute cleaning Kit (QIAGEN, Hilden, Germany). MicroRNA-specific stem-loop primers (Table S1) were used to reverse-transcribe cDNA from the microRNAs. The primers used for PCR are listed in Table S2. The 2-ΔΔCt method was used to calculate the expression levels from qPCR data.
Plasmid Construction
The full length of circSNX29 (Text S1), Wnt5a coding region, and genomic fragments, including miR-744, were separately cloned into pcDNA2.1 (+) and pcDNA3.1 (+) vectors (Invitrogen, Carlsbad, CA, USA). The fragment of the circSNX29 3′ UTR containing the binding site of miR-744 was amplified and inserted into the psiCHECK-2 vector (Promega, Madison, WI, USA) at the 3′ end of the Renilla gene using restriction enzymes Xho I and Not I (TaKaRa, Dalian, China) and T4 DNA ligase. The mutant psiCHECK2-circSNX29-3′ UTR-Mut was obtained by mutating sequences complementary to the seed region of the miR-744 using a mutagenic primer. Consistently, we generated the vectors of psi-CHECK-CaMKIIδ and/or Wnt5a-W/Mut (pck-CaMKIIδ and/or Wnt5a-W/Mut) according to above method. All primer sequences are shown in Table S3.
Cell Culture and Treatments
We isolated and cultured primary bovine myoblasts from bovine longissimus dorsi samples as previously described.67 Myoblasts were cultured in high-glucose DMEM supplemented with fetal bovine serum (Hyclone, USA; 20%) supplemented with double antibiotics (1% penicillin and streptomycin; GM) at 5% CO2, 37°C. To induce myoblast differentiation, cells were switched to DM at approximately 90% confluence. Myoblasts were transfected with pcDNA3.1-miR-744, pcDNA3.1-Wnt5a, si-CaMKIIδ, or pcDNA2.1-circSNX29 when cell confluence reached about 80%. The si-CaMKIIδ sequence is 5′-GATCGAAGCTATCAACAAT-3′ (Ruibo, Guangzhou, China). Myoblasts were treated with the transcription inhibitor actinomycin D (1 μg mL−1; Leagene, Beijing, China) or dimethylsulfoxide as a control to the medium.
Detection of Intracellular Ca2+ Signals with Confocal Microscopy
After transfection for bovine primary myoblasts and induced differentiation with DMEM containing 2% heat-inactivated horse serum for 4 days, the change of calcium concentration was detected by using Fluo-4, AM (Yeasen, Shanghai, China), a probe that accumulates intracellularly and increases its green fluorescence signal on binding Ca2+. Different treatment groups of primary bovine myoblasts were incubated with 4 μM of the cell-permeable Fluo-4, AM fluorescent probe. After treatment for 25 min at 37°C in the dark, cells were washed three times with Hank’s balanced salt solution (HBSS) medium containing 1% Pluronic F-127. Fluorescence signal was assessed using a confocal microscope at a wavelength of 494 nm (FV1000MPE, Olympus Corporation, Japan).
Kinase Activity Assay
CamKII activity was measured with a CaM Kinase II Assay Kit (Colorful Gene, Wuhan, China) according to the manufacturer.
Flow Cytometry for the Cell Cycle Assay
We analyzed the cell cycle of different treatment groups using a cell cycle testing kit (Multisciences, Hangzhou, China). Myoblasts were seeded in 60-mm plates (2 × 106 cells/well) with 3 mL culture medium. 24 h after transfection, we harvested cells and washed them in PBS buffer. After treatment with PI master mix (40 mg/mL of PI and 100 mg/mL of RNase in PBS) at 37°C for 30 min, the cell suspension was subjected to flow cytometry (FACS Canto II, BD Biosciences, USA), and each treatment group had three independent replicates.
Proliferation Assay
For the CCK-8 (Multisciences, Hangzhou, China) assay, primary bovine myoblasts were plated into 96-well culture plates at a density of 1 × 104 cells/well in 100 μL of culture medium per well, and each treatment group had eight independent replicates. Cell proliferation was assessed by using CCK-8 following the manufacturer’s protocol. For the MTT assay, the cells were seeded at 1 × 104 cells per well in 96-well plates and incubated for 0, 6, 12, 18, and 24 h after transfection and then the cells were fixed for MTT according to the manufacturer’s protocol. For the EdU assay, the cells were seeded in 48-well plates and incubated under the standard conditions for 24 h. After incubation with 50 μM of EdU from the Proliferation Assay Kit (Ribobio, Guangzhou, China) for 2 h, cells were fixed and stained for EdU as described in the manufacturer’s protocol. The cell nuclei were counter-stained with Hoechst 3342 for 30 min and detected by fluorescence microscopy (DM5000B, Leica Microsystems, Germany).
Hoechst 33342/PI Dual Staining Assay
Cell apoptosis was tested using Hoechst 33342 and PI double staining (Solarbio, Beijing, China). In brief, after treatment for 24 h, myoblasts were incubated with Hoechst 33342 for 15 min at room temperature. Finally, cells were treated with PI for 10 min at room temperature. Fluorescence signal was captured under a fluorescence microscope (DM5000B, Leica Microsystems, Germany).
Luciferase Activity Assay
For the luciferase reporter assays, miR-744 and pck-circSNX29-Wild or pck-circSNX29-Mut were co-transfected into 293T cells when the cell confluence reached approximately 80%. A miR-744 sensor was created by inserting two consecutive miR-744 complementary sequences into the psiCHECK-2 vector. Similarly, the cells were seeded into 48-well plates in triplicate and co-transfected with a wild-type or mutant construct of CaMKIIδ/Wnt5a with and without miR-744. After transfection for 48 h, the luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega).
Immunofluorescence Staining
After transfection and induced induction of differentiation for 4 days, C2C12 cells were fixed with 4% paraformaldehyde in PBS for 20 min, washed with PBS three times, permeabilized with 0.5% Triton X-100 for 10 min, blocked with 5% BSA at 4°C for 30 min, and then incubated with primary antibody (anti-MyHC diluted 1:250; Abcam, Cambridge, MA) at 4°C overnight. The cells were subsequently washed three times with PBS and incubated with the corresponding fluorescent secondary antibody (rabbit anti-mouse immunoglobulin G [IgG] H&L diluted 1:1,000 with 1% BSA in PBS; Abcam, Cambridge, MA) at 4°C for 2.5 h, then cells were stained with 5 mg/mL DAPI. Images from at least three or more regions in each well were captured by using a fluorescence microscope (DM5000B, Leica Microsystems, Germany).
KEGG Pathway Enrichment Analysis
Pathway enrichment analysis was performed for target genes of miR-744 through the KEGG databases and the Omicsbean online software (http://www.omicsbean.com:88/).
Western Blotting Analysis
The total proteins were extracted from primary bovine myoblasts using protein lysis radioimmunoprecipitation assay (RIPA) buffer containing 1 mM PMSF (Solarbio, Beijing, China). Subsequently, proteins in the supernatant were separated by SDS-PAGE and then transferred to 0.2 μm polyvinylidene fluoride (PDVF) membranes and sealed with 5% skim milk for 2 h at room temperature. After, the membranes were incubated with primary antibodies specific for anti-Wnt5a, anti-CaMKIIδ (Sangon Biotech, Shanghai, China), anti-PKC, anti-PLC, anti-calcineurin, anti-β-catenin, anti-NAFTC1 (Wanleibio, Haerbin, China), anti-CDK2, anti-PCNA, anti-cyclin D1, anti-Bcl2, anti-Bax, anti-p53, anti-MyoD, anti-MyHC, anti-MyoG, and β-actin from Abcam (Cambridge, MA,USA) at 4°C overnight. The PVDF membranes were washed with Tris saline with Tween (TBST) buffer for three times and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2.5 h at room temperature. The enhanced chemiluminescence luminous fluid (ECL) was applied for color development.
Statistical Analysis
The quantitative results are presented as mean ± SEM based on at least three independent experiments. All data in this study were analyzed by ANOVA for p value calculations using SPSS v 19.0 and Prism 6.01 (GraphPad Software, La Jolla, CA, USA). A value of p < 0.05 was considered statistically significant and is indicated with an asterisk, and two asterisks indicated p < 0.01.
Author Contributions
S.P. and H.C. designed and coordinated the project. S.P. performed the experiments and drafted the manuscript. C.S., H.L., and X.C. modified the manuscript. Y.M. and X.W. helped perform the experiments and analyzed the data. Y.H., X.L., and C.L. provided suggestion to the experiments; B.C. helped collect tissue samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 31772574), the Program of National Beef Cattle and Yak Industrial Technology System (CARS-37), and Haixi Project of Qinghai Province: Identification of key genes of growth and high-quality meat in Yak Multi hybrids (Fu Niu).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.03.009.
Supplemental Information
References
- 1.Bentzinger C.F., Wang Y.X., Rudnicki M.A. Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javed R., Jing L., Yang J., Li X., Cao J., Zhao S. miRNA transcriptome of hypertrophic skeletal muscle with overexpressed myostatin propeptide. BioMed Res. Int. 2014;2014:328935. doi: 10.1155/2014/328935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudnicki M.A., Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. BioEssays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- 4.Sassoon D.A. Myogenic regulatory factors: dissecting their role and regulation during vertebrate embryogenesis. Dev. Biol. 1993;156:11–23. doi: 10.1006/dbio.1993.1055. [DOI] [PubMed] [Google Scholar]
- 5.Arnold H.H., Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 1998;8:539–544. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- 6.Lassar A., Münsterberg A. Wiring diagrams: regulatory circuits and the control of skeletal myogenesis. Curr. Opin. Cell Biol. 1994;6:432–442. doi: 10.1016/0955-0674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 7.Wright W.E., Sassoon D.A., Lin V.K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y., Ge Y., Drnevich J., Zhao Y., Band M., Chen J. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. J. Cell Biol. 2010;189:1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legnini I., Morlando M., Mangiavacchi A., Fatica A., Bozzoni I. A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis. Mol. Cell. 2014;53:506–514. doi: 10.1016/j.molcel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang H., Chen X., Li W., Li Z., Nie Q., Zhang X. Circular RNA circSVIL promotes myoblast proliferation and differentiation by sponging miR-203 in chicken. Front. Genet. 2018;9:172. doi: 10.3389/fgene.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Te Pas M.F., Keuning E., Hulsegge B., Hoving-Bolink A.H., Evans G., Mulder H.A. Longissimus muscle transcriptome profiles related to carcass and meat quality traits in fresh meat Pietrain carcasses. J. Anim. Sci. 2010;88:4044–4055. doi: 10.2527/jas.2010-2952. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez S., Risolino M., Mandia N., Talotta F., Soini Y., Incoronato M., Condorelli G., Banfi S., Verde P. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene. 2015;34:3240–3250. doi: 10.1038/onc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W., He H.B., Zhang W.Y., Zhang H.X., Bai J.B., Liu H.Z., Cao J.H., Chang K.C., Li X.Y., Zhao S.H. miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development. Cell Death Dis. 2013;4:e668. doi: 10.1038/cddis.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 15.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 16.Meganck R.M., Borchardt E.K., Castellanos Rivera R.M., Scalabrino M.L., Wilusz J.E., Marzluff W.F., Asokan A. Tissue-dependent expression and translation of circular RNAs with recombinant AAV vectors in vivo. Mol. Ther. Nucleic Acids. 2018;13:89–98. doi: 10.1016/j.omtn.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu M.T., Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 18.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 19.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei X., Li H., Yang J., Hao D., Dong D., Huang Y., Lan X., Plath M., Lei C., Lin F. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017;8:e3153. doi: 10.1038/cddis.2017.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Yang J., Wei X., Song C., Dong D., Huang Y., Lan X., Plath M., Lei C., Ma Y. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J. Cell. Physiol. 2018;233:4643–4651. doi: 10.1002/jcp.26230. [DOI] [PubMed] [Google Scholar]
- 22.Mercken E.M., Majounie E., Ding J., Guo R., Kim J., Bernier M., Mattison J., Cookson M.R., Gorospe M., de Cabo R., Abdelmohsen K. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany N.Y.) 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W., Li Y., Gou S., Xiong J., Wu H., Wang C., Yan H., Liu T. MiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/β-catenin pathway. Oncotarget. 2015;6:37557–37569. doi: 10.18632/oncotarget.5317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Yang X., Wang J., Zhou Z., Jiang R., Huang J., Chen L., Cao Z., Chu H., Han B., Cheng Y. Silica-induced initiation of circular ZC3H4 RNA/ZC3H4 pathway promotes the pulmonary macrophage activation. FASEB J. 2018;32:3264–3277. doi: 10.1096/fj.201701118R. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C.W., Yeh J.C., Fan T.-P., Smith S.K., Charnock-Jones D.S. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2008;365:285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- 29.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 30.Swaminathan P.D., Purohit A., Hund T.J., Anderson M.E. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ. Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinsey T.A., Zhang C.L., Lu J., Olson E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backs J., Backs T., Bezprozvannaya S., McKinsey T.A., Olson E.N. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol. Cell. Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitzki F., Zibat A., König S., Wijgerde M., Rosenberger A., Brembeck F.H., Carstens P.O., Frommhold A., Uhmann A., Klingler S. Tumor stroma-derived Wnt5a induces differentiation of basal cell carcinoma of Ptch-mutant mice via CaMKII. Cancer Res. 2010;70:2739–2748. doi: 10.1158/0008-5472.CAN-09-3743. [DOI] [PubMed] [Google Scholar]
- 34.Cong W., Liu B., Liu S., Sun M., Liu H., Yang Y., Wang R., Xiao J. Implications of the Wnt5a/CaMKII pathway in retinoic acid-induced myogenic tongue abnormalities of developing mice. Sci. Rep. 2014;4:6082. doi: 10.1038/srep06082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J., Li M., Li Z., Xue J., Lan X., Zhang C., Lei C., Chen H. Identification and profiling of conserved and novel microRNAs from Chinese Qinchuan bovine longissimus thoracis. BMC Genomics. 2013;14:42. doi: 10.1186/1471-2164-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kühl M., Sheldahl L.C., Park M., Miller J.R., Moon R.T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 37.He X., Saint-Jeannet J.P., Wang Y., Nathans J., Dawid I., Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 38.Toyofuku T., Hong Z., Kuzuya T., Tada M., Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-β-catenin complex. J. Cell Biol. 2000;150:225–241. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang H., Chen Q., Coles A.H., Anderson S.J., Pihan G., Bradley A., Gerstein R., Jurecic R., Jones S.N. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 41.Torres M.A., Yang-Snyder J.A., Purcell S.M., DeMarais A.A., McGrew L.L., Moon R.T. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J. Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babij P., Kelly C., Periasamy M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: complete nucleotide and protein coding sequence and analysis of the 5′ end of the gene. Proc. Natl. Acad. Sci. USA. 1991;88:10676–10680. doi: 10.1073/pnas.88.23.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z., Sun H., Dai H., Walsh R.M., Imakura M., Schelter J., Burchard J., Dai X., Chang A.N., Diaz R.L. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–2768. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 47.Huang V. Springer Singapore; 2017. Endogenous miRNAa: miRNA-Mediated Gene Upregulation. [DOI] [PubMed] [Google Scholar]
- 48.Lin F., Ding R., Zheng S., Xing D., Hong W., Zhou Z., Shen J. Decrease expression of microRNA-744 promotes cell proliferation by targeting c-Myc in human hepatocellular carcinoma. Cancer Cell Int. 2014;14:58. doi: 10.1186/1475-2867-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang V., Place R.F., Portnoy V., Wang J., Qi Z., Jia Z., Yu A., Shuman M., Yu J., Li L.C. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012;40:1695–1707. doi: 10.1093/nar/gkr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fontana L., Pelosi E., Greco P., Racanicchi S., Testa U., Liuzzi F., Croce C.M., Brunetti E., Grignani F., Peschle C. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat. Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 51.Chen X.F., Liu Y. MicroRNA-744 inhibited cervical cancer growth and progression through apoptosis induction by regulating Bcl-2. Biomed. Pharmacother. 2016;81:379–387. doi: 10.1016/j.biopha.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Li S., Hu C., Li J., Liu L., Jing W., Tang W., Tian W., Long J. Effect of miR-26a-5p on the Wnt/Ca(2+) pathway and osteogenic differentiation of mouse adipose-derived mesenchymal stem cells. Calcif. Tissue Int. 2016;99:174–186. doi: 10.1007/s00223-016-0137-3. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Liu Z., Zhou M., Liu C. MicroRNA-129-5p inhibits vascular smooth muscle cell proliferation by targeting Wnt5a. Exp. Ther. Med. 2016;12:2651–2656. doi: 10.3892/etm.2016.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H., Wei X., Yang J., Dong D., Hao D., Huang Y., Lan X., Plath M., Lei C., Ma Y. circFGFR4 promotes differentiation of myoblasts via binding miR-107 to relieve its inhibition of Wnt3a. Mol. Ther. Nucleic Acids. 2018;11:272–283. doi: 10.1016/j.omtn.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Y., Cao J.-H., Li X.-Y., Zhao S.-H. Inhibition of miR-214 expression represses proliferation and differentiation of C2C12 myoblasts. Cell Biochem. Funct. 2011;29:378–383. doi: 10.1002/cbf.1760. [DOI] [PubMed] [Google Scholar]
- 56.Agley C.C., Lewis F.C., Jaka O., Lazarus N.R., Velloso C., Francis-West P., Ellison-Hughes G.M., Harridge S.D.R. Active GSK3β and an intact β-catenin TCF complex are essential for the differentiation of human myogenic progenitor cells. Sci. Rep. 2017;7:13189. doi: 10.1038/s41598-017-10731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng H., Wang Q., Lou T., Qin J., Jung S., Shetty V., Li F., Wang Y., Feng X.H., Mitch W.E. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat. Commun. 2017;8:1493. doi: 10.1038/s41467-017-01646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baksh D., Boland G.M., Tuan R.S. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 59.Liu N., Shi S., Deng M., Tang L., Zhang G., Liu N., Ding B., Liu W., Liu Y., Shi H. High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J. Bone Miner. Res. 2011;26:2082–2095. doi: 10.1002/jbmr.440. [DOI] [PubMed] [Google Scholar]
- 60.Goljanek-Whysall K., Sweetman D., Münsterberg A.E. microRNAs in skeletal muscle differentiation and disease. Clin. Sci. (Lond.) 2012;123:611–625. doi: 10.1042/CS20110634. [DOI] [PubMed] [Google Scholar]
- 61.Liu N., Bassel-Duby R. Regulation of skeletal muscle development and disease by microRNAs. Results Probl. Cell Differ. 2015;56:165–190. doi: 10.1007/978-3-662-44608-9_8. [DOI] [PubMed] [Google Scholar]
- 62.Williams A.H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J.L., Bassel-Duby R., Sanes J.R., Olson E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 64.Wang K., Long B., Liu F., Wang J.-X., Liu C.-Y., Zhao B., Zhou L.Y., Sun T., Wang M., Yu T. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016;37:2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 65.Greco S., Cardinali B., Falcone G., Martelli F. Circular RNAs in muscle function and disease. Int. J. Mol. Sci. 2018;19:E3454. doi: 10.3390/ijms19113454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., Fatica A., Santini T., Andronache A., Wade M. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell. 2017;66:22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun X., Li M., Sun Y., Cai H., Lan X., Huang Y., Bai Y., Qi X., Chen H. The developmental transcriptome sequencing of bovine skeletal muscle reveals a long noncoding RNA, lncMD, promotes muscle differentiation by sponging miR-125b. Biochim. Biophys. Acta. 2016;1863:2835–2845. doi: 10.1016/j.bbamcr.2016.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.