Abstract
BACKGROUND
Substance abuse is increasingly prevalent among young adults, but data on cardiovascular outcomes remain limited.
OBJECTIVES
The objectives of this study were to assess the prevalence of cocaine and marijuana use in adults with their first myocardial infarction (MI) at ≤50 years and to determine its association with long-term outcomes.
METHODS
The study retrospectively analyzed records of patients presenting with a type 1 MI at ≤50 years at 2 academic hospitals from 2000 to 2016. Substance abuse was determined by review of records for either patient-reported substance abuse during the week before MI or substance detection on toxicology screen. Vital status was identified by the Social Security Administration’s Death Master File. Cause of death was adjudicated using electronic health records and death certificates. Cox modeling was performed for survival free from all-cause and cardiovascular death.
RESULTS
A total of 2,097 patients had type 1 MI (mean age 44.0 ± 5.1 years, 19.3% female, 73% white), with median follow-up of 11.2 years (interquartile range: 7.3 to 14.2 years). Use of cocaine and/or marijuana was present in 224 (10.7%) patients; cocaine in 99 (4.7%) patients, and marijuana in 125 (6.0%). Individuals with substance use had significantly lower rates of diabetes (14.7% vs. 20.4%; p=0.05) and hyperlipidemia (45.7% vs. 60.8%; p<0.001), but they were significantly more likely to use tobacco (70.3% vs. 49.1%; p < 0.001). The use of cocaine and/or marijuana was associated with significantly higher cardiovascular mortality (hazard ratio: 2.22; 95% confidence interval: 1.27 to 3.70; p = 0.005) and all-cause mortality (hazard ratio: 1.99; 95% confidence interval: 1.35 to 2.97; p = 0.001) after adjusting for baseline covariates.
CONCLUSIONS
Cocaine and/or marijuana use is present in 10% of patients with an MI at age ≤50 years and is associated with worse all-cause and cardiovascular mortality. These findings reinforce current recommendations for substance use screening among young adults with an MI, and they highlight the need for counseling to prevent future adverse events.
Keywords: cocaine, marijuana, myocardial infarction, substance abuse, young adults
Substance abuse, including use of cocaine and marijuana, has been increasing nationally, yet its potential cardiovascular consequences are not fully understood. According to the National Institute on Drug Abuse, this increased prevalence is largely driven by a rise in marijuana use (1). Current American College of Cardiology guidelines for the management of acute coronary syndromes recommend that urine toxicology screening be considered when substance abuse is suspected, especially in patients <50 years of age (2). However, there are limited data on the true prevalence of substance abuse in young individuals who experience a myocardial infarction (MI).
Although it is well established that cocaine use is a strong risk factor for MI and injury through its effects on myocardial contractility and oxygen use (3), a recent meta-analysis suggests that there is insufficient evidence on the association of marijuana use with cardiovascular outcomes (4,5). However, it is known that cannabinoid receptors are present in myocardial, endothelial, and smooth muscle cells and that marijuana use has been shown to increase the generation of reactive oxygen species, decrease myocardial contractility, create a proinflammatory endothelial response, and contribute to neointimal proliferation of vascular smooth muscle. As a result, marijuana use has been suggested to be associated with adverse cardiovascular outcomes including stroke, coronary artery dissection, vasospasm, coronary thrombosis, acute coronary syndromes, arrhythmias, vasculitis, myocarditis, and cardiomyopathies (6–8).
The recent increase in substance use among young adults and the legalization of marijuana in multiple states have led to a significant public health debate with an urgent need to understand the health effects of this substance use. Therefore, we sought to determine the prevalence of substance abuse and its association with cardiovascular outcomes in young individuals with their first MI ≤50 years of age.
METHODS
STUDY GROUP.
The design of the YOUNG-MI registry has been previously described (9). This is a retrospective cohort study from 2 large academic medical centers (Brigham and Women’s Hospital and Massachusetts General Hospital in Boston, Massachusetts) that included patients who experienced an MI at or before 50 years of age between 2000 and 2016. The presence and type of MI were separately adjudicated by 2 individuals. In cases of discrepancy that could not be resolved, the final determination was performed by an adjudication panel. The Third Universal Definition of Myocardial Infarction was used (10). For the present analysis, only patients with type 1 MI were included.
SUBSTANCE ABUSE.
Cocaine and/or marijuana use was determined by the review of records for either patient-reported substance abuse within the week before presentation for MI or by substance detection on toxicology screen during admission for MI when the screen was obtained as part of clinical care. Electronic medical records were reviewed for clinic notes before admission, admission history and physical examination, and discharge summaries to determine patient-reported substance use. Urine toxicology screens were also reviewed when such tests were obtained as part of clinical care. Patients who used both cocaine and marijuana were included in the cocaine group for subanalyses. Opioid use was captured; however, this was limited by an inability to distinguish prescription drug use from nonprescription drug use, as well as by the fact that many individuals receive opiates in the emergency room for chest pain, and such use could have affected their toxicology screen results. Given these limitations, the data on opioid use was insufficient for analysis in our cohort. In addition, the use of methamphetamines, benzodiazepines, and barbiturates was captured through toxicology screens. However, these substances accounted for only a few cases (4 patients with methamphetamine use, 2 patients with benzodiazepine use, and no patients with barbiturate use) and therefore were not incorporated into the analysis.
RISK FACTORS.
A detailed review of the electronic medical record was conducted to determine the presence of cardiovascular risk factors during or before the index admission. For each risk factor, we also determined whether it was known before admission or diagnosed during that hospitalization. Diabetes was defined as fasting plasma glucose >126 mg/dl, hemoglobin A1c ≥6.5%, or diagnosis or treatment of diabetes. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or diagnosis or treatment of hypertension. Dyslipidemia was defined as total cholesterol ≥240 mg/dl, serum triglycerides ≥150 mg/dl, high-density lipoprotein cholesterol (HDL-C) <40 mg/dl in men or <50 mg/dl in women, or diagnosis or treatment of dyslipidemia. Obesity was defined as a body mass index ≥30 kg/m2 or a diagnosis of obesity. Smoking was defined as current (tobacco products used within the last month), former, or never. Family history of premature coronary artery disease was defined as fatal MI, nonfatal MI, or coronary revascularization occurring before 55 years of age for first-degree male family members and before 65 years of age for first-degree female family members, and it was captured by a thorough review of the electronic medical records, which included all clinic notes before admission, admission history and physical examination, discharge summaries, and follow-up visit notes. The atherosclerotic cardiovascular disease score was calculated on the basis of data available before MI or at the time of presentation by using the pooled cohort equation, as previously described (9).
MEDICATIONS.
A detailed review of electronic medical records was used to determine the medications prescribed at the time of hospital discharge. Patients who died in hospital were excluded from this specific analysis but were included in all other analyses. Medications captured included aspirin, P2Y12 antiplatelet therapies, statins, beta-blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers.
OUTCOMES.
The outcomes of interest were cardiovascular mortality and all-cause mortality. Vital status was assessed with linkage with the Partners HealthCare electronic medical record system, the Social Security Administration’s Death Master File, and the National Death Index, and it was censored on the date of the latest query. The cause of death was adjudicated by 2 independent cardiologists using electronic health records, records from the Massachusetts Department of Vital Statistics, and death certificates obtained from the National Death Index. In cases of disagreement, consensus for the cause of death was reached by the adjudication committee. The cause of death was categorized into cardiovascular death, noncardiovascular death, or undetermined cause of death. If the cause of death was undetermined, patients were conservatively analyzed as having a noncardiovascular death. The definition of cardiovascular death was adapted from the 2014 American College of Cardiology definition for cardiovascular endpoint events (11), as previously detailed in the study design publication (9). Cardiovascular deaths included death from acute MI, heart failure, sudden cardiac death, ischemic stroke, nontraumatic hemorrhagic stroke, immediate complications of a cardiovascular procedure, cardiovascular hemorrhage, and other cardiovascular causes such as pulmonary embolism or peripheral arterial disease.
Secondary outcomes evaluated included cardiac arrest at presentation and coronary revascularization.
DATA MANAGEMENT.
Study-related data for all patients who met inclusion criteria were stored on our customized secure electronic adjudication system and REDCap, an electronic data capture platform (12). The Institutional Review Board at Partners HealthCare approved the YOUNG-MI registry.
STATISTICAL ANALYSIS.
Categorical variables were reported as frequencies and proportions and were compared with the chi-square or Fisher exact test. Continuous variables were reported as medians or means and compared with Student’s t-tests or the Mann-Whitney U test. Trends over time were modeled using linear regression analysis with the program Tableau version 10.5 (Tableau Software, Seattle, Washington). All analyses were performed using Stata software version 14.2 (StataCorp, College Station, Texas). Cox proportional hazards models were constructed for survival free from all-cause death and cardiovascular death. Proportional hazards assumption was assessed by analyzing the Schoenfeld residuals.
Multivariable risk adjustment was performed using variables that had significant univariate association with the outcome of interest or are known to be associated with either all-cause mortality or cardiovascular death. We also performed a sensitivity analysis using inverse treatment of probability weighting (IPW) with stabilized IPW weights (Online Appendix), and we generated adjusted survival curves by the Cole and Hernan method (13).
RESULTS
PREVALENCE.
Our study cohort consisted of 2,097 patients with a type 1 MI. Use of cocaine and/or marijuana was present in 224 (10.7%) patients. Cocaine use was present in 99 (4.7%) patients, whereas marijuana use was present in 125 (6.0%) patients. There were 36 individuals who used both substances.
BASELINE CHARACTERISTICS.
The baseline characteristics of patients with substance use compared with nonusers are shown in Table 1 and Online Table 1. Patients with substance abuse tended to be younger at the time of their MI (median of 44 years of age vs. 45 years of age; p < 0.001) and male (86.2% vs. 80.1%; p = 0.03), but there were no significant differences in race between the groups. There was a significantly higher proportion of ST-segment elevation MIs (STEMIs) in the substance abuse group (64.7% vs. 52.1%; p < 0.001). When compared with patients without substance abuse, patients with substance abuse had significantly lower rates of prevalent diabetes (14.7% vs. 20.4%; p = 0.05) and hyperlipidemia (45.7% vs. 60.8%; p < 0.001). Notably, rates of tobacco use were significantly higher in the substance abuse group (70.3% vs. 49.1%; p < 0.001).
Table 1.
Baseline Characteristics of Individuals With Substance Use
| No Cocaine or Marijuana Use (n = 1,873, 89.3%) | Cocaine and/or Marijuana Use (n = 224,10.7%) | p Value | |
|---|---|---|---|
| Age at event, yrs | 45.0 (42.0–48.0) | 44.0 (39.0–46.5) | <0.001 |
| Female | 373 (19.9) | 31 (13.8) | 0.031 |
| White | 1,378 (73.6) | 153 (68.3) | 0.095 |
| STEMI | 976 (52.1) | 145 (64.7) | <0.001 |
| Length of stay, days | 3.0 (2.0–5.0) | 3.0 (2.0–6.0) | 0.47 |
| Diabetes | 378 (20.4) | 32 (14.7) | 0.048 |
| Hypertension | 877 (47.3) | 93 (42.1) | 0.15 |
| Hyperlipidemia | 1,128 (60.8) | 101 (45.7) | <0.001 |
| Family history of premature CAD | 511 (27.3) | 71 (31.7) | 0.18 |
| ASCVD score | 4.6 (2.7–7.9) | 5.2 (3.2–7.7) | 0.24 |
| Obesity | 551 (34.2) | 52 (28.6) | 0.14 |
| Current smoker | 910 (49.1) | 156 (70.3) | <0.001 |
| Cardiac arrest at presentation | 66 (3.5) | 18 (8.0) | 0.003 |
| Coronary angiogram performed | 1,767 (96.5) | 204 (92.7) | 0.015 |
| Revascularization | 1,598 (90.4) | 188 (92.2) | 0.53 |
| PCI | 1,429 (76.3) | 176 (78.6) | 0.50 |
| CABG | 164 (8.8) | 13 (5.8) | 0.16 |
| tPA | 108 (5.8) | 5 (2.2) | 0.027 |
| Cardiac rehabilitation | 197 (10.5) | 27 (12.1) | 0.49 |
| Creatinine at presentation | 1.0 ± 0.4 | 1.1 ± 0.4 | 0.081 |
| Normalized troponin | 38.6 (10.2–147.8) | 62.2 (13.0–193.0) | 0.014 |
| Total-C, mg/dl | 193.6 ± 57.1 | 181.3 ± 50.0 | 0.004 |
| LDL-C, mg/dl | 119.9 ± 47.2 | 112.6 ± 43.0 | 0.035 |
| Triglycerides, mg/dl | 149 (102.0–224.0) | 140.0 (103.0–205.0) | 0.22 |
| HDL-C, mg/dl | 37.0 ± 10.3 | 36.4 ± 10.5 | 0.47 |
Values are median (interquartile range), n (%), or mean ± SD.
ASCVD = atherosclerotic cardiovascular disease; CABG = coronary artery bypass graft; CAD = coronary artery disease; HDL-C = high-density lipoprotein cholesterol; IQR = interquartile range; LDL-C = low-density lipoprotein cholesterol; Max = maximum; Min = minimum; PCI = percutaneous coronary intervention; STEMI = ST- segment elevation myocardial infarction; Total-C = total cholesterol; tPA = tissue plasminogen activator.
When analyzed by drug, the cocaine group had similar rates of diabetes and hypertension but significantly lower rates of hyperlipidemia (40.4% vs. 60.8%; p < 0.001) compared with patients without substance abuse (Table 2). In the marijuana group, there were significantly lower rates of diabetes (10.7% vs. 20.4%; p = 0.007), hypertension (34.4% vs. 47.3%; p = 0.006), and hyperlipidemia (60.0% vs. 60.8%; p = 0.022) compared with patients without substance abuse (Table 2).
Table 2.
Baseline Characteristics by Drug of Abuse
| No Cocaine or Marijuana Use (n = 1,873, 89.3%) | Marijuana (n = 125, 6.0%) | p Value (Marijuana vs. No Cocaine or Marijuana Use) | Cocaine (n = 99, 4.7%) | p Value (Cocaine vs. No Cocaine or Marijuana Use) | |
|---|---|---|---|---|---|
| Age at event, yrs | 45 (42.0–48.0) | 44.0 (38.0–47.0) | <0.001 | 44.0 (40.0–46.0) | <0.001 |
| Female | 373 (19.9) | 16 (12.8) | 0.061 | 15 (15.2) | 0.30 |
| White | 1,378 (73.6) | 93 (74.4) | 0.92 | 60 (60.6) | 0.007 |
| STEMI | 976 (52.1) | 79 (63.2) | 0.016 | 66 (66.7) | 0.005 |
| Length of stay, days | 3.0 (2.0–5.0) | 4.0 (3.0–6.0) | 0.089 | 3.0 (2.0–5.0) | 0.42 |
| Diabetes | 378 (20.4) | 13 (10.7) | 0.007 | 19 (19.8) | 1.00 |
| Hypertension | 877 (47.3) | 42 (34.4) | 0.006 | 51 (51.5) | 0.35 |
| Hyperlipidemia | 1,128 (60.8) | 61 (60.0) | 0.022 | 40 (40.4) | <0.001 |
| Family history of premature CAD | 511 (27.3) | 37 (29.6) | 0.60 | 34 (34.3) | 0.13 |
| ASCVD score | 4.6 (2.7–7.9) | 5.0 (3.0–8.0) | 0.62 | 5.3 (3.6–7.4) | 0.20 |
| Obesity | 551 (34.2) | 30 (30.3) | 0.45 | 22 (26.5) | 0.19 |
| Current smoker | 910 (49.1) | 80 (65.0) | <0.001 | 76 (76.8) | <0.001 |
| Cardiac arrest at presentation | 66 (3.5) | 11 (8.8) | 0.007 | 7 (7.1) | 0.091 |
| Coronary angiogram performed | 1,767 (96.5) | 117 (95.1) | 0.45 | 87 (89.7) | 0.003 |
| Revascularization | 1,598 (90.4) | 107 (91.5) | 0.87 | 81 (93.1) | 0.57 |
| PCI | 1,429 (76.3) | 98 (78.4) | 0.66 | 78 (78.8) | 0.63 |
| CABG | 164 (8.8) | 10 (8.0) | 0.87 | 3 (3.0) | 0.042 |
| tPA | 108 (5.8) | 2 (1.6) | 0.043 | 3 (3.0) | 0.37 |
| Cardiac rehabilitation | 197 (10.5) | 17 (13.6) | 0.29 | 10 (10.1) | 1.00 |
| Creatinine at presentation | 1.0 ± 0.4 | 1.1 ± 0.4 | 0.86 | 1.2 ± 0.4 | 0.012 |
| Normalized troponin | 38.6 (10.2–147.8) | 61.2 (11.8–199.8) | 0.11 | 64.4 (15.6–193.0) | 0.042 |
| Total-C, mg/dl | 193.6 ± 57.1 | 184.0 ± 53.7 | 0.084 | 177.8 ± 44.7 | 0.011 |
| LDL-C, mg/dl | 119.9 ± 47.2 | 115.0 ± 47.1 | 0.29 | 109.3 ± 37.1 | 0.039 |
| Triglycerides, mg/dl | 149.0 (102.0–224.0) | 144.0 (103.0–206.0) | 0.60 | 136.0 (102.0–205.0) | 0.18 |
| HDL-C, mg/dl | 37.0 ± 10.3 | 36.1 ± 11.0 | 0.37 | 36.9 ± 9.8 | 0.92 |
Values are median (interquartile range), n (%), or mean ± SD.
Abbreviations as in Table 1.
CARDIAC ARREST AT PRESENTATION.
Out-of-hospital cardiac arrest at presentation occurred in 4.0% of the patients in our cohort. Overall, the incidence of out-of-hospital cardiac arrest was significantly higher in patients with substance abuse compared with patients without substance abuse (8.0% vs. 3.5%; p = 0.003). Subgroup analyses by drug showed that this difference was largely driven by an increased rate of cardiac arrest in the marijuana group (8.8% vs. 3.5%; p = 0.007). There was no significant difference in the cocaine subgroup analysis.
CORONARY REVASCULARIZATION.
Patients with substance abuse were slightly less likely to undergo cardiac catheterization (92.7% vs. 96.5%; p = 0.015), although there were no significant differences in the rates of coronary revascularization. This difference in coronary angiography was mainly driven by patients with cocaine abuse because only 87 patients (89.7%) who used cocaine underwent catheterization compared with 1,884 (96.4%) without substance use (p = 0.003) (Table 2). There were no differences in the rate of percutaneous coronary intervention versus coronary artery bypass grafting or thrombolytic therapy across the 3 groups. However, patients with cocaine abuse were significantly less likely to undergo coronary artery bypass grafting compared with patients without substance abuse (3.0% vs. 8.8%; p = 0.042) (Table 2).
POST-MI THERAPY.
Medications prescribed at discharge in each group are shown in Table 3. Prescription rates were similar between the groups for dual antiplatelet therapy, angiotensin-converting enzyme inhibitors, and statin therapy. Beta-blocker therapy was less frequently prescribed in patients with substance use (n = 180, 84.1%) compared with patients without substance use (n = 1,695, 92.1%) (p < 0.001).
Table 3.
Medical Therapy at Hospital Discharge*
| Medication | No Cocaine or Marijuana Use (n = 1,840, 89.7%) | Cocaine and/or Marijuana Use (n = 214, 10.3%) | p Value |
|---|---|---|---|
| Aspirin | 1,737 (94.4) | 204 (95.3) | 0.75 |
| Beta-blocker | 1,695 (92.1) | 180 (84.1) | <0.001 |
| P2Y12 inhibitor | 1,496 (81.3) | 180 (84.1) | 0.35 |
| ACE inhibitor | 1,132 (61.5) | 135 (63.1) | 0.71 |
| Statin | 1,640 (89.1) | 190 (88.8) | 0.91 |
| Diuretic | 209 (11.4) | 15 (7.0) | 0.063 |
Values are n (%).
Patients who died in hospital were excluded from this specific analysis (n = 43).
ACE = angiotensin-converting enzyme.
TRENDS OVER TIME.
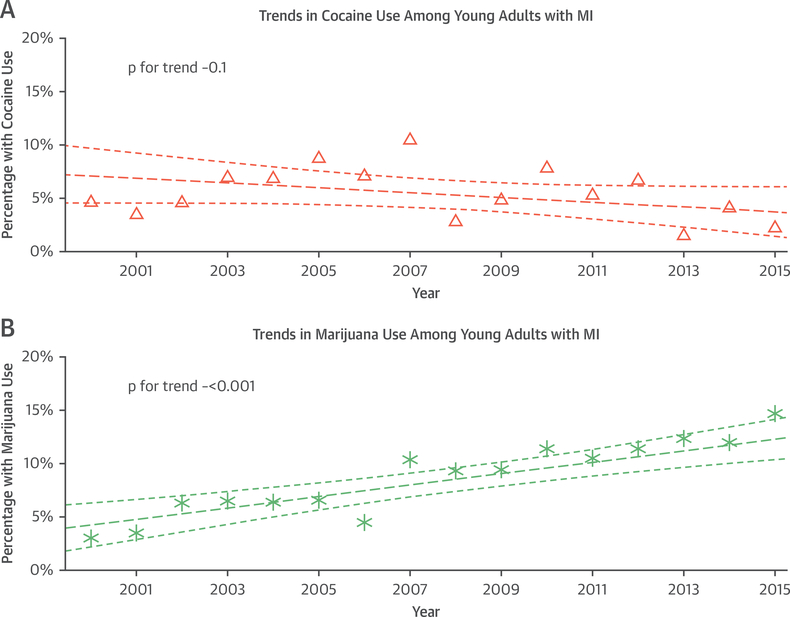
Over the study period of 2000 to 2016, substance abuse in our cohort increased significantly (odds ratio: 1.06 increase per year; p < 0.001). However, this was driven largely by increasing marijuana use (odds ratio: 1.09 increase per year; p < 0.001) because cocaine use stayed relatively constant (Figures 1A and 1B).
FIGURE 1. Trends in Substance Use Over Time.
Trends in (A) cocaine and (B) marijuana use over the course of the study period, 2000 to 2016. The thick dashed line represents a fitted regression Line and the thinner dashed lines are confidence intervals. MI = myocardial infarction. Triangles represent the percentage of patients with MI for the particular year who used cocaine. Asterisks represent the percentage of patients with MI for the particular year who used marijuana.
LONG-TERM OUTCOMES.
All-cause death.
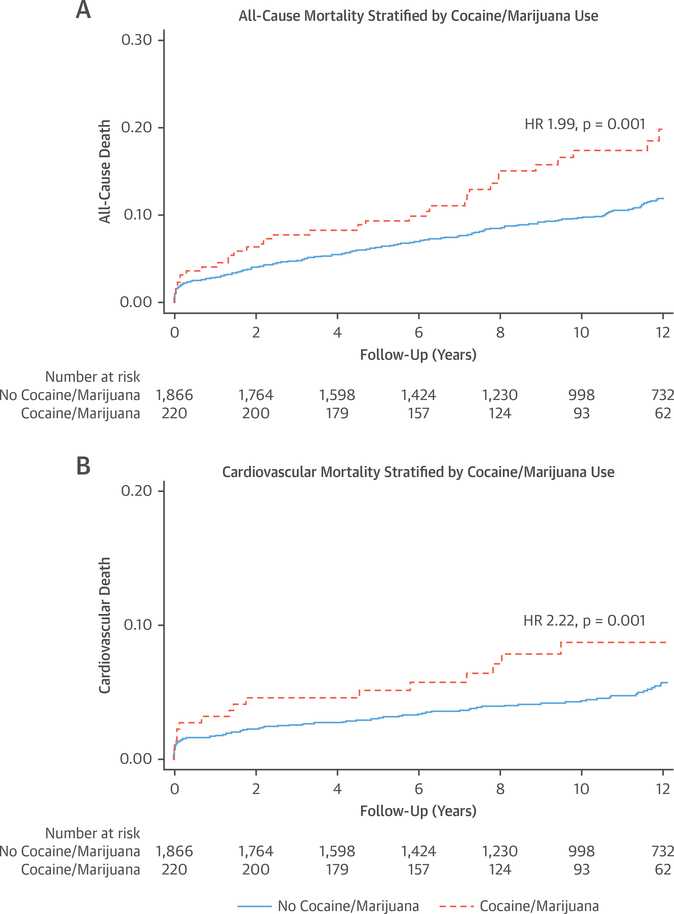
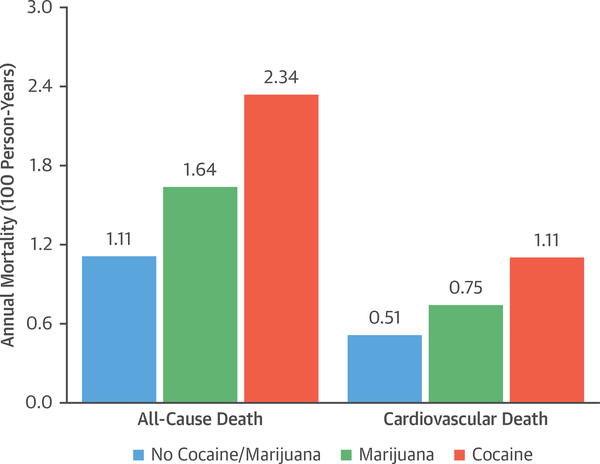
Over a median follow-up time of 11.2 years (interquartile range: 7.3 to 14.2 years), patients with substance use had significantly higher all-cause mortality compared with patients without substance use (18.8% vs. 11.3%; p = 0.001) (Figures 2A and 2B). When comparing annual death rates, the rate was highest in the cocaine use group at 2.34 deaths per 100 person-years, followed by 1.64 deaths in the marijuana use group and 1.11 deaths in the no abuse group (p = 0.002) (Figure 3).
FIGURE 2. All-Cause Mortality and Cardiovascular Mortality in Patients With Substance Abuse.
Kaplan-Meier curves of (A) all-cause mortality and (B) cardiovascular mortality in patients with substance abuse compared with patients without substance abuse. HR = hazard ratio.
FIGURE 3. Annual Incidence Rates for All-Cause Death and Cardiovascular Death.
Observed annual death and cardiovascular death rates are shown by drug of abuse in 100 person-years.
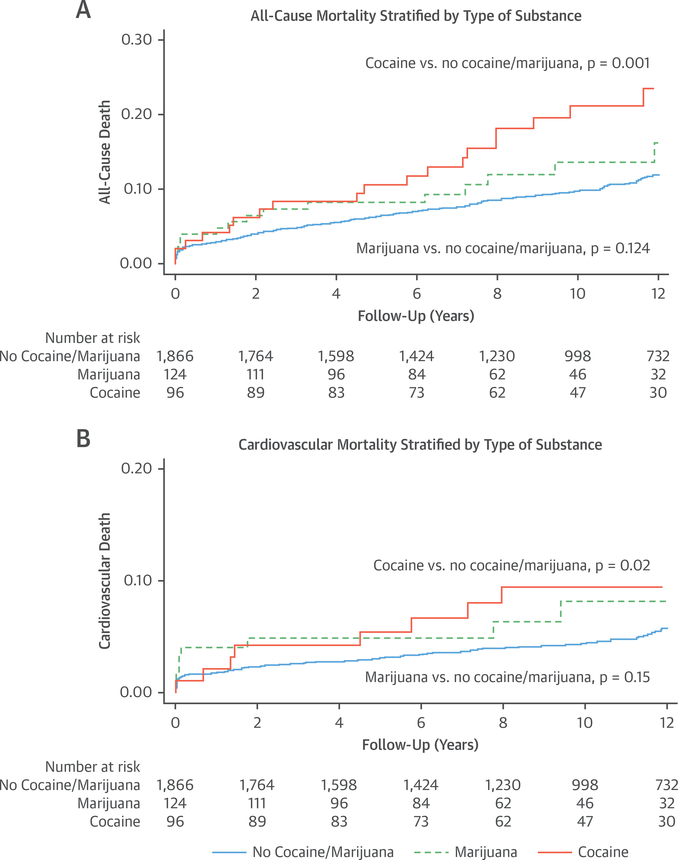
The unadjusted hazard ratio for all-cause death was 1.77 (95% confidence interval [CI]: 1.26 to 2.51; p = 0.001) among substance users. When examining each substance separately, the unadjusted hazard ratio among cocaine users was 2.13 (95% CI: 1.36 to 3.33; p = 0.001), whereas the hazard ratio among marijuana users was 1.47 (95% CI: 0.90 to 2.42; p = 0.012) (Figures 4A and 4B).
FIGURE 4. All-Cause Mortality and Cardiovascular Mortality Stratified by Use of Cocaine or Marijuana.
Kaplan-Meier curves of (A) all-cause mortality and (B) cardiovascular mortality by drug of abuse.
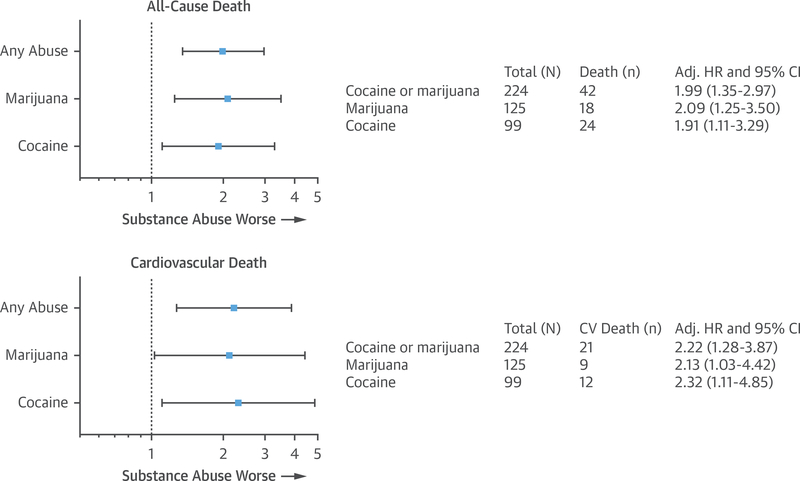
After adjusting for age, sex, presence of diabetes, hypertension, peripheral vascular disease, smoking, HDL-C, triglycerides, revascularization, creatinine, medications at discharge, and length of stay, the adjusted hazard ratio was 1.99 (95% CI: 1.35 to 2.97; p = 0.001) for any substance use, 2.09 (95% CI: 1.25 to 3.50; p = 0.005) for marijuana, and 1.91 (95% CI: 1.11 to 3.29; p = 0.02) for cocaine (Figure 5, Online Table 2).
FIGURE 5. Adjusted Cardiovascular Mortality and All-Cause Death.
Forest plots are shown for adjusted models of all-cause and cardiovascular (CV) death. AU-cause death was adjusted for age, sex, presence of diabetes, hypertension, peripheral vascular disease, smoking, high-density lipoprotein cholesterol, triglycerides, revascularization, creatinine, medications at discharge, and length of stay. CV death was adjusted for age, presence of diabetes, hypertension, peripheral vascular disease, smoking, high-density lipoprotein cholesterol, creatinine, medications at discharge, and length of stay. Adj. HR = adjusted hazard ratio; CI = confidence interval.
Cardiovascular death.
When compared with individuals who were not substance users, patients with substance use had higher cardiovascular mortality (9.4% vs. 5.3%; p = 0.01) (Figures 2A and 2B). When comparing annual cardiovascular death rates, the observed cardiovascular death rate was similarly highest in the cocaine use group, at 1.11 deaths per 100 person-years, followed by 0.75 deaths per 100 person-years in patients with marijuana use and 0.51 deaths in patients without any substance use (p = 0.03) (Figure 3).
The unadjusted hazard ratio for cardiovascular death was 1.90 (95% CI: 1.16 to 3.11; p = 0.01) among substance users overall, 2.18 (95% CI: 1.14 to 4.19; p = 0.02) in cocaine users, and 1.66 (95% CI: 0.84 to 3.29; p = 0.15) among marijuana users (Figures 4A and 4B).
After adjusting for age, presence of diabetes, hypertension, peripheral vascular disease, smoking, HDL-C, creatinine, medications at discharge, and length of stay, the hazard ratio was 2.22 (95% CI: 1.28 to 3.87; p = 0.005) for overall substance abuse, 2.13 (95% CI: 1.03 to 4.42; p = 0.042) for marijuana users, and 2.32 (95% CI: 1.11 to 4.85; p = 0.025) for cocaine users (Figure 5, Online Table 3).
The increased all-cause and cardiovascular death rates observed in patients with either cocaine or marijuana use remained robust after analysis using IPW weights (Online Appendix, Online Figures 1A and 1B).
DISCUSSION
Our study examined the prevalence and outcomes of substance use in a large cohort of individuals who experienced an MI at a young age. We found that approximately 10% of our cohort used cocaine and/or marijuana, and such use was associated with increased all-cause and cardiovascular mortality (Central Illustration). Although patients with substance use in our study were younger and were less likely to have traditional risk factors such as diabetes, hypertension, and hyperlipidemia, they were more likely to have an STEMI at presentation and had higher peak troponin values. The lower burden of traditional risk factors among substance users suggests that their substance abuse provided sufficient risk to overcome their otherwise lower risk profiles than their non–drug user counterparts. Therefore, our results support other data that substance abuse is an important risk factor for developing premature MI and suggest that among patients who experience an MI, the use of cocaine or marijuana is associated with an adverse long-term prognosis.
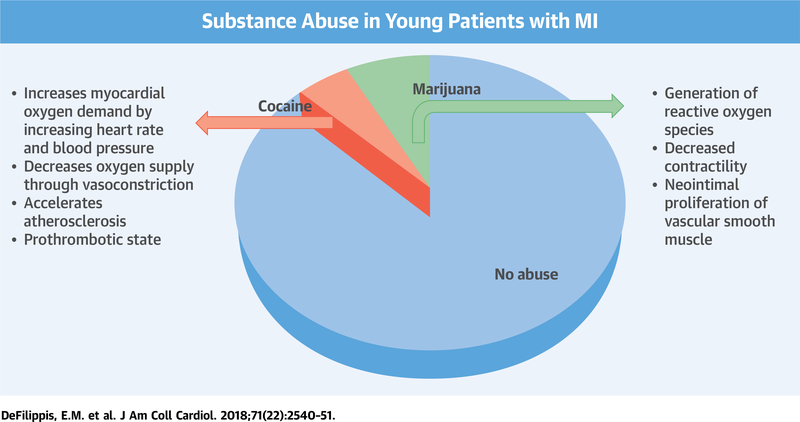
CENTRAL ILLUSTRATION. Cocaine and Marijuana Use in Young Patients With Myocardial Infarction.
We found that in our young patients with myocardial infarction (MI), approximately 10% were using either cocaine or marijuana. Cocaine use increases myocardial oxygen demand, decreases oxygen supply, and accelerates atherosclerosis and can create a prothrombotic state. Marijuana use leads to the generation of reactive oxygen species, decreases myocardial contractility, and leads to neointimal proliferation of vascular smooth muscle cells. Substance abuse was associated with increased all-cause mortality and cardiovascular mortality.
COCAINE.
Cocaine use was found in 5% of our cohort, and it was associated with a higher frequency of STEMI, as well as higher troponin values. Importantly, we observed that patients with cocaine use were less likely to be prescribed beta-blockers but just as likely to receive all other medical therapy following an MI. This has been shown in other cohorts as well (14,15). The underuse of beta-blockers may reflect the theoretical concerns that beta-blockers may exacerbate coronary vasospasm and create “unopposed” stimulation of alpha-adrenergic receptors. However, multiple studies have shown the safety of beta-blocker therapy in these patients (16–19). However, the increased event rate among cocaine users was present even after accounting for these differences in beta-blocker use on discharge.
Cocaine users in our study group were also modestly less likely to undergo coronary angiography during their index hospitalization for MI. However, if coronary angiography was performed, cocaine users were just as likely to require revascularization with percutaneous coronary intervention.
MARIJUANA.
Our study revealed an increase in use of marijuana over time among young adults who experienced an MI. This finding, along with the recent legalization of marijuana, underscores the importance of determining the cardiovascular implications of marijuana use. Although our finding on the increased use of marijuana over time parallels national data from the National Institute of Drug Abuse, it is possible that these results are confounded by increased questioning and testing of individuals over time. A multicenter cohort study of 3,886 MI survivors that followed patients for up to 18 years found an increased, although not statistically significant, mortality rate among marijuana users (5). Our study focused on younger individuals and revealed that marijuana use was associated with a statistically significant difference in all-cause death and cardiovascular deaths, after adjusting for baseline characteristics. Interestingly, we found that patients who used marijuana were more likely to present with cardiac arrest associated with MI. One hypothesis for this finding is that a proarrhythmic state may be induced by a tetrahydrocannabinol-induced increase in circulating catecholamines (20). There is also a potential multisubstance effect that could be influenced by concomitant use of other substances such as alcohol and opioids. These effects may be more prevalent in substance abusers and are not just specific to our study.
As young individuals continue to use cocaine and marijuana, it is important to recognize and educate patients and clinicians alike about the potential cardiovascular consequences of these substances. When young individuals present with an MI, clinicians should assess for potential substance use. This not only allows their care teams to risk stratify these patients, but it also provides an opportunity for patient education and implementation of interventions that may lower these risks. Our findings support the need for aggressive interventions for substance use cessation, as well as aggressive treatment of all other underlying cardiovascular risk factors, among young patients who experience an MI while actively using cocaine or marijuana. Importantly, we found an increase in all-cause death among both cocaine and marijuana users. The reason for this finding may be that individuals who use substances may also engage in other high-risk behaviors that can lead to noncardiovascular mortality.
STUDY LIMITATIONS.
One limitation of our study is its retrospective nature. However, this study design allowed us to examine a large number of individuals who experienced an MI at a young age; enrolling a similar number of patients using a prospective design would take >10 years to conduct. Because our cohort was limited to individuals who experienced an MI, we were not able to determine the prevalence of substance use in the at-risk population. Thus, although we were unable to provide data on the relative risk of cocaine and/or marijuana use for causing MI, our data support that this risk factor is common and that it is associated with adverse events post-MI. Furthermore, because substance abusers had a lower prevalence of other traditional risk factors, our findings support the known causal role of cocaine in the development of MI, but they also support the less commonly recognized risks associated with marijuana use. Our study was underpowered to detect for differences in outcomes between marijuana and cocaine users. Therefore, there remains a need for more research on marijuana and its effects on cardiovascular health.
Our analysis included only type 1 MI. Despite our careful adjudication process, we realize that there is a risk of misclassification bias between type 1 and type 2 MI. In addition, we did not have data on left ventricular systolic function available for our cohort.
Our analysis was limited to cocaine and marijuana. Although we attempted to obtain data on opioid abuse, it was difficult to distinguish nonprescription opioid abuse from medical use, particularly because many patients with an MI may have received morphine in the emergency room for management of chest pain. In addition, alcohol use was not consistently captured. The use of other drugs, such as amphetamines and barbiturates, was infrequent in our sample.
The association between substance abuse and cardiovascular outcomes may be driven by confounders including lifestyle and behavioral factors. Although we adjusted for an extensive array of baseline covariates, including demographics, comorbidities, laboratory values, revascularization procedures, and medications, we realize that other unmeasured confounders may remain.
Finally, we do not have data on participation in substance abuse rehabilitation programs. However, we realize that this information would be helpful in guiding future strategies for treatment and counseling in young patients with substance abuse.
CONCLUSIONS
The use of cocaine and/or marijuana is present in 10% of patients with an MI at age ≤50 years and is associated with higher all-cause and cardiovascular mortality. These findings reinforce current recommendations for screening for substance abuse among adults who experience an MI at a young age and highlight the need for implementation of therapies and counseling that could prevent future adverse events.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Use of cocaine and/or marijuana is prevalent in patients presenting with MI before age 50 years and is associated with high all-cause and cardiovascular mortality.
TRANSLATIONAL OUTLOOK:
Further research is needed to clarify the cardiovascular effects of these commonly used substances, identify characteristics of young patients at greatest risk of developing MI during use, and develop evidence-based treatment strategies to improve outcomes in those patients presenting with acute coronary syndromes.
Acknowledgments
Dr. Gupta is supported by National Institutes of Health grant 5T32HL094301. Dr. Qamar is supported by National Institutes of Health grant T32HL007604. Dr. Januzzi has received grant support from Roche Diagnostics, Abbott, Singulex, and Prevencio; has received consulting income from Roche Diagnostics, Critical Diagnostics, Janssen, and Novartis; and participates in clinical endpoint committees or data safety monitoring boards for Novartis, Amgen, Pfizer, Janssen, AbbVie, and Boehringer-Ingelheim. Dr. Di Carli has received a research grant from Spectrum Dynamics; is on the scientific advisory board of Sanofi; is on the medical advisory board of General Electric Healthcare; and has received consulting honoraria from Sanofi and General Electric Healthcare. Dr. Bhatt is on the advisory boards of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; is on the board of directors of the Boston VA Research Institute and the Society of Cardiovascular Patient Care; is chair of the American Heart Association Quality Oversight Committee; is on the data monitoring committees of the Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, acc.org; vice-chair, American College of Cardiology accreditation committee), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today’s Intervention), and the Society of Cardiovascular Patient Care (secretary/treasurer), WebMD (CME steering committees); is deputy editor of Clinical Cardiology; is chair of the NCDR-ACTION Registry Steering Committee and the VA CART Research and Publications Committee; has received research funding from Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); is a site co-investigator for Biotronik, Boston Scientific, and St. Jude Medical (now Abbott); is a trustee of the American College of Cardiology; and has conducted unfunded research for FlowCo, Merck, PLx Pharma, and Takeda. Dr. Blankstein has served on the advisory board of Amgen; and has received research support from Amgen, Sanofi, and Gilead Sciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Joseph Alpert, MD, served as Guest Editor for this paper. Presented on March 18, 2018, at the American College of Cardiology 2018 Scientific Sessions.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- HDL-C
high-density lipoprotein cholesterol
- IPW
inverse treatment of probability weighting
- MI
myocardial infarction
- STEMI
ST-segment elevation myocardial infarction
Footnotes
APPENDIX For a supplemental sensitivity analysis as well as supplemental tables and a figure, please see the online version of this paper.
REFERENCES
- 1.National Institute for Drug Abuse. Nationwide Trends: Drug Facts. 2015. Available at: https://www.drugabuse.gov/publications/drugfacts/nationwide-trends. Accessed April 12, 2018.
- 2.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-Elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64: e139–228. [DOI] [PubMed] [Google Scholar]
- 3.Moliterno DJ, Willard JE, Lange RA, et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med 1994; 330:454–9. [DOI] [PubMed] [Google Scholar]
- 4.Ravi D, Ghasemiesfe M, Korenstein D, Cascino T, Keyhani S. Associations between marijuana use and cardiovascular risk factors and outcomes: a systematic review. Ann Intern Med 2018;168:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J 2013;165:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018;15:151–66. [DOI] [PubMed] [Google Scholar]
- 7.Renard D, Taieb G, Gras-Combe G, Labauge P. Cannabis-related myocardial infarction and cardioembolic stroke. J Stroke Cerebrovasc Dis 2012;21:82–3. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am J Cardiol 2014;113: 187–90. [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Collins B, Qamar A, et al. Study of young patients with myocardial infarction: design and rationale of the YOUNG-MI Registry. Clin Cardiol 2017;40:955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, et al. Third Universal Definition of Myocardial Infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 11.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–9. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Washam JB, Mountantonakis SE, et al. Characteristics, management, and outcomes of cocaine-positive patients with acute coronary syndrome (from the National Cardiovascular Data Registry). Am J Cardiol 2014;113:749–56. [DOI] [PubMed] [Google Scholar]
- 15.Shitole SG, Kayo N, Srinivas V, et al. Clinical profile, acute care, and middle-term outcomes of cocaine-associated ST-segment elevation myocardial infarction in an inner-city community. Am J Cardiol 2016;117:1224–30. [DOI] [PubMed] [Google Scholar]
- 16.Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med 2010;170:874–9. [DOI] [PubMed] [Google Scholar]
- 17.Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med 2008;51:117–25. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim M, Maselli DJ, Hasan R, Hamilton A. Safety of b-blockers in the acute management of cocaine-associated chest pain. Am J Emerg Med 2013;31:613–6. [DOI] [PubMed] [Google Scholar]
- 19.Fanari Z, Kennedy KK, Lim MJ, Laddu AA, Stolker JM. Comparison of in-hospital outcomes for beta-blocker use versus non-beta blocker use in patients presenting with cocaine-associated chest pain. Am J Cardiol 2014;113: 1802–6. [DOI] [PubMed] [Google Scholar]
- 20.Sidney S Cardiovascular consequences of marijuana use. J Clin Pharmacol 2002;42: 64S–70S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.