Conflict of Interest
The authors declare no conflict of interest.
Ischemic stroke is one of the most common cerebrovascular and neurological diseases leading to significant lifelong morbidity and mortality. Post‐stroke angiogenesis formed by new capillaries is a key event of neurovascular remodeling processes for supply of nutrition and oxygen into injury area. Endothelial progenitor cells (EPC) is found to be involved in post‐stroke angiogenesis, which decreases stroke size and improves behavioral outcomes 1, 2. Therefore, several clinical trials evaluating angiogenesis by EPC in treating ischemic stroke are underway 1.
Nicotinamide adenine dinucleotide (NAD) is a ubiquitous biological molecule that participates in numerous cellular biological reactions, and nicotinamide phosphoribosyltransferase (NAMPT) is the rate‐limiting enzyme for mammalian NAD salvage synthesis 3. We and other groups have showed that neuronal NAMPT is upregulated 4 and released 5 after cerebral ischemia. The inducible NAMPT by cerebral ischemia displays potent neuroprotection in rodent ischemic stroke models 4, 5, 6. Recently, we demonstrated that intracellular NAMPT improves hind‐limb vascular repair by modulating Notch signaling pathway in EPC 7. Overexpression of NAMPT increases deacetylation of Notch‐1 intracellular domain, which inhibits delta‐like ligand‐4‐Notch signaling and thereby upregulates vascular endothelial growth factor receptors in EPC 7. This pro‐angiogenesis action of NAMPT in hind‐limb raises the possibility that NAMPT may promote post‐stroke angiogenesis. However, it should be noted that there exists much difference between skeletal muscle tissue and central nerve system (CNS), which might affect the action of NAMPT in brain angiogenesis after cerebral ischemia.
To test this hypothesis, we subjected two transgenic mice stains (NAMPT‐transgenic and H247A dominant negative NAMPT‐transgenic mice, which are referred as NAMPT‐Tg and DN‐NAMPT‐Tg respectively) 7 with middle cerebral artery occlusion (MCAO), a widely used cerebral ischemia model. The cerebral blood flow (CBF) in ischemic brain area was measured using Laser Doppler Monitoring. As shown in Figure 1, the CBF declined markedly (~20% of control) after MCAO. During the two weeks post ischemia, the CBF gradually recovered (from ~20% to ~40% of control), suggesting a naturally occurred angiogenesis process. Compared with WT mice, NAMPT‐Tg mice exhibited significantly increased CBF recovery at 7th day and 14th day but not at 3rd day post MCAO. In contrast, such phenotype was not observed in DN‐NAMPT‐Tg mice. The CBF recovery in DN‐NAMPT‐Tg mice was even lower than that in WT mice, although there was no significance.
Figure 1.
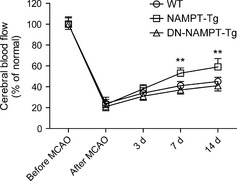
Cerebral blood flow (CBF) in ischemic brain area. CBF at five time‐points (before MCAO, after MCAO, 3rd days post MCAO, 7th days post MCAO and 14th days post MCAO) was measured using Laser Doppler monitoring. The CBF in the contralateral side is deemed to be normal. **P < 0.01 versus WT by one‐way ANOVA. n = 8 per group.
We also determined the cerebral angiogenesis in these three stains using immunohistochemistry. Brain sections were stained by double‐label immunohistochemistry at 14th day after MCAO. IB4‐lectin was applied to stain blood vessels and anti‐Ki‐67 was applied to stain proliferative cells. The IB4‐lectin/Ki‐67 double‐positive (IB4‐lectin+/Ki‐67+) cells were thought to be new‐formed blood vessels, which reflects the post‐ischemic angiogenesis. As shown in Figure 2, the number of IB4‐lectin+/Ki‐67+ cells in brain sections of NAMPT‐Tg mice was more than that in WT mice. However, this change was not observed in DN‐NAMPT‐Tg mice.
Figure 2.
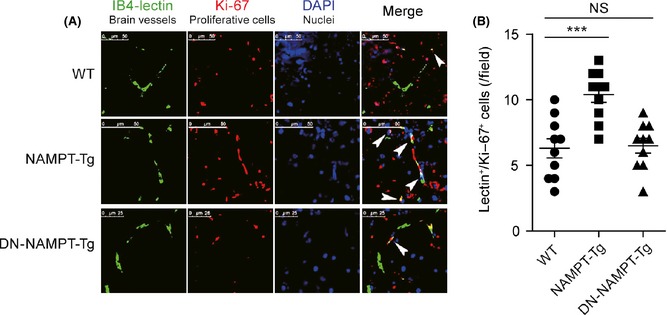
Representative images and quantitative analysis of post‐stroke angiogenesis in mouse brain penumbra tissue at 14th days after MCAO. (A) Brain penumbra tissue was fixed by 4% paraformaldehyde and cut into 20 μM sections, which were stained by Alexa 488‐conjugated IB4‐lectin (Sigma) and mouse monoclonal anti‐Ki‐67 (BD Biosciences). Then, sections were stained by corresponding goat anti mouse Cy3‐conjugated secondary antibody and DAPI (nuclei marker). The images were captured by FV1000 (Olympus) laser scanning confocal microscopes. (B) Quantitative analysis of the number of IB4‐Lectin+/Ki‐67+ cells. At least three brain sections per mouse and ten visual fields per section were analyzed. ***P < 0.001 versus WT by one‐way ANOVA. NS, no significance. n = 8 per group.
The integrity of neurovascular unit plays critical roles in ischemic stroke as well as many other CNS disorders 8. The neurovascular unit involves microvessels, pericytes, astrocytes, neurons, axons, and other supporting cells such as microglia 8. The increased supply of nutrition and oxygen resulted by post‐stroke angiogenesis undoubtedly contributes to restoration of neurological functions. Recently, some circulating angiogenic factors, such as adiponectin and angiopoietin‐1, were reported to improve neurobehavioral outcomes after focal cerebral ischemia 9, 10. Our results support the notion that NAMPT improves post‐stroke angiogenesis. Because neuronal NAMPT is upregulated and released after ischemia 4, 5, we propose that the upregulation of NAMPT may be a beneficial response against ischemic stress. NAMPT may be not only a neuroprotector, but also a promoter for post‐stroke angiogenesis: on the one hand, the NAMPT upregulation helps to protect neurons from ischemic‐induced acute death; on the other hand, the NAMPT upregulation may increase the formation of new capillaries via improving post‐ischemic angiogenesis during the subacute and chronic periods.
The presence of proliferating vascular endothelial cells and enlarged and thin‐walled vessels, termed “mother” vessels, is a marker of cerebral ischemic angiogenesis. When ischemia occurs, EPC were motivated and recruited to ischemic area. Finally, EPCs were incorporated into neovessels to induce post‐ischemic angiogenesis and neurogenesis 1, 2. We propose that the molecular mechanisms underlying the promoting effect of NAMPT on angiogenesis may attribute to its regulation of EPC. Previously, we have demonstrated that NAMPT overexpression improved blood flow recovery and augmented collateral arterioles in the ischemic limb by promoting EPCs 7. This biological function of NAMPT could also work upon brain ischemia and thereby improves EPCs recruitment and enhances EPCs incorporation into cerebral arterioles. All these might lead to improvement of angiogenesis and neurogenesis.
In summary, the results of this study demonstrate that NAMPT facilitates post‐stroke angiogenesis and represents a promising therapeutic target for ischemic stroke.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81373414, 81473208, 81422049 and 81130061) and Shanghai Qimingxing project (14QA1404700).
References
- 1. Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: Therapeutic perspective for ischemic stroke. CNS Neurosci Ther 2013;19:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mao L, Huang M, Chen SC, et al. Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF‐1 axis and improve outcome after stroke. CNS Neurosci Ther 2014;20:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imai S. Nicotinamide phosphoribosyltransferase (Nampt): A link between NAD biology, metabolism, and diseases. Curr Pharm Des 2009;15:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang P, Xu TY, Guan YF, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1‐dependent adenosine monophosphate‐activated kinase pathway. Ann Neurol 2011;69:360–374. [DOI] [PubMed] [Google Scholar]
- 5. Jing Z, Xing J, Chen X, et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J Cereb Blood Flow Metab 2014;34:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Y, Liu XZ, Tian WW, et al. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther 2014;20:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang P, Du H, Zhou CC, et al. Intracellular NAMPT‐NAD+‐SIRT1 cascade improves post‐ischaemic vascular repair by modulating Notch signalling in endothelial progenitors. Cardiovasc Res 2014;104:477–488. [DOI] [PubMed] [Google Scholar]
- 8. Muoio V, Persson PB, Sendeski MM. The neurovascular unit ‐ concept review. Acta Physiol (Oxf) 2014;210:790–798. [DOI] [PubMed] [Google Scholar]
- 9. Miao J, Shen LH, Tang YH, et al. Overexpression of adiponectin improves neurobehavioral outcomes after focal cerebral ischemia in aged mice. CNS Neurosci Ther 2013;19:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan T, Venkat P, Ye X, et al. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther 2014;20:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]


