Abstract
Objectives
To date, Ficus carica L. cultivar Dottato (F. carica) has not been studied from a phototoxic point of view. In the present work, aerial components of F. carica from Italy, were examined to assess their antioxidant and phototoxic activity on human melanoma cells. A relationship between antioxidant, phototoxic activities and chemical composition has also been investigated.
Materials and methods
Coumarin and fatty acid content in F. carica leaves, bark and woody parts were examined and compared by capillary GC and GC/MS. Polyphenolic content was also determined. Linoleic acid peroxidation and DPPH test were used to assess antioxidant activities, and MTT assay was used to evaluate anti‐proliferative activity, on C32 human melanoma cells, after irradiation with a UVA dose of 1.08 J/cm2.
Results
Leaves demonstrated the best antioxidant and anti‐proliferative activity in comparison to bark and wood. In particular, leaves were shown to possess the highest anti‐radical activity and inhibition of peroxidation, with IC 50 values of 64 and 1.48 μg/ml respectively. The leaves had highest anti‐proliferative activity with IC 50 value of 3.92 μg/ml. The phytochemical investigation revealed different composition between the coumarins, psoralen and bergapten, fatty acids, polyphenols and flavonoid content among plant parts.
Conclusions
Data obtained indicate that this type of fig tree may constitute an excellent source of bioactive compounds, such as phenolics, coumarins and fatty acids. This study offers a new perspective in developing others formulations potentially useful in photodynamic therapy for treatment of non‐melanoma skin cancers.
Introduction
Fig (Ficus carica, Moraceae) products are widely used both as food and as medicine in the Middle East. Figs are perhaps the oldest of all cultivated fruit crops and are grown in many areas of the world that have subtropical climates. Latex released on picking the fruits is used to treat skin tumours and warts 1 but the first scientific investigation of activity of fig latex was conducted by Ullman et al. in the 1940s and 50s 2, 3, 4. High doses of fig latex injected into rats were found to be lethal. Smaller doses injected into mice bearing a benzopyrene‐induced sarcoma caused inhibition of growth of tumours and even disappearance of small ones 2. Dialysate of the latex was found to contain the active ingredients. Although isolation of the active components was not pursued further, some pharmacological work has been reported by the same group 3, 4. Fig latex has also been tested for its anti‐helminthic activity, but was found to cause acute toxicity with haemorrhagic enteritis 5. Leaf decoction affected lipid catabolism in hypertriglyceridemic rats and had hypoglycaemic action in type‐I diabetic patients 6, 7. Athnasios et al. 8 have isolated psoralen, γ‐sitosterol, bergapten and taraxasterol from petroleum ether extraction of the leaves and others have isolated triterpenoids 9, 10.
Plant‐derived natural compounds are an important source for development of cancer‐fighting drugs. One of the most critical risk factors in initiation and development of several skin diseases is exposure to UV solar radiation. As time people spend outside during sports and other activities extends, numbers of skin cancers increase 11.
Malignant melanoma and non‐melanoma skin cancers are among the most prevalent cancers in the human population. Free radicals are generated by normal physiological processes, including aerobic metabolism and the inflammatory response, but these may inflict cell damage when their generation is increased and antioxidant defence mechanisms are overwhelmed. Important findings support that the free radical hypothesis in skin carcinogenesis are: (i) reactive oxygen species (ROS) are generated in UVA‐ and UVB‐irradiated skin in excessive doses, (ii) natural cutaneous antioxidant defence is impaired on UV‐exposure, (iii) free radicals are involved in all steps of carcinogenesis, (iv) supplementation with antioxidants can inhibit skin carcinogenesis and (v) conditions that increase ROS generation enhance photocarcinogenesis. These findings provide a promising rationale for development of powerful new antioxidant strategies in prevention and therapy of skin cancer 12.
Over the last few decades, natural compounds have attracted considerable attention as cancer chemopreventive agents, as well as in cancer therapeutics. Research on natural products has recently regained prominence due to greater understanding of their biological significance and growing recognition of origins and functions of their structural diversity. It has been estimated by the World Health Organization that approximately 75–80% of the world's population, uses either in part or solely, plant medicines, either in part or entirely. For many people, this is out of necessity, as they cannot afford high costs of pharmaceutical drugs. In this article, we describe evaluation of phototoxicity of various phytocomplexes obtained from different components of the fig tree. Leaves, bark and wood of F. carica cultivar Dottato (from here on referred to simply as F. carica), from Italy were examined to assess their antioxidant activity on linoleic acid peroxidation, their free radical‐scavenging activity with 1,1‐diphenyl‐2 picrylhydrazyl (DPPH) and their phototoxicity, on C32 melanoma cells. In view of potential pharmaceutical applications, the relationship between antioxidant, phototoxic activities and chemical composition has also been investigated.
Materials and methods
Reagents
The following were obtained from Sigma‐Aldrich Spa. (Milano, Italy): β‐carotene, quercetin, chlorogenic acid, NaCl, ascorbic acid, propyl gallate, DPPH radical, linoleic acid, Tween 20, Folin‐Ciocalteu reagent, RPMI 1640 medium, foetal bovine serum, l‐glutamine, penicillin/streptomycin, trypan blue, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT), Hanks' Balanced Salt Solution and Amelanotic melanoma C32 (ATCC No.: CRL‐1585) cells. All other reagents, of analytical grade, were supplied by VWR International srl. (Milan, Italy).
Plant materials
Leaves, bark and woody parts of F. carica were collected in September 2009 in Calabria (Italy) from the ‘Miracco Lucia’ (Lat.: 16°16′14″; Long.: 39°32′56″) farm, from mature plants. Identification was performed by Dr Uzunov of the Botanic Garden, University of Calabria, Italy, and plants were deposited at the Herbarium CLU of the Natural History Museum of Calabria. Components of the Ficus samples were extracted from the different aerial elements, using a maceration technique with hydroalcoholic solution (70% ethanol) at room temperature. Extraction was repeated three times for 48 h. Hydroalcoholic solutions were combined and dried to obtain total extracts with 9.7, 8.2 and 6.4 of yield per cent of extraction for leaves, bark and woody parts respectively.
GC and GC‐MS analysis
Analysis of lipophilic compounds was performed using a Hewlett‐Packard gas‐chromatograph, model 5890, equipped with a mass spectrometer, model 5972 series II, and controlled by HP software with capillary column 30 m × 0.25 mm, static phase SE30, using programmed temperatures from 60 to 280 °C (rate 16°/min); detector and injector were set at 280 and 250 °C respectively (split vent flow 1 ml/min). Compound identification was verified according to relative retention time and mass spectra with those of Wiley 138 library data of the GC‐MS system (Hewlett‐Packard Co., Milan, Italy). Extracts were analysed, also using the Shimadzu GC17A gas chromatograph system (Columbia, MD, USA). An SE‐30 capillary column (30 m, internal diameter 0.25 mm and film thickness 0.25 μm) was used with nitrogen as the carrier gas. GC oven temperature and conditions were as described above. Percentages utilized for composition of samples analysed were computed by the normalization method from GC peak areas related to GC peak area of external standards, injected into the GC equipment in isothermal conditions, at 180 °C. Percentage of their total area was obtained by their addition. All determinations were performed in triplicate and averaged.
Determination of total phenolic and flavonoid content
Total phenolic content of Ficus samples was assessed using Folin‐Ciocalteau reagent 13. The extract (100 μl) was mixed with 0.2 ml Folin‐Ciocalteau reagent (Sigma‐Aldrich) and 2 ml of distilled water, and flasks were shaken vigorously. Subsequently, 1.0 ml of 15% sodium carbonate solution was added and combinations were mixed thoroughly again. Mixtures were allowed to rest for 2 h, protected from light. Absorbance of blue colouration produced was measured using a spectrophotometer (UV‐Vis Jenway 6003, Staffordshire, UK) at 765 nm. Levels of total phenolic contents were determined in triplicate. Chlorogenic acid was used as standard and total phenolic contents was expressed as chlorogenic acid equivalents in mg/g of dried material.
Flavonoid content was determined spectrophotometrically using a method based on formation of flavonoid‐aluminium complex 14. One millilitre of extract was added to a 10 ml volumetric flask. Distilled water was added to produce a volume of 5 ml. At time zero, 0.3 ml of 5% (w/v) sodium nitrite was added to the flask. After 5 min, 0.6 ml 10% (w/v) AlCl3 was added, then at 6 min, 2 ml of 1 m NaOH was also added to the mixture, followed by addition of 2.1 ml distilled water. Absorbance at 510 nm was measured immediately. Quercetin was chosen as standard and levels of total flavonoid content were determined in triplicate and expressed in mg/g of extract.
Free radical scavenging activity assay
Free radical scavenging activity was rapidly evaluated using a TLC screening method based on reduction of a methanolic solution of the coloured free radical DPPH. After application of hydroalcoholic solution of extracts, developing (mobile phase CHCl3/MeOH 8:2) and drying, TLC plates were sprayed with a 0.2% DPPH solution in MeOH and examined after 30 min. Samples with antioxidant activity yielded yellow spots against a purple background; positive samples were assayed for their radical scavenging potency as described by Wang et al. 15. Two hundred microlitres of test sample solution (1–1000 μg/ml) was added to 800 μl of 10−4 m ethanolic solution of DPPH; reaction mixtures were vigorously shaken and kept in the dark for 30 min. Absorbance of resulting solutions was measured in 1 cm cuvettes using a Perkin Elmer Lambda 40 UV/VIS spectrophotometer (Milan, Italy) at 517 nm against blank (without DPPH). All tests were run in triplicate and mean values were calculated. Ascorbic acid was used as positive control.
β‐Carotene bleaching‐linoleic acid assay
Antioxidant activity was determined using the β‐carotene bleaching test 16 with modifications. Briefly, 1 ml of chloroform β‐carotene solution (0.2 mg/ml) was added to 0.02 ml of linoleic acid and 0.2 ml of 100% Tween 20. After evaporation of chloroform and dilution with 100 ml of water, 5 ml of the emulsion was transferred into different test tubes containing 0.2 ml of samples, in 70% ethanol, at different concentrations. Propyl gallate at the same concentration was used as positive control. Tubes were then gently shaken and placed in a water bath at 45 °C for 60 min. Absorbances of the samples, standard and control, were measured at 470 nm using a Perkin Elmer Lambda 40 UV/VIS spectrophotometer against a blank, consisting of emulsion without β‐carotene. Measurement was carried out at initial time (t = 0) and successively at 30 and 60 min. All samples were assayed in triplicate and mean value was calculated. Antioxidant activity was measured in terms of successful prevention of β‐carotene bleaching, using the following equation:
where A 0 and A°0 are absorbance values measured at initial incubation time for samples/standard and control, respectively, while A t and A°t are absorbance values measured in samples/standard and control respectively, at t = 30 min and t = 60 min.
Phototoxicity
The phototoxicity test was adapted from Barraja et al. 17 with modifications. Amelanotic melanoma cells (C32) were grown in RPMI‐1640 medium (Sigma‐Aldrich) supplemented with 1% l‐glutamine, 1% of penicillin/streptomycin and 10% foetal calf serum. Individual wells of a 96‐well tissue culture microtitre plate (Falcon BD, Milan, Italy) were inoculated with 100 μl of complete medium containing 1 × 104 cells. Plates were incubated at 37 °C in a humidified 5% incubator for 24 h prior to experiments. After medium removal, 100 μl drug solution, dissolved in DMSO and diluted with Hanks' balanced salt solution (HBSS, pH 7.2), was added to each well and incubated at 37 °C for 30 min, then irradiated. HPW 125 Philips lamps, mainly emitting at 365 nm, were used for irradiation experiments. Spectral irradiance of the source was 4.0 mW/cm2 as measured at sample level, by a Cole‐Parmer Instrument Company radiometer (Niles, IL, USA), equipped with a 365‐CX sensor. Cells were irradiated for 1 h at a dose of 1.08 J/cm2. After irradiation, the solution was replaced with medium, and plates were incubated for 48 h. Cell viability was assayed by MTT [(3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5 diphenyl tetrazolium bromide)] test, as previously described 18.
Statistical analysis
Data are expressed as means ± SD. Statistical significance was assessed with one‐way analysis of variance (ANOVA) followed by Bonferroni post‐hoc testing. Differences were considered significant at P ≤ 0.05. Inhibitory concentration 50% (IC50) was calculated from the dose–response curve obtained by plotting percentage of inhibition versus concentration using Prism statistical software (www.graphpad.com).
Results and discussion
Phytochemical analysis
Chemical composition varies from plant to plant and within different parts of the same plant. To identify non‐polar components of F. carica extracts, GC‐MS and GC analyses were carried out. Coumarins and fatty acids were identified and quantified as major constituents in all samples. Among coumarins, psoralen and bergapten are two of the major active components identified in F. carica extracts. Psoralen concentrations were significantly different between plant parts (P < 0.05), following the order bark>leaves>woody components (mean values of 23.3%, 19.2% and 8.6% respectively) (Fig. 1). In the case of bergapten, concentrations also were different (P < 0.05), following the order: leaves>bark>woody components (mean values of 8.2%, 6.6% and 2.6% respectively).
Figure 1.
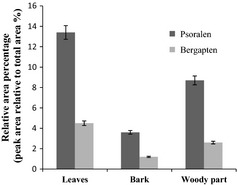
Coumarin composition of F. carica cv. Dottato extracts. Data, mean ± SD (n = 3).
Composition of fatty acids of F. carica extracts is reported in Table 1. Extracts were characterized by linolenic acid methyl and ethyl ester and linoleic acid ethyl ester, as the most abundant components. Leaf extracts were characterized by linolenic acid methyl ester as the most abundant constituents of leaves (12.4%), while linolenic acid ethyl ester and linoleic acid ethyl ester were the most abundant compounds in bark and woody parts samples.
Table 1.
Composition of fatty acids of F. carica cv. Dottato extracts
| Compounda | Leaves | Bark | Woody part |
|---|---|---|---|
| Palmitic acid methyl ester | 0.5 | ND | 0.5 |
| Palmitic acid ethyl ester | 1.9 | 0.5 | 5.6 |
| Palmitic acid | 3.7 | 2.1 | 6.9 |
| Linolenic acid methyl ester | 12.4 | 2.4 | tr |
| Linoleic acid | 1.6 | 0.3 | 5.4 |
| Linolenic acid ethyl ester | ND | 9.8 | 9.7 |
| Linoleic acid ethyl ester | 6.5 | 7.9 | 7.5 |
| Stearic acid ethyl ester | ND | 5.2 | 0.6 |
tr, trace; ND, not detected.
Relative area percentage (peak area relative to total peak area%).
Ethyl esters of fatty acids were artefacts formed during extraction with hydroalcoholic solution (70% ethanol).
Total phenolic content was significantly different among the three plant parts (P < 0.05) (Table 2). In particular, leaves possessed higher quantities of phenolic compounds. Leaves also were characterized by major content of total flavonoid. Some studies have described presence of several phenolic compounds in F. carica 19, 20, 21, 22 and their antioxidant activity 23. Composition and antioxidant activities of leaves from two Portuguese F. carica varieties have also been evaluated 24, however, metabolic profiles of bark and woody components had not been compared.
Table 2.
Polyphenol and flavonoid content of F. carica cv. Dottato extracts
| Sample | Polyphenolsa (mg/g) | Flavonoidsb (mg/g) | Flavonoids/polyphenols |
|---|---|---|---|
| Leaves | 3.6 ± 0.18 | 1.0 ± 0.05 | 0.3 |
| Bark | 2.8 ± 0.14 | 1.3 ± 0.06 | 0.5 |
| Woody parts | 1.4 ± 0.07 | 0.8 ± 0.04 | 0.6 |
Chlorogenic acid equivalents/g of dried material.
Quercetin equivalents/g of dried material.
Antioxidant activity
Many studies have shown that natural antioxidants are closely related to their bio‐functionalities, such as reduction in chronic conditions such as mutagenesis, carcinogenesis, DNA damage and more. Thus, antioxidant capacity is widely used as a parameter to characterize food or medicinal plants and their bioactive components. Antioxidant properties, especially free radical scavenging activities, are very important due to the deleterious role of free radicals in foods and biological systems. Reduction in DPPH absorption is indicative of capacity of the extracts to scavenge free radicals, independently of any enzymatic activity.
Figure 2 illustrates DPPH free radical scavenging ability (IC50 values) of different parts of plants. Ascorbic acid was used as free radical scavenger reference. Leaves, bark and woody component extracts exhibited DPPH scavenging capacity, in a concentration‐dependent manner, leaves being the most effective material, with IC50 value of 100 μg/ml. Thus the samples were revealed to have a protective effect for lipid peroxidation, the effect being concentration‐dependent (Fig. 3). As happened for DPPH, leaves exhibited the strongest capacity with IC50 value of 1.48 μg/ml after 30 min incubation (Fig. 3a).
Figure 2.
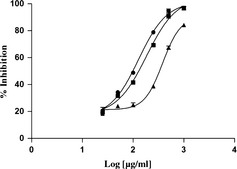
Free radical scavenging activity on DPPH of F. carica cv. Dottato extracts. ●: leaves; ■: bark; ▲: woody parts. Data, mean ± SEM (n = 3). Ascorbic acid (IC 50 value of 2 ± 0.01 μg/ml) was used as positive control.
Figure 3.
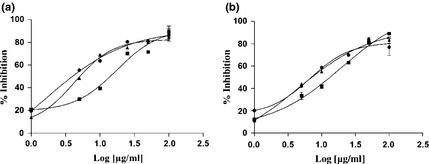
Lipid peroxidation inhibition activity using β‐carotene‐linoleic acid system after 30 and 60 min of incubation of F. carica cv. Dottato extracts. ●: leaves; ■: bark; ▲: woody parts; a: 30 min; b: 60 min. Data represent mean ± SEM (n = 3). Propyl gallate (IC 50 = 1 ± 0.02 μg/ml) was used as positive control.
Figure 4.
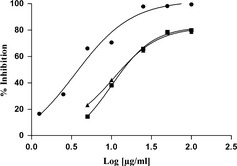
Phototoxic effects exerted by total extracts and fractions of F. carica cv. Dottato extracts on UVA‐induced A375 cells. ●: leaves; ■: bark; ▲: woody parts. Data represent mean ± SEM (n = 4). Bergaptene (IC 50 = 0.0416 ± 0.008 μg/ml) was used as positive control.
Overall, results obtained in the two assays revealed that leaves possess the strongest antioxidant potential and bark, the weakest. These results may be partially explained due to the highest amounts of phenolic compounds occurring in leaves. Antioxidant capacity of phenolic compounds is based on their ability to scavenge free radicals, chelate pro‐oxidant metal‐ions and to inhibit some enzymes 25, 26. Nevertheless, the contribution of organic acids cannot be ignored. Previous studies have shown the antioxidant activity of F. carica leaf extract (10 mg/ml), which had approximately 50% free radical scavenging activity, using the DPPH test 27. Results obtained in our study however, reveal that leaf extract was very potent. Oliveira et al. 24 compared anti‐free radical activity of pulp, peel and leaves of two Portuguese F. carica varieties and the authors showed that leaf extract, obtained by boiling in water, was the most effective material.
Phototoxicity
Phototoxicity of F. carica samples was investigated here, for the first, time on the human tumour cell line C32 (amelanotic melanoma). Table 3 shows IC50 value, concentration that induces 50% inhibition of cell population growth, of leaves, bark and woody part extracts, after irradiation at the specific UVA dose of 1.08 J/cm2. Control experiments using UVA light and parallel treatment groups of non‐irradiated cells, were carried out without significant cytotoxic effects.
Table 3.
IC 50 values (μg/ml) of antioxidant and phototoxic activities of F. carica cv. Dottato extracts
| Sample | DPPH | Lipid peroxidation inhibition | Phototoxicity | ||
|---|---|---|---|---|---|
| 30 min | 60 min | With UV | Without UV | ||
| Leaves | 100 ± 2.51c | 1.48 ± 0.07d | 6.61 ± 0.33b | 3.92 ± 0.19c | 100 ± 2.75a |
| Bark | 161 ± 3.04b | 18.36 ± 0.92a | 18.9 ± 0.94a | 10.03 ± 0.57b | ND |
| Woody part | 360 ± 3.89a | 4.02 ± 0.21c | 4.92 ± 0.25bc | 10.69 ± 0.53b | ND |
For antioxidant activities, data are expressed as means ± SD (n = 3). For photodynamic activity, data are expressed as mean ± SD (n = 4). Different letters along column (DPPH test), or within the two columns (β‐carotene test and MTT) indicate statistically significant differences at P < 0.05 (Bonferroni test).
ND, not detectable.
Ficus carica leaves had best anti‐proliferative activity in comparison with bark and woody components, with IC50 values of 3.92, 10.0 and10.69 μg/ml, respectively.
The reason for this is in their composition. All extracts contain polyphenolic compounds such as flavonoids, however, individual components and their concentrations differ. Huang et al. came to similar conclusions when they studied effects of extracts of black raspberries, strawberries and blueberries on UV‐induced damage, to a mouse epidermal cell line 28.
Anti‐proliferative activity may be attributed also to presence of furanocoumarins, which represent a novel class of potentially effective natural drugs for treatment of several types of cancer and skin disease 29.
A modern approach in chemoprevention is to formulate a mixture of active compounds or prepare extracts/fractions from plant material with protective activity. A mixture of various phytochemicals has a number of advantages, for example, extracts and fractions are cheaper and easier to prepare than pure formulations, as individual components may provide for each other additive and/or synergistic effects, and lower concentration of constituents lessens their possible toxic effects.
Conclusion
This is the first study comparing F. carica leaves, bark and woody parts. Strong relationships observed between psoralen and bergapten within and between different plant structures support the idea that these are probably metabolically dynamic compounds with a cycle of synthesis, translocation and transformation in the plant 30.
Concentrations of coumarin compounds found in Ficuc carica cv. Dottato reaffirm early findings on presence of psoralen and bergapten in different structures of this species. Utilization of the leaves may contribute to prevention of diseases in which homeostasis is impaired by oxidative features. In addition, the leaves were characterized as having higher quantities of psoralen and bergapten.
This study confirms that plant‐derived natural compounds are an important source for development of anticancer drugs. F. carica cv. Dottato leaves could provide a new perspective in developing new formulations, potentially useful in PUVA therapy for treatment of malignant melanoma and other diseases. Further experiments are necessary to examine bioavailability of the active components, to exclude any possible toxic effects they may have and to clarify their photoprotective activity in vivo.
Acknowledgements
We thank Mrs Miracco Lucia for supplying the herbal samples used in this study, and Dr Dimitar Uzunov for their identification.
References
- 1. Ghazanfar SA (1994) Handbook of Arabian Medicinal Plants, p. 148 Boca Raton: CRC Press. [Google Scholar]
- 2. Ullman SB, Halberstaedter L, Leibowitz J (1945) Some pharmacological and biological effects of the latex of Ficus carica . Exp. Med. Surg. 3, 11–23. [Google Scholar]
- 3. Ullman SB (1952) The inhibitory and necrosis‐inducing effects of the latex of Ficus carica L. on transplanted and spontaneous tumours. Exp. Med. Surg. 10, 26–49. [PubMed] [Google Scholar]
- 4. Ullman SB, Clark GM, Roan KM (1952) The effects of the fraction R3 of the latex of Ficus carica L. on the tissues of mice bearing spontaneous mammary tumors. Exp. Med. Surg. 10, 287–305. [PubMed] [Google Scholar]
- 5. De‐Amorin A, Borba HR, Carauta JP, Lopes D, Kaplan MA (1999) Anthelmintic activity of the latex of Ficus species. J. Ethnopharmacol. 64, 255–258. [DOI] [PubMed] [Google Scholar]
- 6. Perez C, Canal JR, Campillo JE, Romero A, Torres MD (1999) Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother. Res. 13, 188–191. [DOI] [PubMed] [Google Scholar]
- 7. Serraclara A, Hawkins F, Perez C, Dominguez E, Campillo JE, Torres MD (1998) Hypoglycemic action of an oral fig‐leaf decoction in type‐I diabetic patients. Diabetes Res. Clin. Pract. 39, 19–22. [DOI] [PubMed] [Google Scholar]
- 8. Athnasios AK, El Kholy IE, Soliman G, Shaban MAM (1962) Constituents of the leaves of Ficus carica, L. Part I Isolation of psoralen, bergapten, γ‐taraxasterol and β‐sitosterol. Int. J. Chem. Soc. 62, 4253–4254. [Google Scholar]
- 9. Abu‐Mustafa EA, El Tawil BAH, Fayez MBE (1964) Constituents of local plants IV. Ficus carica, F. sycomorus, and F. salicifolia leaves. Phytochemistry 3, 701–703. [Google Scholar]
- 10. Wasim A, Abdul‐Qasim K, Abdul M (1988) Two triterpenes from the leave of Ficus carica . Planta Med. 54, 481. [DOI] [PubMed] [Google Scholar]
- 11. Moehrle M (2008) Outdoor sports and skin cancer. Clin. Dermatol. 26, 12–15. [DOI] [PubMed] [Google Scholar]
- 12. Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ (2004) Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 43, 326–335. [DOI] [PubMed] [Google Scholar]
- 13. Menichini F, Tundis R, Bonesi M, Loizzo MR, Conforti F, Statti G et al (2009) The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 114, 553–560. [Google Scholar]
- 14. Yoo KM, Lee CH, Lee H, Moon BK, Lee CY (2008) Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 106, 929–936. [Google Scholar]
- 15. Wang M, Li J, Rangarajan M, Shao Y, La Voie EJ, Huang TC et al (1998) Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 46, 4869–4873. [Google Scholar]
- 16. Ismail A, Marjan ZM, Foong CW (2004) Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 87, 581–586. [Google Scholar]
- 17. Barraja P, Diana P, Montalbano A, Dattolo G, Cirrincione G, Viola G et al (2006) Pyrrolo[2,3‐h]quinolinones: a new ring system with potent photoantiproliferative activity. Bioorg. Med. Chem. 24, 8712–8728. [DOI] [PubMed] [Google Scholar]
- 18. Tubaro A, Florio C, Luxich E, Vertua R, Della Loggia R, Yasumoto T (1996) Suitability of the MTT‐based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 34, 965–974. [DOI] [PubMed] [Google Scholar]
- 19. Teixeira DM, Patão RF, Coelho AV, Costa CT (2006) Comparison between sample disruption methods and solid‐liquid extraction (SLE) to extract phenolic compounds from Ficus carica leaves. J. Chromatogr. A 1103, 22–28. [DOI] [PubMed] [Google Scholar]
- 20. Vaya J, Mahmood S (2006) Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliquia L.) and pistachio (Pistacia lentiscus L.). Biofactors 28, 1–7. [DOI] [PubMed] [Google Scholar]
- 21. Guarrera PM (2005) Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 76, 1–25. [DOI] [PubMed] [Google Scholar]
- 22. Jeong WS, Lachance PA (2001) Phytosterols and fatty acids in Fig (Ficus carica, var. Mission) fruit and tree components. J. Food Sci. 66, 278–281. [Google Scholar]
- 23. Solomon A, Golubowicz S, Yablowicz Z, Grossman S, Bergman M, Gottlieb H et al (2006) Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 54, 7717–7723. [DOI] [PubMed] [Google Scholar]
- 24. Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB (2009) Ficus carica L.: metabolic and biological screening. Food Chem. Toxicol. 47, 2841–2846. [DOI] [PubMed] [Google Scholar]
- 25. Silva BM, Andrade PB, Valentão P, Ferreres F, Seabra RM, Ferreira MA (2004) Quince (Cydonia oblonga Miller) fruit (pulp, peel and seed) and jam: antioxidant activity. J. Agric. Food Chem. 52, 4705–4712. [DOI] [PubMed] [Google Scholar]
- 26. Halliwell B, Clement MV, Long H (2000) Hydrogen peroxide in the human body. FEBS Lett. 486, 10–13. [DOI] [PubMed] [Google Scholar]
- 27. Bae H, Batt HP (2005) Evaluation of antioxidant activity of fig tree (Ficus carica) leaf extract, IFT Annual Meeting, July 15–20 – New Orleans, Louisiana.
- 28. Conforti F, Marrelli M, Menichini F, Bonesi M, Statti G, Provenzano E et al (2009) Natural and synthetic furanocoumarins as treatment for vitiligo and psoriasis. Curr. Drug Ther. 4, 38–58. [Google Scholar]
- 29. Huang C, Zhang D, Li J, Tong Q, Stoner GD (2007) Differential inhibition of UV‐induced activation of NFκB and AP‐1 by extracts from Black raspberries, strawberries and blueberries. Nutr. Cancer 58, 205–212. [DOI] [PubMed] [Google Scholar]
- 30. Harborne JB (1991) The chemical basis of plant defenses In: Palo RT, Robins CT, eds. Plant Defenses Against Mammalian Herbivory, p. 4559 Boca Raton: CRC. [Google Scholar]


