Abstract
Membrane asymmetry is a key organizational feature of the plasma membrane. Type IV P-type ATPases (P4-ATPases) are phospholipid flippases that establish membrane asymmetry by translocating phospholipids, such as phosphatidylserine (PS) and phospatidylethanolamine, from the exofacial leaflet to the cytosolic leaflet. Saccharomyces cerevisiae expresses five P4-ATPases: Drs2, Neo1, Dnf1, Dnf2, and Dnf3. The inactivation of Neo1 is lethal, suggesting Neo1 mediates an essential function not exerted by the other P4-ATPases. However, the disruption of ANY1, which encodes a PQ-loop membrane protein, allows the growth of neo1Δ and reveals functional redundancy between Golgi-localized Neo1 and Drs2. Here we show Drs2 PS flippase activity is required to support neo1Δ any1Δ viability. Additionally, a Dnf1 variant with enhanced PS flipping ability can replace Drs2 and Neo1 function in any1Δ cells. any1Δ also suppresses drs2Δ growth defects but not the loss of membrane asymmetry. Any1 overexpression perturbs the growth of cells but does not disrupt membrane asymmetry. Any1 coimmunoprecipitates with Neo1, an association prevented by the Any1-inactivating mutation D84G. These results indicate a critical role for PS flippase activity in Golgi membranes to sustain viability and suggests Any1 regulates Golgi membrane remodeling through protein-protein interactions rather than a previously proposed scramblase activity.
Keywords: phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, trafficking, Golgi apparatus, transport
Phospholipid asymmetry is a fundamental property of the eukaryotic cell plasma membrane. For instance, phosphatidylcholine (PC) and sphingolipids are enriched in the exofacial leaflet, and aminophospholipids such as phosphatidylserine (PS) and phosphatidylethanolamine (PE) are confined to the cytofacial leaflet (1–3). Membrane asymmetry is regulated through the transbilayer movement of amphipathic phospholipids by the concerted activities of three types of transporters: floppases, flippases, and scramblases (4). Floppases belong to the ABC transporter family and translocate phospholipid substrates to the exofacial leaflet of membranes (5). Flippases in the type IV P-type ATPase (P4-ATPase) family transport primarily aminophospholipids (PS and PE) to the cytofacial leaflet of membranes in an ATP-dependent manner (6–8), although some members transport either PC, lyso-PC, or glucosylceramide (9–12). Membrane asymmetry is thought to be produced from the net effect of the relatively slow and nonspecific outward transport of phospholipids by ABC transporters, coupled with a rapid inward transport of aminophospholipids (13). Scramblases in the TMEM16F and Xkr8 protein families are phospholipid channels that open in response to a Ca2+ influx (TMEM16F) or caspase cleavage (Xkr8) to dissipate the phospholipid gradient (14–17). These scramblases play important roles in blood clotting and the removal of apoptotic cell corpses, respectively. In mammals, the loss of P4-ATPase activity is associated with liver disease (18), diabetes (19–21), hearing loss (22), increased risk of myocardial infarction (23, 24), hemolytic anemia (25), neurodegeneration (26), and intellectual disability (27). Thus, perturbations in membrane asymmetry likely contribute to these pathologies.
Membrane remodeling events within the Golgi complex play an important role in establishing plasma membrane asymmetry. Sphingolipids and glycosphingolipids are synthesized in the luminal (exofacial) leaflet of the Golgi and are exported by vesicular transport to the exofacial leaflet of the plasma membrane (28, 29). Aminophospholipids are flipped by P4-ATPases from the luminal leaflet of the Golgi to the cytosolic leaflet and subsequently move to the cytosolic leaflet of the plasma membrane by either vesicular or nonvesicular routes. These events are also coupled to sterol loading in late compartments of the Golgi complex to transition the bilayer from an ER-like composition and organization at the cis-face to a plasma membrane-like composition and organization at the trans-face (30, 31). P4-ATPases also localize to the plasma membrane and endosomal compartments to ensure asymmetry is maintained as membrane fluxes through the endocytic pathway and recycles back to the plasma membrane (32).
While differences in subcellular localization may confer unique functions, it remains unclear why a single-celled organism such as Saccharomyces cerevisiae expresses five different P4-ATPases. Dnf1, Dnf2, Dnf3, and Drs2 form an essential group with both overlapping and nonoverlapping functions. For example, cells harboring drs2Δ grow well at 30°C but fail to grow at 20°C or below because the Dnf P4-ATPases can partially compensate for the loss of Drs2 at higher temperatures but not at lower temperatures. Even at permissive growth temperatures, drs2Δ cells display protein-trafficking defects between Golgi and endosomal membranes. Drs2 localizes to the trans-Golgi network, but Neo1, Dnf1, and Dnf3 also localize significantly to the trans-Golgi network and yet fail to support these trafficking pathways in the absence of Drs2 (33–35). Likewise, neo1Δ cells cannot grow at any temperature, suggesting that this essential P4-ATPase has a unique function that none of the other P4-ATPases can perform (35, 36). Consistently, Drs2 and Neo1 regulate different vesicular transport pathways: early-endocytic/late-secretory and early-secretory pathways, respectively (35, 37–39).
The phospholipid substrate specificity of budding yeast P4-ATPases appears to be the major determinant of their specific roles in the cell. Drs2 and its mammalian orthologues (ATP8A1 and ATP8A2) are primarily PS flippases, although they are also capable of flipping PE (7, 8, 40). drs2Δ cells, accordingly, display a loss of plasma membrane PS and PE asymmetry (41). Substrates of Dnf1 and Dnf2 include lyso-PC and lyso-PE, phospholipids lacking one of the two fatty acyl chains, and glucosylceramide, which is not endogenously produced (10, 12). Thus, these P4-ATPases may function in nutrient scavenging and membrane repair (10). We previously mapped residues that conferred substrate specificity differences between Dnf1 and Drs2 and identified mutations that alter their specificity (12, 42–45). Separation-of-function mutations in Drs2 were isolated that abrogate PS recognition without measurably perturbing PE recognition (Drs2PS−). Conversely, gain-of-function mutations were isolated in Dnf1 that allow it to flip diacyl-PS without perturbing the recognition of its normal substrates (Dnf1PS+) (42–44). Remarkably, drs2Δ cells expressing Drs2PS− display trafficking defects, but normal trafficking and growth at low temperatures are restored in drs2Δ cells expressing Dnf1PS+ (but not Dnf1) (30, 38). These observations imply that Drs2 is the primary PS flippase in the cell, and the other P4-ATPases lack sufficient PS flippase activity to compensate for the loss of Drs2.
However, both Drs2 and Neo1 (orthologues of mammalian ATP9A and ATP9B) are involved in regulating PS/PE plasma membrane asymmetry, and these P4-ATPases become functionally redundant when ANY1 is deleted (39, 46, 47). The inactivation of Neo1ts more substantially perturbs PE asymmetry, but these cells also aberrantly expose PS (39). By contrast, the inactivation of the Caenorhabditis elegans Neo1 orthologue Tat-5 causes exposure of PE without the loss of PS asymmetry (48). The possibility that Neo1 and Drs2 have similar substrate preferences is supported by the observation that the PQ-loop membrane protein Any1 enforces separate functions for Drs2 and Neo1 in the Golgi/endosomal membranes (47, 49). In the absence of Any1, PS and PE membrane asymmetry is restored in neo1ts (neo1ts any1Δ) to WT levels. Moreover, Drs2 can bypass the essential requirement for Neo1, as neo1Δ any1Δ viability depends on a WT copy of DRS2 (47). Any1 was proposed to function as a scramblase in the Golgi, acting to dissipate the PS/PE gradients formed by Drs2 and Neo1 to segregate their functions (47). However, the mechanism by which Any1 antagonizes Neo1 has not been determined.
In this study, we provide evidence that the essential role of Drs2 and Neo1 in Golgi membranes lacking Any1 is to flip PS. These any1Δ strains also allowed us to individually compare the influence of Drs2, Neo1, or Dnf1PS+ variants on membrane asymmetry, and these studies suggest a comparable ability to flip PS but that Neo1 has the greatest impact on PE asymmetry. The overexpression of Any1 is toxic to cells deficient in P4-ATPase activity, but excess Any1 does not disrupt membrane asymmetry. This result suggests that Any1 is not a scramblase and might antagonize Neo1 by a different mechanism. We found that Any1 interacts with Neo1, suggesting that it might inhibit Neo1 activity through this interaction. Thus, this study supports the proposed PE/PS flippase activity of Neo1 reported earlier and defines a protein-interaction network that facilitates membrane remodeling events in the secretory pathway.
MATERIALS AND METHODS
Reagents
Papuamide A (PapA) was purchased from the University of British Columbia Depository (Vancouver, Canada). Duramycin, quinacrine dihydrochloride, calcofluor white, neomycin sulfate, monoclonal FLAG antibody, and EzView FLAG beads were purchased from Sigma-Aldrich (St. Louis, MO). 5-Fluoroorotic acid (5-FOA) was purchased from US Biologicals (Salem, MA). Monoclonal GST (clone D1 and F5) antibody was purchased from Vanderbilt Antibody and Protein Resource (Nashville, TN), and polyclonal GST (clone D1 and F5) antibody was purchased from Torrey Pines Biolabs (Secaucus, NJ). Anti-Arf1, anti-Mnn1, and anti-Drs2 antibodies have been previously described (34). IRDye 800CW goat anti-rabbit IgG (H+L) and Starbrite Blue goat anti-rabbit IgG (H+L) secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE) and Bio-Rad (Hercules, CA), respectively.
Strains and plasmid construction
The strains and plasmids used are listed in supplemental Table 1. Yeast strains were grown and transformed using standard media and transformation techniques (50–52). For growth and plasmid-shuffling assays, 0.1 OD600 cells and 10-fold serial dilutions were spotted onto synthetic media or synthetic media containing 5-FOA and other toxic agents. Yeast knockout and knock-in strains were generated as previously described (53). For coimmunoprecipitation experiments, a GST-tag cassette was amplified from pFA6a-GST-His3MX6 and integrated at the 3′ end of ANY1 in pRS313-ANY1 or pRS313-Any1[D84G] plasmids using Gibson assembly (54). pRS315-NEO1-9XGLY-5XFLAG was generated by the Gibson assembly-mediated insertion of the 9XGLY-5XFLAG tag into pRS315-PNEO1-NEO1. pRS315-NEO1-9XGLY-5XFLAG or pRS315-NEO1 along with pRS313-ANY1-GST or pRS313-Any1[D84G]GST were transformed into MTY10S (neo1Δ any1Δ). Any1 or Neo1 mutants were generated using 3-fragment and 2-fragment Gibson assembly according to the manufacturer’s instructions.
Toxin-sensitivity assays
For toxin-sensitivity assays, 0.1 OD600 mid-log cells were distributed to each well of a 96-well plate with or without the toxin using four independent biological replicates. Plates were incubated at 30°C for 20 h. Concentrations of the cells were measured in OD600/ml with a Multimode Plate Reader Synergy HT (BioTek, Winooski, VT). Sigmodial curve-fitting modality from GraphPad Prism7 was used to fit the data point from all samples when R2 values were equal to or greater than 0.8 for all samples.
Immunoprecipitation and Western blot analysis
Mid-log-phase cells were lysed and subjected to Western blot analysis as described previously (39). For expression analysis, mouse mono-clonal GST (1:1000) and monoclonal FLAG (1:5000) antibodies were used. Total protein concentrations were assessed by the Bio-Rad stain-free gel system. Both Neo1 and Any1 tend to form SDS-resistant aggregates and migrate as high-molecular-weight smears by SDS-PAGE. To mitigate this problem, samples were heated at 50°C for 10 min in SDS/urea sample buffer. In addition, freezing and thawing samples seem to exacerbate the gel-migration artifact; thus, samples were stored at 4°C prior to SDS-PAGE. Total lysates from yeast spheroplasts under native conditions were prepared as described previously (34). Protein concentrations of total lysates used for immunoprecipitations were quantified using a BCA assay. Immunoprecipitations of FLAG-tagged bait were performed from 10 mg of total input (native cell lysate) using 50 µl EzView FLAG beads for 2 h at 4°C. The beads were washed three times with washing buffer I and two times with washing buffer II (34) prior to elution with SDS/urea sample buffer and SDS-PAGE.
RESULTS
PS flippase activity is required in any1-deficient Golgi membranes
Neo1, Drs2, and Dnf1 have nonredundant functions in the Golgi complex despite their similar effects on plasma membrane asymmetry (39) (see Fig. 1A and supplemental Table 2 for an overview of the proteins analyzed in this work). Removing Any1 reveals a redundancy between Neo1 and Drs2, although it was not clear whether a PS or PE flippase activity, or both, were required to support viability in a neo1Δ any1Δ drs2Δ strain. To test this, we expressed WT Neo1, WT Drs2, Drs2PS− (Drs2[QQ→GA]), WT Dnf1, or Dnf1PS+ (Dnf1[N550S]) in neo1Δ drs2Δ any1Δ cells expressing WT NEO1 on a URA3-marked plasmid. We counter-selected pURA3-NEO1 on 5-FOA plates that maintained selection for the HIS3-marked plasmids bearing the P4-ATPase variants to be tested and incubated the plates at 30°C (Fig. 1B). As previously reported (47), WT Neo1 or Drs2 was sufficient to complement neo1Δ drs2Δ any1Δ synthetic lethality and support growth, but the empty vector and extra copy of WT Dnf1 failed to suppress this synthetic lethality. Importantly, while Drs2PS− failed to complement the growth defect, Dnf1PS+ fully suppressed the neo1Δ drs2Δ any1Δ synthetic lethality (Fig. 1A). Thus, bypassing neo1Δ in cells lacking Any1 requires a PS flippase activity that can be provided by either Drs2 or Dnf1.
Fig. 1.
PS translocation in Golgi membranes is required for viability. A: Interacting network of proteins important for membrane organization whose function in membrane asymmetry and cell viability is explored in this study. B: Neo1, Drs2, and Dnf1[N550S] can support the growth of neo1Δ drs2Δ any1Δ cells. neo1Δ drs2Δ any1Δ + pRS416-NEO1 (MTY10-615M2DS) expressing single-copy (cen) NEO1 (pRS313-NEO1), empty vector (pRS313), cen DRS2 (pRS313-DRS2), cen Drs2[QQ→GA] (pRS313-Drs2[QQ→GA]), cen DNF1 (pRS313-DNF1), or cen Dnf1[N550S] (pRS313-Dnf1[N550S]), respectively, were spotted onto minimal media plates, and the pRS416-NEO1 plasmid was counter-selected on 5-FOA. C: Loss of Kes1 failed to suppress the synthetic lethality of neo1Δ drs2Δ any1Δ. neo1Δ drs2Δ any1Δ kes1Δ + pRS416-NEO1 (MTY10-615M2DS) expressing single-copy (cen) NEO1 (pRS313-NEO1), empty vector (pRS313), cen DRS2 (pRS313-DRS2), and cen Drs2[QQ→GA] (pRS313-Drs2[QQ→GA), respectively, were spotted onto minimal media plates, and the pRS416-NEO1 plasmid was counter-selected on 5-FOA. These images are representative of four independent growth assays.
The cold-sensitive growth defect of drs2Δ can be fully suppressed by deleting KES1/OSH4, which encodes an ergosterol/phosphatidylinositol-4-phosphate exchange protein involved in Golgi membrane remodeling (Fig. 1A) (31, 55). Therefore, we tested whether the synthetic lethality between neo1Δ and drs2 alleles could be suppressed by kes1Δ. The same plasmid-shuffling strategy was used to express WT Neo1, WT Drs2, Drs2PS−, and empty vector control in neo1Δ any1Δ drs2Δ and neo1Δ any1Δ drs2Δ kes1Δ cells (Fig. 1B). In this experiment, kes1Δ failed to suppress the neo1Δ any1Δ drs2 synthetic lethality (empty vector and Drs2[QQ→GA]), and only WT Neo1 or Drs2 were able to support the growth of neo1Δ drs2Δ any1Δ kes1Δ cells (Fig. 1C).
Loss of Any1 does not suppress membrane asymmetry defects of P4-ATPase null mutants
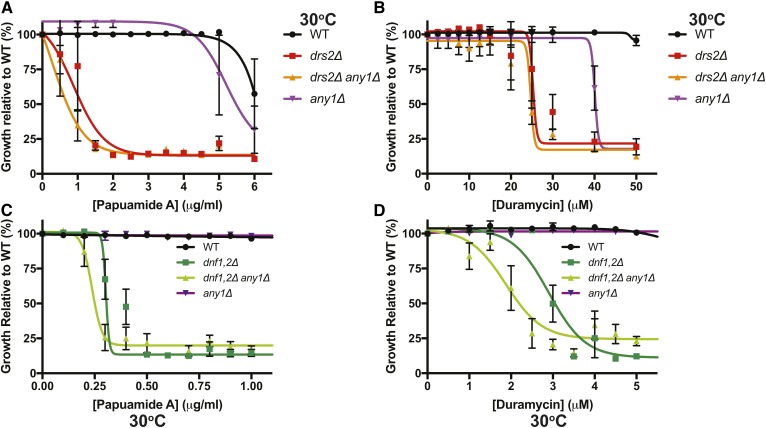
We previously found that neo1ts mutants expose both PS and PE in the exofacial leaflet at semipermissive growth temperatures. The loss of asymmetry can be probed by hypersensitivity to pore-forming toxins that specifically target PS (PapA) or PE (Duramycin) exposed on the exofacial leaflet of the plasma membrane (56, 57). The loss of neo1ts plasma membrane asymmetry is completely suppressed in neo1ts any1Δ cells (47). Here we examined membrane asymmetry in strains carrying neo1Δ, any1Δ, and drs2Δ null alleles. WT and any1Δ cells were resistant to both PS-binding (PapA) and PE-binding (Duramycin) toxins. Although any1Δ cells were partially sensitive to Duramycin at the highest concentration tested (Fig. 2A, B). The neo1Δ drs2Δ any1Δ cells expressing Neo1, Drs2, or Dnf1PS+ were hypersensitive to PapA and showed a similar level of sensitivity with Dnf1[N550S], displaying a slightly lower capacity to establish PS asymmetry relative to Drs2 or Neo1 (Fig. 2A). Thus, none of these P4-ATPases could prevent PS exposure in the plasma membrane outer leaflet independently in this strain background. A comparison of NEO1 ANY1 drs2Δ to NEO1 any1Δ drs2Δ seemed to show that any1Δ weakly suppressed drs2Δ PS exposure, but these data were not significantly different (Fig. 2A). Note that we cannot analyze the neo1Δ ANY1 DRS2 strain because it is inviable. Dnf1[N550S], Drs2, and Neo1 conferred increasing resistance to Duramycin, with Neo1 restoring PE asymmetry to a significantly greater extent than Drs2 or Dnf1[N550S] in neo1Δ drs2Δ any1Δ cells. Again, none of the P4-ATPases were able to restore PE asymmetry to WT levels on their own, and any1Δ did not suppress PE exposure caused by drs2Δ (Fig. 2B). These findings indicate that Drs2, Neo1, and Dnf1PS+ have a near-equivalent ability to support the growth of neo1Δ any1Δ drs2Δ cells and a comparable ability to support PS asymmetry, whereas Neo1 has a more substantial impact on PE asymmetry than Drs2 or Dnf1PS+.
Fig. 2.
None of the Golgi P4-ATPases can individually restore membrane asymmetry when expressed in neo1Δ any1Δ drs2Δ cells. The strains indicated were grown with increasing concentrations of PapA (A) or Duramycin (B) to assess the exposure of PS or PE, respectively. Growth (OD600) after 20 h at 30°C is plotted relative to untreated WT cells (n ≥ 4; error bars indicate ± SEM).
To further explore these genetic relationships, we tested whether any1Δ could suppress growth phenotypes in strains deficient for several different P4-ATPases. Even though any1Δ did not measurably suppress the loss of membrane asymmetry in drs2Δ, it was able to partially suppress the cold-sensitive growth defect of drs2Δ (Fig. 3A). Hypomorphic neo1-1 and neo1-2 alleles are synthetically lethal with drs2Δ (37) (Fig. 3A), but any1Δ effectively suppressed this synthetic lethality. The more stringent neo1-1 allele still displayed a temperature-sensitive growth phenotype in the neo1-1 any1Δ drs2Δ background, while neo1-2 was suppressed across the full growth temperature range (Fig. 3A). However, any1Δ could not suppress the lethality caused by drs2Δ dnf1,2,3Δ (Fig. 3B). Dnf1 and Dnf2 form heterodimers with Lem3 (Fig. 1A, supplemental Table 2), and lem3Δ cells grow well but are deficient for both Dnf1 and Dnf2 activities. Here we show that lem3Δ is synthetically lethal with neo1-1 and neo1-2 (Fig. 3C, top), but the growth defects of neo1ts lem3Δ and neo1Δ lem3Δ are substantially, but not completely, suppressed by any1Δ (Fig. 3C, bottom).
Fig. 3.
any1Δ genetic interactions with P4-ATPase mutants. A: any1Δ suppresses the cold-sensitive growth of drs2Δ, high-temperature growth defects of neo1-1 and neo1-2, and the synthetic lethality between drs2 and neo1ts alleles. Both neo1-1ts and neo1-2ts can support the viability of any1Δ drs2Δ cells at all temperatures tested except for 37°C. B: Loss of Any1 failed to suppress the synthetic lethality of dnf1,2,3Δ drs2Δ. C: Loss of Any1 can suppress the synthetic lethality of neo1Δ lem3Δ or neo1ts lem3Δ at all temperatures tested except for 37°C. These images are representative of four independent growth assays.
To test the influence of Any1 on the loss of membrane asymmetry caused by Drs2 and Dnf deficiency, we performed pore-forming toxin-sensitivity assays with WT, drs2Δ, drs2Δ any1Δ, dnf1Δ dnf2Δ, dnf1Δ dnf2Δ any1Δ, and any1Δ cells. drs2Δ and drs2Δ any1Δ were equally sensitive to PapA and equally sensitive to Duramycin (Fig. 4A, B). We further examined membrane asymmetry in WT, dnf1Δ dnf2Δ, and dnf1Δ dnf2Δ any1Δ cells. Again, no significant difference in sensitivity to the toxins was observed with or without Any1 (Fig. 4C, D). In summary, any1Δ suppresses growth and membrane asymmetry defects caused by hypomorphic neo1 alleles and bypasses the essential function of Neo1 in a Drs2-dependent manner. any1Δ modestly suppresses the cold-sensitive growth defect of drs2Δ but does not enhance the ability of the remaining P4-ATPases to restore PS/PE asymmetry in these cells. Likewise, any1Δ does not enhance the ability of remaining P4-ATPases to restore membrane asymmetry defects caused by dnf1,2Δ.
Fig. 4.
Deletion of Any1 failed to suppress the loss of PS and PE asymmetry in drs2Δ or dnf1,2Δ mutants, respectively. The strains indicated were grown with increasing concentrations of Papa A (A, C) or Duramycin (B, D) to assess the exposure of PS or PE, respectively. Growth (OD600) after 20 h at 30°C is plotted relative to untreated WT cells (n ≥ 4; error bars indicate ± SEM).
Any1 overexpression is toxic to P4-ATPase mutants
We also tested whether the overexpression of Any1 would perturb cell growth and/or alter membrane organization, as would be expected if it inhibits Neo1 and/or is a scramblase. ANY1 was placed under transcriptional control of the GAL promoter (PGAL), which is repressed by glucose and strongly induced by galactose. This PGAL-ANY1 construct was expressed in WT and neo1-2 any1Δ strains, and the growth on galactose or glucose media was examined over a range of temperatures (Fig. 5A). Any1 overexpression caused a mild growth defect in WT cells relative to the empty vector control. In contrast, neo1-2 was very sensitive to Any1 overexpression and failed to grow at any temperature on the galactose plates. The robust growth of neo1-2 any1Δ on glucose plates as well as galactose plates with empty vector at high temperatures shows the strong suppression conferred by the absence of Any1. The deletion of ANY1 also suppresses growth defects caused by mutations in the Neo1-interacting proteins Dop1 and Mon2 (Figs. 1A,). dop1-1 is temperature-sensitive for growth, but the dop1-1 any1Δ double mutant (empty vector) grows well at 37°C (47) (Fig. 5A). Similarly, mon2Δ mutants grow slowly, but this phenotype is suppressed in mon2Δ any1Δ strains (47). Like neo1-2, the growth of dop1-1 and mon2Δ is severely inhibited by Any1 overexpression (Fig. 5A). We also found that the growth of drs2Δ and dnf1,2Δ strains is inhibited by Any1 overexpression (Fig. 5B), but not as potently as with neo1, dop1, and mon2 mutants. Thus, cells deficient for Neo1/Dop1/Mon2 function are most sensitive to changes in Any1 levels within the cell.
Fig. 5.
ANY1 overexpression partially inhibits the growth of drs2Δ and dnf1,2Δ mutants but is most detrimental to neo1ts, dop1ts, and mon2Δ mutants. A: ANY1 overexpression completely inhibits the growth of neo1ts any1Δ, dop1ts any1Δ, and mon2Δ any1Δ mutants. Growth assays were performed with WT, neo1ts any1Δ, dop1ts any1Δ, and mon2Δ any1Δ mutants expressing empty vector or pBY011-ANY1 (PGAL1-ANY1) at 27, 34, and 37°C. B: ANY1 overexpression partially inhibits the growth of drs2Δ and dnf1,2Δ mutants to a greater extent than WT cells. WT, drs2Δ, and dnf1,2Δ expressing empty vector or pBY011-ANY1 (PGAL1-ANY1) were spotted onto the minimal media plates containing glucose or galactose at 30°C. These images are representative of four independent growth assays.
If Any1 were functioning as a scramblase, we might expect that overexpression would cause a loss of membrane asymmetry. However, WT and drs2Δ showed no difference in papuamide B sensitivity between cells harboring PGAL-ANY1 and empty vector grown on galactose (Fig. 6A, B). WT cells harboring PGAL-ANY1 displayed a small increase in sensitivity to Duramycin, although no significant difference was observed for drs2Δ cells with or without ANY1 overexpression (Fig. 6C,). These experiments suggest that if Any1 is a scramblase or phospholipid transporter of some type, it may be specific for PE.
Fig. 6.
ANY1 overexpression induces a modest loss of PE asymmetry but does not increase PS exposure in WT or drs2Δ cells. A, B: ANY1 overexpression does not induce a loss of PS asymmetry in WT and drs2Δ cells. C, D: ANY1 overexpression induces a partial but significant loss of PE asymmetry in WT and drs2Δ cells. WT and drs2Δ cells expressing empty vector (pRS416) or pRS416-PGAL1-ANY1 were grown to mid-log phase in the minimal media containing glucose. Cells (0.2 OD600) were shifted to minimal medium containing galactose or glucose (control) in the presence of pore-forming toxins PapA or Duramycin. Growth relative to the vehicle control was plotted. Student’s t-test was performed for each concentration tested between WT and drs2Δ cells (n ≥ 2).
Interaction between Neo1 and Any1
Two spontaneous point mutations, G80R and D84G, were identified in Any1 that were capable of suppressing mon2Δ growth defects (47). Interestingly, we found that any1-G80R did not suppress neo1Δ, but any1-D84G suppressed neo1Δ as effectively as deleting the ANY1 gene (Fig. 7A). We also overexpressed these Any1 mutants in WT, drs2Δ, and dnf1,2Δ cells using the strong GAL1 promoter. The PGAL-ANY1 construct used in this experiment was on a different vector backbone than the construct used in Fig. 5 and caused a more substantial growth defect in WT cells grown on galactose (compare Fig. 7B to). This is likely due to slight differences in expression between the two constructs. In contrast, PGAL-ANY1[D84G] caused only a minor growth defect in WT and dnf1,2Δ cells but was still able to perturb the growth of drs2Δ cells. Again, the influence of PGAL-ANY1[G80R] expression on the growth of these strains was indistinguishable from PGAL-ANY1. These results indicate D84G partially inactivates Any1 function in suppressing the growth of WT or dnf1,2Δ cells, while the G80R variant is fully functional in these assays. We suspect that the drs2Δ cells are more sensitive to Neo1 inhibition by Any1, which is why Any1[D84G] is still capable of inhibiting the growth of drs2Δ.
Fig. 7.
Effect of Any1 point mutations on its ability to antagonize Neo1 and inhibit the growth of P4-ATPase mutants. A: Any1[D84G] suppressed neo1Δ lethality, whereas Any1[G80R] inhibited growth comparably to WT ANY1. Plasmid shuffling assays were performed with neo1Δ any1Δ + pRS416-NEO1 (MTY10) expressing single copy NEO1 (pRS313-NEO1), empty vector (pRS313), ANY1 (pRS313-ANY1), ANY1[D84G] (pRS313-Any1[D84G]) or ANY1[G80R] (pRS313-Any1[G80R]); each strain was then spotted onto minimal media plates (SD) and the pRS416-NEO1 plasmid was counter selected on 5-fluorotic acid (5-FOA). B: Overexpression of ANY1 using pRS416-PGAL1-ANY1 construct inhibits the growth of WT, drs2Δ, and dnf1,2Δ mutants. WT, drs2Δ, and dnf1,2Δ expressing pRS416 (empty vector) or pRS416-PGAL1-ANY1 were spotted onto the minimal media plates containing glucose or galactose at 30°C. C, D: Growth inhibition due to ANY1 overexpression is suppressed by the D84G mutation but not the G80R mutation.
To test for a physical interaction between Any1 and Neo1, we immunoprecipitated Neo1-5XFLAG from cells expressing either Any1-GST or Any1[D84G]GST (58). The Any1-GST and Any1[D84G]GST variants were expressed at comparable levels in these cells (Fig. 8A). An interaction between Neo1 and Any1 can be clearly detected in the immunoprecipitates from Neo1-FLAG cells relative to the control sample from cells lacking the 5XFLAG tag (Fig. 8B). Strikingly, the inactivating D84G mutation completely abrogated the Neo1-Any1 interaction. A minor fraction of total Any1 in the cell immunoprecipitated with Neo1-FLAG, and the recovery of Any1 was only slightly enhanced by the addition of a membrane-permeable cross-linker (DSP) to the cells prior to lysis and immunoprecipitation. We also probed these Neo1-FLAG immunoprecipitates with anti-Arf1 as an additional control and found no significant difference in the background binding of Arf1 to the beads with or without Neo1-FLAG (Fig. 8B). Thus, we identify a weak but significant association between Neo1 and Any1 that is disrupted by the D84G mutation in Any1.
Fig. 8.
Any1 physically interacts with Neo1, and the [D84G] mutation abolishes this interaction. A: Expression of Neo1-FLAG, Any1-GST, and Any1[D84G]GST in cells used for coimmunoprecipitation experiments. neo1Δ any1Δ (MTY10S) cells expressing pRS315-NEO1 or pRS315-NEO1-5XFLAG along with pRS313-ANY1-GST or pRS313-Any1[D84G]GST were lysed in SDS/urea and probed for Neo1-FLAG and Any1-GST expression. B: Any1 interacts with Neo1. neo1Δ any1Δ (MTY10S) cells expressing pRS315-NEO1 or pRS315-NEO1-5XFLAG along with pRS313-ANY1-GST or pRS313-Any1[D84G]GST were spheroplasted and lysed in the presence of detergent. Immunoprecipitations were performed using these total lysates in the presence or absence of the DSP cross-linker. Immunoprecipitations were also probed for Arf1 as a negative control. Immunoprecipitations were performed two independent times.
DISCUSSION
Regulating membrane asymmetry requires a complex network of membrane remodeling machineries (Fig. 1A). P4-ATPases are membrane remodelers that generate a steep gradient of specific glycerophospholipids in biological membranes. Prior studies have shown that the Golgi P4-ATPases Neo1 and Drs2 execute nonredundant biological functions despite their apparently similar substrate preferences and localizations, and the PQ-loop protein Any1 somehow enforces these separate functions (39). In this study, we found that PS flippase activity is essential in supporting the viability of neo1Δ drs2Δ any1Δ cells. Even a Dnf1PS+ variant (Dnf1[N550S]) is capable of providing the PS flippase activity required for viability in this background, while WT Dnf1 or a Drs2PS− variant cannot. While none of the flippases can fully restore PS or PE asymmetry in neo1Δ drs2Δ any1Δ mutant cells, Drs2 and Neo1 establish an equivalent degree of PS asymmetry. In contrast, Neo1 displays a greater ability than Drs2 or Dnf1PS+ in preventing PE exposure in the extracellular leaflet in this background.
Our data show that PS flipping by a P4-ATPase in the Golgi/endosomal system of budding yeast is essential for growth, yet strains lacking the sole PS synthase (cho1Δ) are viable. How do we explain this apparent discrepancy? We favor the idea that PS in the lumenal leaflet antagonizes vesicle budding from Golgi membranes, while the translocation of this negatively charged phospholipid and its concentration in the cytosolic leaflet promotes vesicle budding. Failure to flip PS will increase luminal leaflet PS and reduce cytosolic leaflet PS, with both effects likely contributing to the strong trafficking defect observed in drs2 and neo1 mutants (37–41). The cho1Δ mutant would obviously lack the luminal leaflet PS, thus relieving negative effects, and compensatory changes in the lipidome to this perturbation might provide sufficient anionic phospholipids in the cytosolic leaflet to support vesicular transport, which appears normal in this mutant (40). In addition, the transport of other phospholipid substrates by Drs2 and Neo1 may increase significantly in cho1Δ cells in the absence of competition by PS, providing another compensatory mechanism to support vesicular transport.
We have previously shown a requirement for PS flipping to support protein trafficking in the Golgi/endosomal system using the same Drs2PS− and Dnf1PS+ variants described here (38). PS flipping increases the positive curvature (bending into the cytosol) and negative charge needed to recruit the ArfGAP Gcs1 via its ArfGAP lipid-packing sensor motif onto Golgi/endosomal membranes from the cytosol (38). In mammalian cells, PS flipping by ATP8A1 or ATP8A2 facilitates the recruitment of EHD1, a membrane fission factor, to the cytosolic leaflet of recycling endosomes (59). Thus, the concentration of PS in the cytosolic leaflet has positive roles in vesicle budding. A negative role for PS in the luminal leaflet is supported by the observation that endocytic recycling defects in the C. elegans tat-1 mutant (orthologue of Drs2/ATP8A1/ATP8A2) are suppressed by the depletion of PS synthase (60). This result suggests that the accumulation of PS in the luminal leaflet of tat-1 endosomes inhibits vesicle budding and the depletion of PS can relieve this inhibition. In budding yeast, drs2Δ cho1Δ cells grow very poorly and transport defects are not suppressed (40), suggesting that the loss of PS in the luminal leaflet is insufficient to overcome the deficit in the cytosolic leaflet in this case.
The loss of Any1 has been shown previously to suppress deficiencies in Neo1 and Drs2/Dnf P4-ATPases (47, 49). Yamamoto et al. (49) found that any1Δ (also called cfs1Δ) suppressed drs2Δ and cdc50Δ cold-sensitive growth and the synthetic lethality of lem3Δ cdc50Δ crf1Δ, astrain deficient for the β-subunits needed for the activity of Dnf1-Lem3, Dnf2-Lem3, Dnf3-Crf1, and Drs2-Cdc50 heterodimers (supplemental Table 2). On the contrary, we found that the loss of Any1 failed to suppress the dnf1,2,3Δ drs2Δ synthetic lethality, a mutant that should be equivalent to lem3Δ cdc50Δ crf1Δ. This raises the possibility that lem3Δ cdc50Δ crf1Δ cells retain some residual activity of P4-ATPase α-subunits to allow suppression by any1Δ/cfs1Δ. However, it is also possible that some minor difference in the strain background was responsible for the different results, particularly considering that lem3Δ cdc50Δ crf1Δ any1Δ cells grew very slowly (49). We also found that neo1 alleles are synthetically lethal with lem3Δ, but neo1Δ any1Δ lem3Δ cells grow similarly to WT and must rely on Drs2 for growth. In total, these genetic interactions suggest that Any1 segregates the functions of multiple P4-ATPases and eliminating Any1 allows Drs2, in particular, to carry out the essential function of the P4-ATPase group.
The deletion of KES1 or ANY1 similarly suppresses the growth and trafficking defects of drs2Δ/cdc50Δ mutants (30, 31), raising the possibility that Any1 and Kes1 are performing comparable tasks. kes1Δ mutants display a substantial increase in the anionic phosphatidylinositol-4-phosphate in the cytosolic leaflet, which is likely the reason kes1Δ suppresses drs2 and sec14. any1Δ does not suppress sec14and kes1 does not suppress neo1 (49), suggesting that the mechanism for suppressing P4-ATPase deficiency is distinct for any1Δ and kes1Δ.
The biochemical function of Any1 remains enigmatic, although this study provides some insight into potential roles for this protein in membrane organization. PQ-loop proteins share this simple PQ motif in a cytosolic loop and a similar membrane topology with seven or eight transmembrane segments. Beyond this, the PQ-loop proteins share little sequence homology. The best-characterized PQ-loop proteins include the KDEL receptor, the sweet and semisweet sugar transporters in plants, and basic amino-acid transporters in lysosomes/vacuoles (61). The potential transporter activity of Any1 and its ability to antagonize Neo1 function led to the proposal that Any1 is a scramblase that disrupts the phospholipid gradient formed by Neo1. However, it is also possible that Any1 antagonizes Neo1 through protein interactions. Consistent with this latter possibility, Any1 and Neo1 were found to interact based on a coimmunoprecipitation assay. The observation that an inactivating mutation in the second transmembrane domain of Any1, D84G, causes a loss of interaction with Neo1 supports the specificity and physiological relevance of this interaction. In addition, the influence of any1Δ on membrane asymmetry is most profound when assayed with hypomorphic neo1ts alleles, which could be explained by the removal of a protein interaction that negatively regulates Neo1 flippase activity. Any1 overexpression is particularly toxic to neo1ts mutants and slightly increases PE exposure in the outer leaflet of WT cells, a result more consistent with the inhibition of Neo1 activity by Any1.
The potential role of Any1 as a negative regulator of Neo1 activity does not fully explain the role of this PQ-loop protein, as it seems to actively inhibit growth when NEO1 is deleted. It could be that additional unknown protein interactions or a transport activity of Any1 becomes dysregulated in the absence of Neo1. Dop1 and Mon2 interact with Neo1 and are critical for the growth of yeast, but they have no known biochemical function. Potential interactions between Any1 and Dop1 or Mon2 have not yet been explored and could help to define the full nature of this regulatory network. If Any1 can scramble phospholipids as a monomer or homo-oligomer, we would expect to see a more substantial impact on membrane organization when highly expressed from the strong GAL promoter or disrupted in P4-ATPase-deficient backgrounds. However, it remains possible that Any1 is a scramblase if it is part of a hetero-oligomeric complex and/or tightly regulated. For example, the majority of Any1 expressed from the Gal promoter could be inactive if another subunit is required, or Any1 scramblase activity may be turned on only as a pressure-relief valve to counterbalance excessive flippase activity.
In summary, we identified Any1 as a key regulator that enforces separate functions for Drs2 and Neo1 in Golgi membranes. When Any1 is deleted, essential Golgi function can be provided by either Neo1 or variants of Drs2 or Dnf1 that are able to flip PS. This result emphasizes the importance of PS translocation for Golgi function and supports the possibility that Neo1 can flip PS. Our studies do not support the proposed scramblase function for Any1, and we also provide evidence that Neo1 and Any1 form a complex in cells. However, further work is needed to determine whether this interaction directly inhibits Neo1 activity or if Any1 acts upstream of Neo1 to limit substrate availability (for both Neo1 and Drs2) or downstream of Neo1 to dissipate phospholipid gradients in the membrane. The possibility that Neo1 suppresses Any1 activity will also be an interesting avenue to pursue.
Supplementary Material
Acknowledgments
The authors thank Charles Boone for providing strains and plasmids and members of the Graham laboratory for advice during the course of these studies. Antibodies were generated by the Vanderbilt Antibody and Protein Resource. The Vanderbilt Antibody and Protein Resource is supported by the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485).
Footnotes
Abbreviations:
- PapA
- papuamide A
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PGAL
- GAL promoter
- PS
- phosphatidylserine
- P4-ATPase
- type IV P-type ATPase
- 5-FOA
- 5-fluoroorotic acid
This work was supported by National Institutes of Health Grant GM107978 (T.R.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gordesky S. E., Marinetti G. V., and Love R.. 1975. The reaction of chemical probes with the erythrocyte membrane. J. Membr. Biol. 20: 111–132. [DOI] [PubMed] [Google Scholar]
- 2.Verkleij A. J., Zwaal R. F., Roelofsen B., Comfurius P., Kastelijn D., and van Deenen L. L.. 1973. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta. 323: 178–193. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher M. S. 1972. Asymmetrical lipid bilayer structure for biological membranes. Nat. New Biol. 236: 11–12. [DOI] [PubMed] [Google Scholar]
- 4.Daleke D. L. 2003. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 44: 233–242. [DOI] [PubMed] [Google Scholar]
- 5.López-Marques R. L., Poulsen L. R., Bailly A., Geisler M., Pomorski T. G., and Palmgren M. G.. 2015. Structure and mechanism of ATP-dependent phospholipid transporters. Biochim. Biophys. Acta. 1850: 461–475. [DOI] [PubMed] [Google Scholar]
- 6.Seigneuret M., and Devaux P. F.. 1984. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA. 81: 3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X., and Graham T. R.. 2009. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc. Natl. Acad. Sci. USA. 106: 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman J. A., Kwok M. C., and Molday R. S.. 2009. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem. 284: 32670–32679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., and Holthuis J. C.. 2003. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 14: 1240–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riekhof W. R., and Voelker D. R.. 2009. The yeast plasma membrane P4-ATPases are major transporters for lysophospholipids. Biochim. Biophys. Acta. 1791: 620–627. [DOI] [PubMed] [Google Scholar]
- 11.Roland B. P., and Graham T. R.. 2016. Decoding P4-ATPase substrate interactions. Crit. Rev. Biochem. Mol. Biol. 51: 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roland B. P., Naito T., Best J. T., Arnaiz-Yepez C., Takatsu H., Yu R. J., Shin H. W., and Graham T. R.. 2019. Yeast and human P4-ATPases transport glycosphingolipids using conserved structural motifs. J. Biol. Chem. 294: 1794–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux P. F. 1992. Protein involvement in transmembrane lipid asymmetry. Annu. Rev. Biophys. Biomol. Struct. 21: 417–439. [DOI] [PubMed] [Google Scholar]
- 14.Segawa K., Kurata S., Yanagihashi Y., Brummelkamp T. R., Matsuda F., and Nagata S.. 2014. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 344: 1164–1168. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki J., Umeda M., Sims P. J., and Nagata S.. 2010. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 468: 834–838. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki J., Denning D. P., Imanishi E., Horvitz H. R., and Nagata S.. 2013. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 341: 403–406. [DOI] [PubMed] [Google Scholar]
- 17.Bevers E. M., and Williamson P. L.. 2016. Getting to the outer leaflet: physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 96: 605–645. [DOI] [PubMed] [Google Scholar]
- 18.Klomp L. W., Vargas J. C., van Mil S. W., Pawlikowska L., Strautnieks S. S., van Eijk M. J., Juijn J. A., Pabon-Pena C., Smith L. B., DeYoung J. A., et al. 2004. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 40: 27–38. [DOI] [PubMed] [Google Scholar]
- 19.Dhar M., Webb L. S., Smith L., Hauser L., Johnson D., and West D. B.. 2000. A novel ATPase on mouse chromosome 7 is a candidate gene for increased body fat. Physiol. Genomics. 4: 93–100. [DOI] [PubMed] [Google Scholar]
- 20.Irvin M. R., Wineinger N. E., Rice T. K., Pajewski N. M., Kabagambe E. K., Gu C. C., Pankow J., North K. E., Wilk J. B., Freedman B. I., et al. 2011. Genome-wide detection of allele specific copy number variation associated with insulin resistance in African Americans from the HyperGEN study. PLoS One. 6: e24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura M., Takahashi A., Yamauchi T., Hara K., Yasuda K., Grarup N., Zhao W., Wang X., Huerta-Chagoya A., Hu C., et al. 2016. Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nat. Commun. 7: 10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapelbroek J. M., Peters T. A., van Beurden D. H., Curfs J. H., Joosten A., Beynon A. J., van Leeuwen B. M., van der Velden L. M., Bull L., Oude Elferink R. P., et al. 2009. ATP8B1 is essential for maintaining normal hearing. Proc. Natl. Acad. Sci. USA. 106: 9709–9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks A. A., Pramstaller P. P., Johansson A., Vitart V., Rudan I., Ugocsai P., Aulchenko Y., Franklin C. S., Liebisch G., Erdmann J., et al. 2009. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genet. 5: e1000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kengia J. T., Ko K. C., Ikeda S., Hiraishi A., Mieno-Naka M., Arai T., Sato N., Muramatsu M., and Sawabe M.. 2013. A gene variant in the Atp10d gene associates with atherosclerotic indices in Japanese elderly population. Atherosclerosis. 231: 158–162. [DOI] [PubMed] [Google Scholar]
- 25.Arashiki N., Takakuwa Y., Mohandas N., Hale J., Yoshida K., Ogura H., Utsugisawa T., Ohga S., Miyano S., Ogawa S., et al. 2016. ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica. 101: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X., Libby R. T., de Vries W. N., Smith R. S., Wright D. L., Bronson R. T., Seburn K. L., and John S. W.. 2012. Mutations in a p-type ATPase gene cause axonal degeneration. PLoS Genet. 8: e1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onat O. E., Gulsuner S., Bilguvar K., Nazli Basak A., Topaloglu H., Tan M., Tan U., Gunel M., and Ozcelik T.. 2013. Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur. J. Hum. Genet. 21: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holthuis J. C., and Menon A. K.. 2014. Lipid landscapes and pipelines in membrane homeostasis. Nature. 510: 48–57. [DOI] [PubMed] [Google Scholar]
- 29.van Meer G., Voelker D. R., and Feigenson G. W.. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hankins H. M., Sere Y. Y., Diab N. S., Menon A. K., and Graham T. R.. 2015. Phosphatidylserine translocation at the yeast trans-Golgi network regulates protein sorting into exocytic vesicles. Mol. Biol. Cell. 26: 4674–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthusamy B. P., Raychaudhuri S., Natarajan P., Abe F., Liu K., Prinz W. A., and Graham T. R.. 2009. Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol. Biol. Cell. 20: 2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebastian T. T., Baldridge R. D., Xu P., and Graham T. R.. 2012. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta. 1821: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C. Y., Ingram M. F., Rosal P. H., and Graham T. R.. 1999. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147: 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu K., Surendhran K., Nothwehr S. F., and Graham T. R.. 2008. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol. Biol. Cell. 19: 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua Z., Fatheddin P., and Graham T. R.. 2002. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell. 13: 3162–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prezant T. R., Chaltraw W. E. Jr., and Fischel-Ghodsian N.. 1996. Identification of an overexpressed yeast gene which prevents aminoglycoside toxicity. Microbiology. 142: 3407–3414. [DOI] [PubMed] [Google Scholar]
- 37.Hua Z., and Graham T. R.. 2003. Requirement for Neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum. Mol. Biol. Cell. 14: 4971–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu P., Baldridge R. D., Chi R. J., Burd C. G., and Graham T. R.. 2013. Phosphatidylserine flipping enhances membrane curvature and negative charge required for vesicular transport. J. Cell Biol. 202: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takar M., Wu Y., and Graham T. R.. 2016. The essential Neo1 protein from budding yeast plays a role in establishing aminophospholipid asymmetry of the plasma membrane. J. Biol. Chem. 291: 15727–15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natarajan P., Wang J., Hua Z., and Graham T. R.. 2004. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. USA. 101: 10614–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S., Wang J., Muthusamy B. P., Liu K., Zare S., Andersen R. J., and Graham T. R.. 2006. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic. 7: 1503–1517. [DOI] [PubMed] [Google Scholar]
- 42.Baldridge R. D., and Graham T. R.. 2012. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc. Natl. Acad. Sci. USA. 109: E290–E298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldridge R. D., and Graham T. R.. 2013. Two-gate mechanism for phospholipid selection and transport by type IV P-type ATPases. Proc. Natl. Acad. Sci. USA. 110: E358–E367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldridge R. D., Xu P., and Graham T. R.. 2013. Type IV P-type ATPases distinguish mono- versus diacyl phosphatidylserine using a cytofacial exit gate in the membrane domain. J. Biol. Chem. 288: 19516–19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roland B. P., and Graham T. R.. 2016. Directed evolution of a sphingomyelin flippase reveals mechanism of substrate backbone discrimination by a P4-ATPase. Proc. Natl. Acad. Sci. USA. 113: E4460–E4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y., Takar M., Cuentas-Condori A. A., and Graham T. R.. 2016. Neo1 and phosphatidylethanolamine contribute to vacuole membrane fusion in Saccharomyces cerevisiae. Cell. Logist. 6: e1228791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Leeuwen J., Pons C., Mellor J. C., Yamaguchi T. N., Friesen H., Koschwanez J., Usaj M. M., Pechlaner M., Takar M., Usaj M., et al. 2016. Exploring genetic suppression interactions on a global scale. Science. 354: aag0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wehman A. M., Poggioli C., Schweinsberg P., Grant B. D., and Nance J.. 2011. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr. Biol. 21: 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T., Fujimura-Kamada K., Shioji E., Suzuki R., and Tanaka K.. 2017. Cfs1p, a novel membrane protein in the PQ-loop family, is involved in phospholipid flippase functions in yeast. G3 (Bethesda). 7: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gietz R. D., and Schiestl R. H.. 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2: 31–34. [DOI] [PubMed] [Google Scholar]
- 51.Sherman F. 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- 52.Sikorski R. S., and Hieter P.. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Driessche B., Tafforeau L., Hentges P., Carr A. M., and Vandenhaute J.. 2005. Additional vectors for PCR-based gene tagging in Saccharomyces cerevisiae and Schizosaccharomyces pombe using nourseothricin resistance. Yeast. 22: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 54.Gibson D. G., Young L., Chuang R. Y., Venter J. C., Hutchison C. A. III, and Smith H. O.. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 6: 343–345. [DOI] [PubMed] [Google Scholar]
- 55.de Saint-Jean M., Delfosse V., Douguet D., Chicanne G., Payrastre B., Bourguet W., Antonny B., and Drin G.. 2011. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol. 195: 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwamoto K., Hayakawa T., Murate M., Makino A., Ito K., Fujisawa T., and Kobayashi T.. 2007. Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys. J. 93: 1608–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parsons A. B., Lopez A., Givoni I. E., Williams D. E., Gray C. A., Porter J., Chua G., Sopko R., Brost R. L., Ho C. H., et al. 2006. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 126: 611–625. [DOI] [PubMed] [Google Scholar]
- 58.Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., and Tanaka K.. 2004. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell. 15: 3418–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S., Uchida Y., Wang J., Matsudaira T., Nakagawa T., Kishimoto T., Mukai K., Inaba T., Kobayashi T., Molday R. S., et al. 2015. Transport through recycling endosomes requires EHD1 recruitment by a phosphatidylserine translocase. EMBO J. 34: 669–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsson L., Jonsson E., and Tuck S.. 2011. Caenorhabditis elegans numb inhibits endocytic recycling by binding TAT-1 aminophospholipid translocase. Traffic. 12: 1839–1849. [DOI] [PubMed] [Google Scholar]
- 61.Saudek V. 2012. Cystinosin, MPDU1, SWEETs and KDELR belong to a well-defined protein family with putative function of cargo receptors involved in vesicle trafficking. PLoS One. 7: e30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.