Summary
Aims
The postictal suppression (PS) is a common and important period following an epileptic seizure but has not been well studied. This study was designed to determine whether interleukin‐1β (IL‐1β) is involved in the PS.
Methods
The effects of IL‐1β on the PS were tested in three independent seizure models induced by hippocampal kindling, maximal electroshock seizure (MES), and 4‐aminopyridine, respectively.
Results
IL‐1R1 knockout or IL‐1RA enhanced the seizure refractory phenomenon without influencing the baseline seizure threshold in intermittent MES model. IL‐1β attenuated the seizure refractory phenomenon without affecting the severity of the preceding seizures in hippocampal kindling model, while IL‐1RA enhanced it. Besides, IL‐1β reduced the postictal EEG suppression period, while IL‐1RA prolonged it. And IL‐1β showed no further effect on the postictal EEG suppression and seizure refractory phenomenon in IL‐1R1 knockout mice. In addition, 30 min after intrahippocampal injection of 4‐aminopyridine, IL‐1β increased the incidence of SE, while IL‐1RA prolonged the intervals between recurrent seizures.
Conclusions
This study provides the first direct evidence that IL‐1β is key regulatory factor for the PS, and its receptor IL‐1R1 may be a potential target for adjuvant treatment of postictal problems.
Keywords: Epilepsy, Inflammation, Interleukin‐1 beta, Interleukin‐1 Receptor Type I, Seizures
Introduction
Following a seizure, there is often a “postictal suppression (PS)” or “postictal refractory” period, which is characterized by resistance to induction of further seizures and depression of electroencephalogram (EEG) or neural activity 1, 2. Previous studies regarded the PS as an endogenous anticonvulsant phenomenon that contributes to limiting seizure duration and inhibiting further recurrent seizures 3, 4. However, increasing experimental evidence and clinical observations indicate that the PS may serve as “a double‐edged sword” for patients with epilepsy; that is, a weakened PS may deteriorate seizures and even induce status epilepticus (SE) 5, while a prolonged PS may result in postictal problems 6, 7 and even sudden unexpected death after a seizure 8, 9. So far, the underlying mechanisms of the PS are only incompletely understood, and hence, there is still no reliable approach to modulating the PS in clinic. Thus, it is a pressing need to develop the regulatory targets for the PS.
The proinflammatory cytokine IL‐1β is a key regulator of acute inflammatory processes in the CNS 10, 11.The production of IL‐1β is increased in epileptic tissues, which is involved in seizure generation and propagation 12, 13. Both experimental and clinical evidence indicate that CNS IL‐1β may contribute to epileptic seizures 14. For example, an increase in the level of IL‐1β in the CNS exacerbates acute seizures induced by bicuculline, while inhibition of interleukin‐1 receptor type 1 (IL‐1R1) results in anticonvulsant effect in a rodent model 15. However, few studies attempt to make it clear that which period of a seizure, the initiation, termination, or PS, is influenced by IL‐1β. Recently, Librizzi et al. 16 have found interleukin‐1 receptor antagonist (IL‐1RA) reduces the seizure duration and prevents seizure recurrence in isolated guinea pig brain, suggesting that IL‐1β might affect the postictal period. In addition, the fact that the IL‐1β in the CNS may rapidly be released after a seizure also leads us to hypothesize that IL‐1β is a modulator of the PS.
In this study, we used the classical intermittent MES procedure 17 and developed a new intermittent kindling procedure to evaluate the effects of IL‐1β on the PS. The effects of IL‐1β on further seizure recurrence were introduced to test in an acute recurrent seizure model induced by 4‐aminopyridine (4‐AP). In addition, a recombinant human IL‐1RA and IL‐1R1‐knockout (IL‐1R1‐KO) mice were also used.
Materials and Methods
Animals
Age‐matched C57BL/6 mice and IL‐1R1‐KO mice (generated on a C57BL/6 background, from Jackson Laboratory, stock number: 003245) were used in this study. All animals were maintained in individual cages with a 12‐h light/dark cycle (lights on from 8:00 to 20:00). Water and food were given ad libitum. All experiments were carried out in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were carried out between 10:00 and 17:00.
The Threshold for Maximal Electroshock Seizures (MES)
The MES threshold was determined by an “up‐and‐down” method 18, 19; that is, the stimulus current intensity began at 5 mA and the current was lowered 1 mA if the preceding shock caused tonic hindlimb extension or was raised 1 mA if not. The interval between trials is about 2 min. The electrical stimulation (0.2 second at 50 Hz) was delivered through ear clip using a rodent shocker (Hugo Sachs Elektronik, March‐Hugstetten, Germany).
Intermittent MES Procedure
Mice were subjected to a series of six supramaximal seizures induced by intermittent MES (0.2 second at 50 Hz, 15 mA) at 3‐min intervals. The convulsion patterns were scored as follows: 0, the absence of forelimb extension; 1, complete forelimb extension with the absence of hindlimb extension; 2, complete forelimb extension with partial hindlimb extension; and 3, complete fore‐ and hindlimb extension.
Surgery
The protocol of surgery is similar to previous studies 20, 21. Briefly, under anesthesia by sodium pentobarbital (60 mg/kg i.p.), mice were fixed in a stereotaxic apparatus (512600, Stoelting, Wood Dale, IL, USA). The electrodes were implanted into the right ventral hippocampus (AP: −2.9, L: −3.0, V: −3.0) based on the atlas Paxinos & Franklin 22. The electrodes were made of twisted stainless steel teflon‐coated wires (791500, A.M. Systems, Carlsborg, WA, USA) insulated except at the tip 0.5 mm and separated about 0.5 mm. A ground screw was fixed above the prefrontal cortex (AP: +2, L: −1.5) and a referent screw above the cerebellar cortex (AP: −4.5, L: 0). For intracerebroventricular injection, a guide cannula (62003, RWD Life Science, Shenzheng, China) was stereotaxically implanted into the lateral ventricle (AP: −1.0, L: −1.5, V: −2). For intrahippocampal injection, a bipolar stimulation electrode was adhered to a guide cannula with 0.5–1 mm beyond the tip of the guide cannula, which was implanted into the right ventral hippocampus. Location of electrodes and cannulas was histologically verified.
Hippocampal kindling
One week after surgery, the prekindling afterdischarge threshold (ADT) was determined by a constant current stimulator (SEN‐7203, Nihon Kohden, Tokyo, Japan). The stimulation intensity began at 20 μA (20 Hz; 2 second trains; 1 mseconds monophasic square wave) and was subsequently increased by 20 μA steps every 1 min. The minimal intensity that produced afterdischarge duration (ADD) ≥5 seconds was designated the prekindling ADT. (The mice with prekindling ADT higher than 200 μA were excluded). Then, the mice received 10 stimulations daily (400 μA, once every 30 min). Behavioral seizures were scored according to Racine 23: (1) facial movement, (2) head nodding, (3) unilateral forelimb clonus, (4) bilateral forelimb clonus and rearing and (5) bilateral forelimb clonus and rearing and falling. Seizure stages 1–3 indicate focal seizures, while stages 4–5 are generalized seizures 24. Once a mouse was fully kindled, the postkindled ADT was determined, and the generalized seizure threshold (GST) was determined by continuously increasing the current intensity in steps until a generalized seizure was elicited.
Intermittent kindling procedure
The fully kindled mice were subjected to a series of six suprathreshold kindling stimulations (400 μA); that is, five additional kindling stimulations were carried out at 1, 3, 5, 15, and 30 min after the termination of the seizure induced by the first kindling stimulation. The seizure stage and ADDs were recorded after each stimulation.
Drugs
Recombinant human IL‐1β (Prospec‐Tany Techno Gene), IL‐1RA (Prospec‐Tany Techno Gene), and 4‐aminopyridine (Sigma) were injected through a 30‐gauge needle inserted through the guide cannula and secured to ensure no movement. The injection volume is 1 μL. The injection duration was 10 min, and the needle would be remained for another 5 min after finishing the injection. Drugs were injected into the lateral ventricles in MES model for it is a seizure model without specific epileptic focus or into the hippocampus in the other models for the hippocampus is the epileptic focus in these models.
EEG recording and analysis
Raw EEG signals were recorded with band‐pass filters spanning DC to 200 Hz and sampled at 1000 Hz with a digital amplifier (NuAmps, Neuroscan System, Charlotte, NC, USA) similar as our previous study 25. Recorded EEGs were offline analyzed by Scan 4.5. The entire EEG time series was DC‐shifted, digitally band‐pass‐filtered from 0.3 to 100 Hz. Finally, the EEG data were imported into Labchart 7.0 software (ADInstruments, Bella Vista, NSW, Australia), where the power spectrum and the total power (0–50 Hz) were calculated using fast Fourier transform with a Hanning window.
Effect of IL‐1R1 inhibition on changes in seizure severity during intermittent MES procedure
Before the intermittent MES procedure, WT mice were divided into 4 groups to evaluate whether IL‐1RA has an anticonvulsive effect. The MES tests were performed at 15, 30 and 60 min after injection of IL‐1RA (100 ng) in groups 1–3, respectively.
To test the effect of IL‐1RA on the seizure severity during intermittent MES procedure, WT mice were divided into two groups matched for their MES thresholds: One group was treated with IL‐1RA (100 ng.), while the other group was treated with saline. Both groups were subject to the intermittent MES procedure 15 min after injection.
To test the effect of IL‐1R1‐KO mice on seizure severity during intermittent MES procedure, all mice were subject to the intermittent MES procedure one day after the MES threshold tests.
Effect of IL‐1RA on the postictal EEG suppression in kindled mice
Fully kindled WT mice confirmed by three additional continuous stage 5 seizures were divided into four groups. All mice were treated saline for the baseline tests, and the ADD (ADD1) and duration of the postictal EEG suppression (duration1) were recorded. The postictal EEG suppression was identified if the power density of EEG (0–50 Hz) is lower than one‐tenth of the baseline. Three hours later, mice in groups 1–2 were injected with 1 ng and 10 ng IL‐1β, respectively; animals in group 3 were injected with IL‐1RA (100 ng) and those in group 4 were injected with saline. The ADD (ADD2) and duration of the postictal EEG suppression (duration2) were recorded again. Finally, the change of ADD and postictal EEG suppression duration were calculated by the equations “(ADD2‐ADD1)/ADD1*100%” and “(duration2‐duration1)/duration1*100%,” respectively.
Effect of IL‐1RA on changes in seizure severity during intermittent kindling procedure
WT mice were divided into three groups matched with GSTs and were treated with IL‐1β (1 ng), IL‐1RA (100 ng), and saline, respectively. All mice were subject to the intermittent kindling procedure 30 min after drug injection.
Effect of IL‐1R1 knockout on the kindling acquisition and expression
IL‐1R1‐KO mice and their WT littermates were used. Kindling acquisition was according to the kindling procedure. Once mice were fully kindled, the ADTs of both groups were redetermined twice (once daily, five new kindled WT mice were used here to avoid the influence of repeated stage 5 seizures): The stimulation interval was 1 min for the first test and 5 min for the second test. For investigating the effect of IL‐1R1 knockout on the kindling expression, all mice received 5 electrical stimulations to evoke fully kindled with a 30‐min interval 24 h after ADT redetermining and the postictal EEG suppression was measured after the sixth kindling stimulation. Finally, mice were subject to intermittent kindling procedure after 3 days of recovery.
Effect of IL‐1R1 inhibition on acute recurrent seizures induced by intrahippocampal injection of 4‐AP
The potassium channel blocker 4‐AP (40 nmol) was injected into the right ventral hippocampus to provoke seizure‐like discharges. IL‐1RA (100 ng) or IL‐1β (1 ng) was administered 10 min after seizure initiation. The seizure duration and seizure intervals were calculated 20 min after drug administration.
Statistical analysis of data
Two‐way analysis of variance (ANOVA) for repeated measures was used for analysis of group differences in intermittent MES, kindling acquisition, and intermittent kindling test. Paired t‐test for paired data were also used in intermittent MES and intermittent kindling tests. In the case of comparing the incidence of generalized seizure and the incidence of detectable ADT, the chi‐square test was used. To compare seizure scores or stage among groups, Mann–Whitney U‐test was used. For other data, one‐way ANOVA with LSD post hoc or Student's t‐test was used. Data are presented as mean ± SEM. For all analyses, the tests were two‐sided and a P < 0.05 was considered significant.
Results
IL‐1RA treatment and IL‐1R1‐KO mice enhanced the PS induced by intermittent MES
We first tested weather inhibition of IL‐1R1 would affect the PS in the classical intermittent MES procedure. IL‐1RA had no effect on the seizure severity induced by MES 15, 30, and 60 min after its injection (Figure 1A). The seizure severity induced by the first MES in IL‐1RA‐treated mice was also similar to saline‐treated mice (P > 0.05; Figure 1B). However, intermittent MES produced a progressive decrease in seizure severity, and IL‐1RA enhanced this effect: (1) The seizure severity only decreased at the sixth MES in the control group, while it decreased from the second to the sixth MES in IL‐1RA group (Figure 1B), and (2) the seizure severity induced by the sixth MES in IL‐1RA group was lower than that in control group (P < 0.05; Figure 1B).
Figure 1.
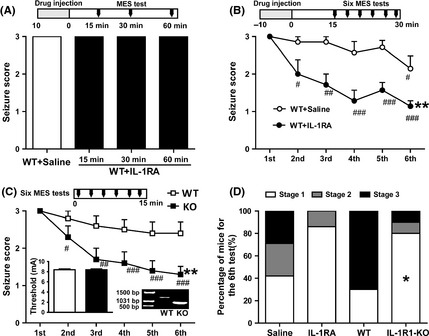
IL‐1RA treatment and IL‐1R1‐KO mice enhanced the postictal suppression induced by intermittent MES in mice. (A) The seizure score induced by MES (15 mA) did not changed 15, 30, and 60 min after the injection of IL‐1RA. (B) IL‐1RA enhanced the postictal suppression induced by intermittent MES (n = 7 for each group). (C) IL‐1R1 knockout enhanced the postictal suppression induced by intermittent MES, while IL‐1R1 knockout did not affect the baseline threshold of MES (showed in the histogram inset, n = 10 for each group). The IL‐1R1 knockout mice were identified by PCR analysis. (D) The percentage distribution of mice at different seizure scores in each group induced by the sixth (last) MES test. One‐way ANOVA with LSD post hoc was used in panel A; two‐way ANOVA for repeated measures and paired t‐test for paired data were used in panels B and C; Student's t‐test was used for comparing the threshold of MES in panel C. *P < 0.05 and **P < 0.01 represent differences from the control or WT group, respectively. # P < 0.05, ## P < 0.01, and ### P < 0.001 represent differences from the first MES test, respectively.
The MES threshold was not significantly different between the IL‐1R1‐KO mice and their WT littermates (P > 0.05; Figure 1C). The seizure severity induced by the first MES in IL‐1R1‐KO mice and their WT littermates was also similar (P > 0.05; Figure 1C). However, IL‐1R1 knockout enhanced the progressive decrease in seizure severity induced by intermittent MES: (1) The seizure severity slightly but not significantly decreased even at the sixth MES in WT mice, while it decreased from the second to the sixth MES in IL‐1R1‐KO mice (Figure 1C), and (2) the seizure severity induced by the sixth MES in IL‐1R1‐KO mice was lower than that in WT mice (P < 0.05; Figure 1C). The percentage distribution of mice at different seizure stages in each group is shown in Figure 1D.
IL‐1β Reduced the Postictal EEG Suppression Period in Kindled Mice
To evaluate the effect of IL‐1β on the postictal period in epileptic condition, we recorded and analyzed the EEG during and after a seizure in kindled mice. After the seizure‐like AD, there is a postictal EEG suppression period (Figure 2C). IL‐1β (1 or 10 ng) and IL‐1RA (100 ng) had no significant effect on the incidence of generalized seizures and behavioral seizure stages in kindled mice (all mice showed stage 5 seizure in both pre‐ and posttreatment tests). Interestingly, 1 ng IL‐1β shortened postictal EEG suppression duration (P < 0.05; Figure 2B) without influencing the ADDs (P > 0.05; Figure 2A), and 10 ng IL‐1β simultaneously prolonged the ADDs (P < 0.05; Figure 2A) and shortened postictal EEG suppression duration (P < 0.001; Figure 2B); in contrast, IL‐1RA (100 ng) shortened the ADDs (P < 0.01; Figure 2A) and prolonged the postictal EEG suppression duration (P < 0.001; Figure 2B). The representative EEG power density spectra for IL‐1β (10 ng) and IL‐1RA (100 ng) treatment are shown in Figure 2C.
Figure 2.
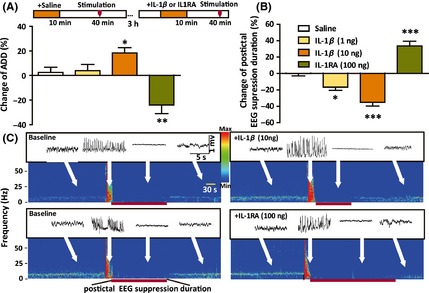
IL‐1β reduced the postictal EEG suppression period in kindled mice. The effects of IL‐1β (1 or 10 ng) and IL‐1RA on ADD (A) and postictal EEG suppression duration (B) in kindled mice (n = 6 for each group). (C) The representative EEG power density spectra. One‐way ANOVA with LSD test was used. *P < 0.05, **P < 0.01, and ***P < 0.001 represent differences from the control.
IL‐1β Attenuated the Postictal Seizure Suppression in Kindled Mice
We also developed a novel intermittent kindling procedure to test the effect of IL‐1β on the postictal suppression in epileptic condition. All mice showed stage 5 seizures in response to the first kindling simulation. However, the severity of seizures was inhibited at postictal 1, 3, and 5 min, indicated by lower incidence of generalized seizures, lower mean seizure stage, and shorter ADD than the first seizure in control kindled mice (Figure 3A–C). Interestingly, IL‐1β (1 ng) attenuated this postictal inhibition evidenced by the increased incidence of generalized seizures, increased seizure stage, and prolonged ADD of seizures at postictal 1 and 3 min (Figure 3A–C). In contrast, IL‐1RA (100 ng) lowered the incidence of generalized seizures, decreased the seizure stage, and shortened the ADD of seizures at postictal 3 and 5 min (Figure 3A–C), indicating the enhancement of postictal inhibition.
Figure 3.
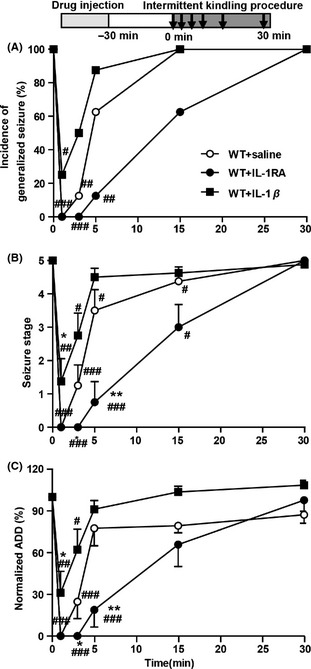
IL‐1β attenuated the postictal seizure inhibition in kindled mice. Compared with saline treatment, intrahippocampal injection of IL‐1β enhanced, while IL‐1RA (100 ng) reduced the incidence of generalized seizure (A), mean seizure stage (B), and normalized ADD (n = 8 for each group). The chi‐square test was used for A; two‐way ANOVA with LSD test was used for B and C. *P < 0.05 and **P < 0.01 represent differences from the control; # P < 0.05, ## P < 0.01, and ### P < 0.001 represent differences from the first test.
IL‐1R1 Knockout Impaired the Kindling Acquisition and Expression
We used IL‐1R1‐KO mice to further confirm our pharmacological finding. IL‐1R1 knockout slowed the progression of behavioral seizure stages (P < 0.01; Figure 4A) and shortened the corresponding ADDs (P < 0.05; Figure 4B) during kindling acquisition. We further calculated the number of stimulations needed to reach each seizure stage. IL‐1R1 knockout increased the number of days to progress to stages 4 and 5 (P < 0.05; Figure 4C). When determining the postkindling ADT in kindled mice, we found kindling stimulation at a stimulation interval of 1 min failed to induce any ADs in most IL‐1R1‐KO mice (4 of 6), even the intensity was increased up to 400 μA (Figure 4D and E). However, when we extended the stimulation interval from 1 to 5 min, the ADTs of all mice were determined by intensities below 400 μA (Figure 4D and E). In addition, fully kindled IL‐1R1‐KO mice showed lower incidence of generalized seizures than their WT littermates when receiving five kindling stimulations at 30‐min interval (P < 0.05; Figure 4F).
Figure 4.
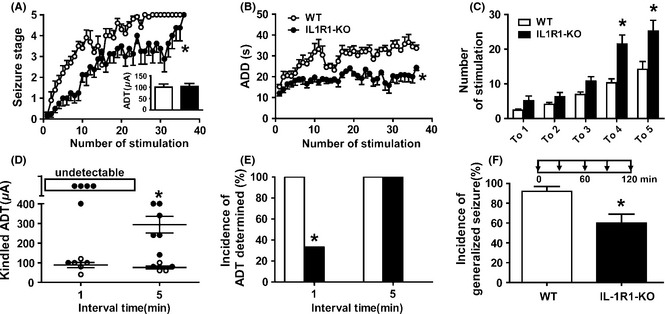
IL‐1R1‐KO mice impaired the kindling acquisition and expression. Effects of IL‐1R1‐KO on behavioral stage of seizures (A) and afterdischarge duration (ADD) (B) during hippocampal kindling acquisition (n = 10 for WT and n = 8 for IL‐1R1‐KO groups). The inset of (A) shows the prekindling afterdischarge threshold (ADT) of each group. (C) Effect of IL‐1R1 knockout on the number of stimulations required to reach each seizure stage. (D) The mean postkindling ADT determined by stimulations with an interstimulation interval of 1 or 5 min (n = 5 for WT and n = 6 for IL‐1R1‐KO groups, the WT mice were just fully kindled and used here to avoid the influence of repeated stage 5 seizures). (E) The incidence of ADT that can be determined by stimulations with an interstimulation interval of 1 or 5 min. (F) The incidence of generalized seizures induced by repeated kindling stimulation (at 400 μA) with a 30‐min interval (n = 5 for WT and n = 6 for IL‐1R1‐KO groups). Two‐way analysis of variance (ANOVA) for repeated measures was used for A and B; Student's t‐test for paired data was also used for comparing the thresholds between the IL‐1R1‐KO and WT mice in A and D; the chi‐square test was used for E and F. *P < 0.05 represents differences from the WT group.
IL‐1β did not Affect the ADD and PS in Kindled IL‐1R1‐KO Mice
IL‐1β (10 ng) or IL‐1RA (100 ng) had no significant effects on the seizure stages, ADDs and postictal EEG suppression duration in IL‐1R1‐KO mice (all mice showed stage 5 seizures in both pre‐ and posttreatment tests; P > 0.05 for all; Figure 5A–C). Similarly, all mice showed stage 5 seizures after receiving the first simulation of intermittent kindling procedure. However, compared with their WT littermates, IL‐1R1‐KO mice showed lower incidence of generalized seizures (P < 0.001 for all; Figure 5D–F), lower mean seizure stage, and shorter ADDs at postictal 5 min. Again, IL‐1β (10 ng) or IL‐1RA (100 ng) had no effect on this postictal inhibition in IL‐1R1‐KO mice (P > 0.05; Figure 5D–F).
Figure 5.
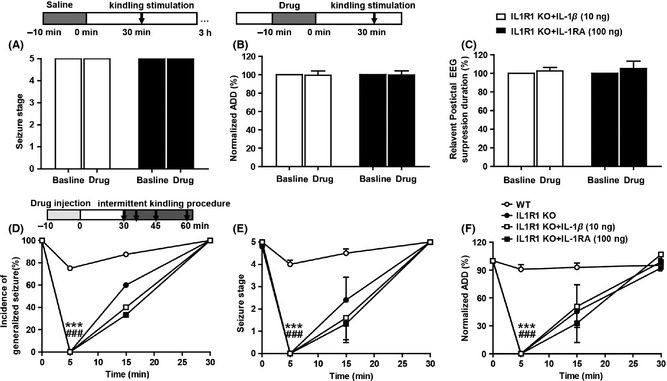
IL‐1β did not affect the ADD and postictal suppression in kindled IL‐1R1‐KO mice. Intrahippocampal injection of IL‐1β (10 ng) or IL‐1RA(100 ng) had no significant effects on mean seizure stage (A), ADD (B), and postictal EEG suppression duration (C) in IL‐1R1‐KO mice. IL‐1R1‐KO mice showed lower incidence of generalized seizure (D), lower mean seizure stage (E), and shorter ADDs (F) at postictal 5 min comparing with their WT littermates. Intrahippocampal injection of IL‐1β (10 ng) or IL‐1RA(100 ng) had no effect on incidence of generalized seizures (D), mean seizure stage (E), and normalized ADD in IL‐1R1‐KO mice (F). Paired t‐test was used for B and C; two‐way ANOVA with LSD test was used for E and F; the chi‐square test was used for D. ***P < 0.001 represents differences from the control; ### P < 0.001 represents differences from the first test.
IL‐1RA Inhibited Recurrent Seizures Induced by 4‐AP
We further investigate whether IL‐1RA and IL‐1β influencing the recurrent seizures by regulating the PS in a 4‐AP induced hippocampal recurrent seizure model. Recurrent ictal discharges were induced by intrahippocampal injection of 4‐AP. Spontaneous ictal discharges occurred periodically for up to 2 h, and the interseizure interval was about 50∼200 seconds (Figure 6A). IL‐1RA increased the interseizure interval (P < 0.001; Figure 6B) and reduced the duration of seizures (P < 0.05; Figure 6C). By contrast, IL‐1β induced higher incidence of SE (4 of 6 in IL‐1β group vs. 0 of 7 in saline group; P < 0.05; Figure 6D).
Figure 6.
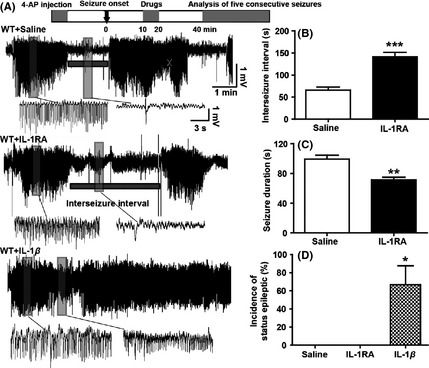
IL‐1RA inhibited recurrent seizures in 4‐AP seizure model. (A) Representative EEGs induced by intrahippocampal injection of 4‐AP (40 nmol in 1 μL) in each group (n = 7 for saline, n = 6 for both IL‐1RA and IL‐1β). (B) IL‐1RA increased the interseizure interval and (C) reduced the cumulative duration of seizure‐like activity (B). IL‐1β induced higher incidence of status epileptic (D). Student's t‐test was used for B and C; the chi‐square test was used for D. *P < 0.05, **P < 0.01 and ***P < 0.001 represent differences from the control.
Discussion
In the present study, we demonstrated for the first time that the PS can be modulated by the pro‐inflammatory cytokine IL‐1β via its receptor IL‐1R1. Both the postictal EEG suppression and seizure refractory phenomenon of the PS was attenuated by acute treatment of IL‐1β but enhanced by IL‐1RA or IL‐1R1‐KO mice. However, IL‐1β showed no effects on postictal EEG suppression and seizure refractory phenomenon in IL‐1R1‐KO mice. These results together suggest that the IL‐1R1 may be a potential target for modulation of the PS and adjuvant treatment of postictal problems.
Although many previous studies have found that IL‐1β contributes to epileptic seizures and its antagonist IL‐1RA shows anticonvulsant effect 11, 26, few of them attempted to make it clear which period of a seizure was influenced. Here, we found IL‐1RA treatment or IL‐1R1‐KO mice enhanced the postictal seizure suppression induced by intermittent MES or kindling stimulations. Furthermore, the expression level of IL‐1β was increased during the postictal depression period compared to preictal control period in kindled mice, and pretreatment of IL‐1β (1 ng or 10 ng) dose dependently shortened the duration of postictal EEG suppression, while IL‐1RA prolonged it. Thus, these results clearly showed that IL‐1β system (IL‐1β, IL‐1RA, and IL‐1R1) can bilaterally regulate the PS period. On the other hand, lower dose of IL‐1β (1 ng) had no significant effect on both seizure severity and duration; higher dose of IL‐1β (10 ng) as well as IL‐1RA (100 ng) also had no effect on behavioral seizure stages (slightly affected the ADDs as discussed below); and IL‐1R1‐KO mice did not influence the MES threshold and hippocampal prekindling ADT. Besides, we also found the postictal EEG suppression duration has no correlation with the ADD (Figure S3). These results indicate the effect of IL‐1β system (IL‐1β, IL‐1RA, and IL‐1R1) on the PS seems not due to their impact on the severity of the preceding seizures. Our data are in accordance with the previous report showing that inhibition of IL‐1β biosynthesis has slightly inhibitory but no significant effect on the kindled seizures in rats 27. Recently, several studies showed that pre‐treatment of IL‐1β does not affect the latency of seizure initiation following intrahippocampal injection of KA in mice 28, 29, which supports IL‐1β may not affect the hippocampal seizure onset. In addition, IL‐1β did not show any further effect on postictal EEG suppression and seizure refractory phenomenon in IL‐1R1‐KO mice. Taken together, these results suggesting IL‐1R1 mediates the effect of IL‐1β and may be the key in IL‐1β system to regulate the PS.
So far, all PS‐related endogenous substances reported previously, such as adenosine, H+ 5, neuropeptide Y, opioids 30, and histamine 17, are inhibitory and contribute to the maintaining of PS. Here, we provide the first evidence that IL‐1β acts as anti‐PS endogenous substance. The appearance of PS usually indicates the termination of a seizures 1, so a delayed or attenuated PS may prolong the preceding seizure or convert it into SE. Meanwhile, the PS is also related to the “postictal seizure protection” phenomenon 3, 17, 30, which may induce a reduction in the severity of subsequent convulsions. Thus, as the PS is related to the limitation of seizure duration and inhibition of further seizures recurrence, the anti‐PS property of IL‐1β is also supported by the following indirect evidence in the present study: (1) IL‐1β (10 ng) slightly prolonged, while IL‐1RA shortened the hippocampal ADD in kindled mice and (2) IL‐1β increased the incidence of SE, while IL‐1RA reduced seizure‐like activities and prolonged interseizure intervals in seizure model induced by 4‐AP. Recently, Ravizza et al. 31 showed there exists a positively correlation between the expression of IL‐1β in epileptogenic brain tissues and the frequency of seizures in patients with epilepsy, which supports our findings. The anti‐PS property of IL‐1β may be also supportive of the proposal that the preceding inflammation lesion results in SE 32. Thus, these results suggest excessive amounts of IL‐1β may exacerbate epileptic seizures by attenuating the PS. On the other hand, extensive PS in specific brain areas may bring with many problems following a seizure 33, which is hard to treat by traditional AEDs 34. For some patients, postictal problems may be more disturbing than the seizure itself. Recently, clinical studies showed a specific PS that influenced global brain areas, named postictal generalized EEG suppression (PGES), is associated with respiratory dysfunction 6, 35and sudden unexpected death 8 in epilepsy cases. Here, we found IL‐1β (1 ng) shortened the postictal EEG suppression without prolonged the ADD (Figure 2) and slightly alleviated the inhibition of breathing frequency during postictal depression (Figure S2) in kindled mice. Although we have no further data, it is likely that a certain amount of IL‐1β may be required for the brain to maintain an appropriate strength of the PS, and properly activating IL‐1R1 may be a potential way to the treatment of postictal problems. These results also suggest that the postictal side effects (e.g., drowsiness, nausea, and headache) need to be concerned in the development and use of AEDs related with IL‐1β system, such as IL‐1β‐converting enzyme inhibitors 36, 37 and some traditional Chinese medicine 38. Although we have no further data, the mechanism of IL‐1β attenuating the PS may be due to its effect on both glutamatergic and GABAergic transmission according to previous studies; that is, (1) IL‐1β may attenuate the GABAergic transmission by inhibiting GABA‐mediated Cl‐ fluxes 39, 40, (2) IL‐1β can enhance NMDA receptor‐mediated intracellular calcium increase by the activation of Src kinase‐mediated tyrosine phosphorylation of the NR2B subunit 41 and (3) IL‐1β also inhibits the astrocytic reuptake of glutamate 42.
In addition, previous studies mainly evaluated the PS using the seizure refractory phenomenon induced by intermittent MES 17, 30. However, this seizure refractory phenomenon is induced by several times of electroshock, which may impair other brain functions rather than enhance the mechanisms underlying the PS. In the present study, to minimize this influence, we modified the intermittent MES model by adopting a protocol that only induced a weak seizure refractory phenomenon in control mice. Furthermore, we also developed an intermittent kindling procedure for evaluating the seizure refractory phenomenon in kindled mice. We found it can clearly result in the seizure refractory phenomenon immediately after a generalized seizure in kindled mice. Indeed, a generalized seizure is sufficient to induce the seizure refractory phenomenon in either epileptic 2 or nonepileptic patients 43. Thus, this intermittent kindling model is prepared under epileptic condition and seems more close to clinical observations. Although the effect of IL‐1β on the both hippocampal kindling seizures and PS seems dose dependent in present study (10 ng IL‐1β acted stronger effect than 1 ng IL‐1β), a few recent studies showed IL‐1β may have a dose‐independent or even protective effect on amygdala‐kindling seizure 44. These contradictions may be due to IL‐1β many may act different roles in various brain areas. Additionally, several interesting findings may also be related to the PS, which may extend the significance of the PS. Firstly, kindling is a classical animal model supporting the “seizures beget seizures” phenomenon 45, which represents that each seizure increases the risk for further seizures. In contrast, the PS helps to inhibit further seizures. Here, we found IL‐1R1‐KO mice impaired kindling acquisition as well as enhanced the PS. Thus, these results supporting that inhibition of IL‐1R1 has antiepileptogenic effect 27, 46, and enhancing PS may be one possible mechanism. Secondly, in the present study, the postkindling ADTs were undetectable in most kindled IL‐1R1‐KO mice using the protocol with 1‐min interstimulation intervals, but they are all determined in all kindled WT mice. Interestingly, using the protocol with 5‐min interval, the postkindling ADTs of all kindled IL‐1R1‐KO mice were determined (Figure 4). It is likely some PS‐related mechanisms might be recruited by repeated subthreshold electrical stimulation, such as the release of adenosine 47.
Taken together, this study provides direct evidence that the PS is modulated by IL‐1β via its receptor IL‐1R1. Overactivation of IL‐1R1 by excessive amounts of IL‐1β may attenuate the PS and exacerbate seizures, while overinhibition of IL‐1R1 may prolong the postictal period, which is related to many postictal problems. Thus, it is likely IL‐1β is a key regulatory factor for the PS, and IL‐1R1 may be a potential target for adjuvant treatment of postictal problems.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. The expression of IL‐1β in mice hippocampus in preictal control and postictal depression condition.
Figure S2. IL‐1β alleviated the inhibition of breathing frequency during postictal depression in kindled mice.
Figure S3. The postictal EEG suppression duration has no correlation with the ADD.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (91332202, 81273492, and 81302749), the China Science Fund for Creative Research Groups (81221003), and the Program for Zhejiang Leading Team of S&T Innovation (2011R50014).
References
- 1. Loscher W, Kohling R. Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav 2010;19:105–113. [DOI] [PubMed] [Google Scholar]
- 2. Truccolo W, Donoghue JA, Hochberg LR, et al. Single‐neuron dynamics in human focal epilepsy. Nat Neurosci 2011;14:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. During MJ, Spencer DD. Adenosine: A potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 1992;32:618–624. [DOI] [PubMed] [Google Scholar]
- 4. Sato M, Moriwake T. Postictal seizure inhibition in amygdaloid‐kindled cats. Epilepsia 1984;25:545–550. [DOI] [PubMed] [Google Scholar]
- 5. Ziemann AE, Schnizler MK, Albert GW, et al. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 2008;11:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure‐associated respiratory dysfunction but not postictal apnea. Epilepsia 2012;53:825–831. [DOI] [PubMed] [Google Scholar]
- 7. Bozorgi A, Chung S, Kaffashi F, et al. Significant postictal hypotension: Expanding the spectrum of seizure‐induced autonomic dysregulation. Epilepsia 2013;54:e127–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case‐control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68:787–796. [DOI] [PubMed] [Google Scholar]
- 9. Poh MZ, Loddenkemper T, Reinsberger C, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 2012;78:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diamond ML, Ritter AC, Failla MD, et al. IL‐1beta associations with posttraumatic epilepsy development: A genetics and biomarker cohort study. Epilepsia 2014;55:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol 2011;7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pernot F, Heinrich C, Barbier L, et al. Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia 2011;52:2315–2325. [DOI] [PubMed] [Google Scholar]
- 13. Rijkers K, Majoie HJ, Hoogland G, Kenis G, De Baets M, Vles JS. The role of interleukin‐1 in seizures and epilepsy: A critical review. Exp Neurol 2009;216:258–271. [DOI] [PubMed] [Google Scholar]
- 14. Vezzani A, Granata T. Brain inflammation in epilepsy: Experimental and clinical evidence. Epilepsia 2005;46:1724–1743. [DOI] [PubMed] [Google Scholar]
- 15. Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL‐1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A 2000;97:11534–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Librizzi L, Noe F, Vezzani A, de Curtis M, Ravizza T. Seizure‐induced brain‐borne inflammation sustains seizure recurrence and blood‐brain barrier damage. Ann Neurol 2012;72:82–90. [DOI] [PubMed] [Google Scholar]
- 17. Jin CL, Zhuge ZB, Wu DC, et al. Lesion of the tuberomammillary nucleus E2‐region attenuates postictal seizure protection in rats. Epilepsy Res 2007;73:250–258. [DOI] [PubMed] [Google Scholar]
- 18. Fischer W, Kittner H, Regenthal R, Malinowska B, Schlicker E. Anticonvulsant and sodium channel blocking activity of higher doses of clenbuterol. Naunyn Schmiedebergs Arch Pharmacol 2001;363:182–192. [DOI] [PubMed] [Google Scholar]
- 19. Hu WW, Fang Q, Xu ZH, et al. Chronic h1‐antihistamine treatment increases seizure susceptibility after withdrawal by impairing glutamine synthetase. CNS Neurosci Ther 2012;18:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin M, Dai Y, Xu C, Wang Y, Wang S, Chen Z. Effects of meclofenamic acid on limbic epileptogenesis in mice kindling models. Neurosci Lett 2013;543:110–114. [DOI] [PubMed] [Google Scholar]
- 21. Xu ZH, Wu DC, Fang Q, et al. Therapeutic time window of low‐frequency stimulation at entorhinal cortex for amygdaloid‐kindling seizures in rats. Epilepsia 2010;51:1861–1864. [DOI] [PubMed] [Google Scholar]
- 22. Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates, 2nd edn San Diego: Academic Press, 2001. [Google Scholar]
- 23. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 24. Sato M, Racine RJ, McIntyre DC. Kindling: Basic mechanisms and clinical validity. Electroencephalogr Clin Neurophysiol 1990;76:459–472. [DOI] [PubMed] [Google Scholar]
- 25. Xu Z, Wang Y, Jin M, et al. Polarity‐dependent effect of low‐frequency stimulation on amygdaloid kindling in rats. Brain Stimul 2013;6:190–197. [DOI] [PubMed] [Google Scholar]
- 26. Vezzani A, Bartfai T, Bianchi M, Rossetti C, French J. Therapeutic potential of new antiinflammatory drugs. Epilepsia 2011;52(Suppl 8):67–69. [DOI] [PubMed] [Google Scholar]
- 27. Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL‐1beta production. Neurobiol Dis 2008;31:327–333. [DOI] [PubMed] [Google Scholar]
- 28. Claycomb RJ, Hewett SJ, Hewett JA. Neuromodulatory role of endogenous interleukin‐1beta in acute seizures: Possible contribution of cyclooxygenase‐2. Neurobiol Dis 2012;45:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vezzani A, Conti M, De Luigi A, et al. Interleukin‐1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J Neurosci 1999;19:5054–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long JB, Tortella FC. Effects of adrenalectomy and hypophysectomy on postictal seizure protection. Brain Res 1987;402:155–159. [DOI] [PubMed] [Google Scholar]
- 31. Ravizza T, Boer K, Redeker S, et al. The IL‐1beta system in epilepsy‐associated malformations of cortical development. Neurobiol Dis 2006;24:128–143. [DOI] [PubMed] [Google Scholar]
- 32. Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation‐mediated status epilepticus. Lancet Neurol 2011;10:99–108. [DOI] [PubMed] [Google Scholar]
- 33. Beniczky S, Jovanovic M, Atkins MD, et al. Postictal inhibition of the somatosensory cortex. Neurol Sci 2011;32:147–149. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt D, Noachtar S. Outlook: The postictal state–future directions for research. Epilepsy Behav 2010;19:191–192. [DOI] [PubMed] [Google Scholar]
- 35. Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia 2013;54:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravizza T, Lucas SM, Balosso S, et al. Inactivation of caspase‐1 in rodent brain: A novel anticonvulsive strategy. Epilepsia 2006;47:1160–1168. [DOI] [PubMed] [Google Scholar]
- 37. Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 2013;103:2–30. [DOI] [PubMed] [Google Scholar]
- 38. Ho TY, Tang NY, Hsiang CY, Hsieh CL. Uncaria rhynchophylla and rhynchophylline improved kainic acid‐induced epileptic seizures via IL‐1beta and brain‐derived neurotrophic factor. Phytomedicine 2014;21:893–900. [DOI] [PubMed] [Google Scholar]
- 39. Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 2008;22:797–803. [DOI] [PubMed] [Google Scholar]
- 40. Wang S, Cheng Q, Malik S, Yang J. Interleukin‐1beta inhibits gamma‐aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther 2000;292:497–504. [PubMed] [Google Scholar]
- 41. Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin‐1beta enhances NMDA receptor‐mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 2003;23:8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. NeuroImmunoModulation 2000;7:153–159. [DOI] [PubMed] [Google Scholar]
- 43. Nobler MS, Luber B, Moeller JR, et al. Quantitative EEG during seizures induced by electroconvulsive therapy: Relations to treatment modality and clinical features. I. Global analyses. J ECT 2000;16:211–228. [DOI] [PubMed] [Google Scholar]
- 44. Sayyah M, Beheshti S, Shokrgozar MA, et al. Antiepileptogenic and anticonvulsant activity of interleukin‐1 beta in amygdala‐kindled rats. Exp Neurol 2005;191:145–153. [DOI] [PubMed] [Google Scholar]
- 45. Berg AT, Shinnar S. Do seizures beget seizures? An assessment of the clinical evidence in humans. J Clin Neurophysiol 1997;14:102–110. [DOI] [PubMed] [Google Scholar]
- 46. Noe FM, Polascheck N, Frigerio F, et al. Pharmacological blockade of IL‐1beta/IL‐1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis 2013;59:183–193. [DOI] [PubMed] [Google Scholar]
- 47. Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation‐mediated attenuation of tremor. Nat Med 2008;14:75–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The expression of IL‐1β in mice hippocampus in preictal control and postictal depression condition.
Figure S2. IL‐1β alleviated the inhibition of breathing frequency during postictal depression in kindled mice.
Figure S3. The postictal EEG suppression duration has no correlation with the ADD.


