Summary
Clobazam is an oral 1,5‐benzodiazepine used worldwide for the treatment of many types of epilepsies, although it is currently only approved for Lennox–Gastaut syndrome in the USA. This anticonvulsant and anxiolytic therapeutic has repeatedly demonstrated great efficacy and a high safety profile in refractory epilepsy as well as in a few monotherapy trials in both children and adults. Clobazam allosterically activates the GABAA receptor, and it binds less to subunits that mediate sedative effects than other benzodiazepines. It acts quickly, maintaining a therapeutic effect for a long duration due to its active metabolite, N‐desmethylclobazam. Dosage is between 5 mg and 40 mg a day, depending on patient weight, efficacy, and tolerability. Efficacy tolerance has not been a problem in the best studies. Clobazam has provided many benefits to epileptic patients. It should be used by clinicians early as an adjuvant therapy in the treatment of refractory epilepsy and even considered as monotherapy in a broad spectrum of epilepsy syndromes.
Keywords: Anticonvulsants, Benzodiazepines, Clobazam, Epilepsy
Epilepsy
Epilepsy is a serious condition that affects approximately 1% of the world population 1. The disorder is characterized by recurrent seizures, but people with epilepsy often suffer from other comorbid conditions as well. In fact, 11–25% of people with epilepsy suffer from a diagnosis of anxiety, which is found in only 7–11% of the general population 2.
Unfortunately, approximately one‐third of patients who use antiepileptic drugs (AEDs) fail to have seizures controlled for at least 1 year 3, 4. There is a need for more effective and safe treatments for refractory epilepsy. In pharmaceuticals, it is difficult to find a clear efficacy difference between many AEDs in part because of insufficient well‐designed epilepsy randomized controlled trials 5. However, the choice of a particular AED should not just depend on efficacy, but also other drug‐related properties such as pharmacokinetics, tolerability, safety, and drug interactions 5. Drug selection is also influenced by patient characteristics such as age, gender, comorbidities, and economic issues.
Clobazam is a promising therapeutic, as it has demonstrated positive outcomes in many criteria. Although it has been available in many countries (but not all) for epilepsy and anxiety for more than four decades, its relatively recent approval for use in the United States made us believe a review would be beneficial.
Clobazam History
Clobazam, also known by many brand names including Onfi, Frisium, Urbanyl, and others, is an AED with the chemical name 7‐chloro‐1‐methyl‐5‐phenyl‐1,5‐benzodiazepine. It contains nitrogen atoms in the 1 and 5 positions in the B ring, which distinguishes it from other classic 1,4‐benzodiazepines (Figure 1).
Figure 1.
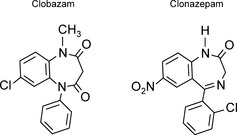
Structure of clobazam and clonazepam. Clobazam, a 1,5‐benzodiazepine, is shown here in comparison to clonazepam, a 1,4‐benzodiazepine.
The development of the benzodiazepines (including clobazam) began as an exploration for better tranquilizers in the 1950s, following the introduction of the revolutionary psychoactive drug, chlorpromazine 6. Leo Sternbach, a medicinal chemist, was working at Hoffmann‐La Roche, trying to quickly create many novel biologically active compounds 6, 7. However, other important tasks arose, and the work was set aside for a couple years. One day during a laboratory cleanup, coworker Earl Reeder found a well‐crystallized base and its hydrochloride that had been left over from the initial project. After the compound, later named chlordiazepoxide, was submitted for testing to Lowell Randall in the pharmacology division, it was discovered to have a sedating, muscle‐relaxing, and anticonvulsant effect on mice with low toxicity 6. Sternbach and his team developed many related compounds, the benzodiazepines, and they obtained a patent in 1959 for chlordiazepoxide (Librium). Clobazam was among the many compounds later produced, and it was shown to have less sedative effects than other benzodiazepines 8.
Clobazam was originally established as a nonsedative agent to treat anxiety in Australia in 1970 and epilepsy in France in 1974 9. It is now approved in many countries as an adjunctive therapy to treat epilepsy and anxiety 10. In December 2008, clobazam was granted orphan drug status by the FDA for treatment of Lennox–Gastaut syndrome 11. By October 2011, the FDA had approved clobazam as an adjunctive treatment for Lennox–Gastaut syndrome in people older than 2 years.
Clobazam has been tested extensively in over 50 studies on more than 3000 epileptic patients, and it has provided great clinical benefit with only mild side effects 12.
Mechanism
Clobazam binds allosterically to the GABAA receptor to exert its anticonvulsant and anxiolytic effects 9. This action increases the frequency of chloride channel opening, allowing chloride to enter and hyperpolarize the neuron. Clobazam also increases the production of GABA transporter 3 13.
GABAA receptors are made up of five subunits: typically two α‐subunits, two β‐subunits, and one γ‐subunit 9. Each subunit has multiple types. Receptors located in the synapse are most likely to be composed of α1βγ2, α2βγ2, or α3βγ2 14. The α1 subunit mediates sedation effects, the α2 subunit controls anxiolytic effects, and all α subunits mediate anticonvulsant effects 9, 15. Clobazam binds in the junction between the α and γ2 subunits (Figure 2) 15. It has a greater selectivity for α2 subunits over α1 subunits than the 1,4‐benzodiazepines, which decreases the likelihood of causing sedation 15. This selective binding may also explain the decreased tendency for efficacy tolerance to develop.
Figure 2.
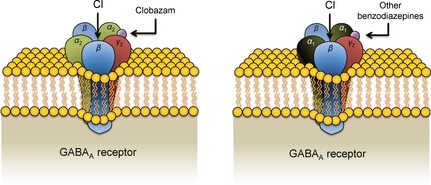
Clobazam and other benzodiazepine binding sites on the GABAA receptor. Clobazam binds between the α2 and γ2 subunits on the GABAA receptor, while other benzodiazepines bind between the α1 and γ2 subunits. This selectivity allows clobazam to mediate less sedative effects than other benzodiazepines.
Clinical Pharmacology and Pharmacokinetics
Clobazam is taken orally, and it requires 1 to 4 h to reach its highest concentration in the blood 16. The lipophilic drug becomes evenly distributed throughout the body, excluding the gastrointestinal tract 17. Food does not affect the extent of absorption 18. Eighty‐five percent of clobazam binds to protein in the blood, so its free concentration can be influenced by hepatic disease or other substances with a high affinity for plasma protein binding sites 8.
Clobazam is hepatically metabolized via the cytochrome P450 pathway, and it has a half‐life that averages 18 h 19. CYP3A4, and to a lesser extent CYP2C19, demethylates clobazam to the pharmacodynamically active N‐desmethylclobazam 20, 21. N‐desmethylclobazam has a half‐life of about 50 h, which is more than twice as long as clobazam's half‐life 19. The metabolite is finally transformed into the inactive 4′‐hydroxy‐N‐clobazam, predominantly by CYP2C19 21.
As CYP2C19 plays a role in the metabolism of clobazam and N‐desmethylclobazam, mutations in this gene can increase N‐desmethylclobazam concentration, which leads to a higher risk of side effects 22. This is especially important in Asian populations, where it is estimated that 35% of people carry the mutated allele 22.
Interactions with Other Drugs
Patients with epilepsy often take multiple medications for their condition and possible other disorders, so it is important to minimize harmful drug–drug interactions when prescribing treatments. Although clobazam is highly protein bound, it is unlikely to displace other bound drugs because its concentration is so low in the plasma. In fact, clobazam has not been found to have enzyme inducing or inhibiting properties, and pharmacokinetic studies have found no change in the levels of other AEDs when clobazam was added 21, 23, 24, 25, 26, 27.
Some CYP3A4 and CYP2C19 inducers, such as carbamazepine, phenobarbital, and phenytoin, may increase clobazam metabolism. Yamamoto et al. found that these three drugs, taken alone or in combination, lowered the clobazam concentration to dose ratio by a mean of 60.8% in adults and 44.3% in children when levels were compared to patients who only took clobazam 28. A retrospective study also found a decreased level to dose ratio of clobazam with each of these drugs 23. However, these medications have been shown to also increase levels of the therapeutically active N‐desmethylclobazam, negating the possible reduction of clinical efficacy 29. Stiripentol, a CYP2C19 inhibitor, also increases N‐desmethylclobazam levels by inhibiting the metabolite's hydroxylation 10.
In contrast, other studies found that patients taking clobazam and stable dosages of carbamazepine, phenobarbital, phenytoin, valproate, felbamate, or oxcarbazepine had no change in the oral clearance of clobazam and the pharmacokinetics of N‐desmethylclobazam 21. Regardless of other drugs' effects on clobazam or lack thereof, the 1,5‐benzodiazepine has a sufficiently wide therapeutic window that small differences in metabolism might not make a clinically significant change in response.
Although blood levels of both clobazam and N‐desmethylclobazam can be obtained, a well‐defined target or “therapeutic” range has not been established. Dosing is dictated more by clinical response.
Clinical Trials
Refractory Epilepsy
A shortage of controlled randomized blinded studies makes assessment of efficacy and adverse effects somewhat limited. However, an extensive literature exists and will be described.
Clobazam has often been used as an adjunctive therapy. It has been very effective in patients with refractory epilepsy, including those with focal (partial onset), generalized tonic–clonic, myoclonic, and absence seizures 12. Koeppen et al. led a double‐blind crossover study in which 129 therapy‐resistant patients who mainly had complex partial seizures took clobazam or a placebo for two intervals of 3 months, separated by a 1‐month switch over period 30. They found that 19% of patients were freed from their seizures, compared to 0% on the placebo. An open, retrospective study by Montenegro et al. found that 57% of 97 adult patients with refractory partial epilepsy achieved a >50% improvement in seizure control over a mean of 16.7 months 31. Clobazam has been similarly effective in children, with 52% of patients experiencing a 50% reduction in seizure frequency while on the drug 25. Kalra et al. found clobazam to be very effective against all types of epilepsy in 88 children: 60.2% of patients achieved complete seizure control 32.
Long‐term studies in refractory epilepsy have also yielded positive results for clobazam. One open‐ended study by Scott et al. in 30 patients over 2–3 years found that clobazam greatly reduced seizure frequency in 43% of patients, with few adverse effects 33. Buchanan reports that 75% of his 56 patients who took the drug for up to 8 years (mean of 3 years) experienced more than a 50% reduction in number of seizures 34. He recommends that if a patient fails to show improvement on clobazam after the first 1–3 months, it is unlikely that clobazam will be effective, and the drug should be withdrawn. This experience resembles that of Schmidt after using the drug for decades, and he recommends that clobazam be tried if first‐line drugs fail 35.
Lennox–Gastaut Syndrome
Studies looking specifically at Lennox–Gastaut syndrome found that clobazam greatly decreased seizure frequency with little adverse effects. In a Phase II, randomized, double‐blind study, 68 patients with Lennox–Gastaut syndrome were given clobazam at either 0.25 or 1.0 mg/kg/day as an adjunctive therapy (OV‐1002) 36. Over the course of a couple months, the frequency of drop and nondrop seizures diminished in both the high‐ and low‐dose groups. A dose–response relation was found. Participants in both groups experienced a similar frequency of mild to moderate adverse effects, and the most common ones were somnolence, lethargy, and sedation.
Successful results continued in Phase III trials (OV‐1012). In the 125 patients who completed the study, average weekly drop seizure rates decreased 12.1%, 41.2%, 49.4%, and 68.3% for the placebo, 0.25, 0.5, and 1.0 mg/kg/day clobazam dosages, respectively 37. Somnolence continued to be the most common adverse event, along with pyrexia and upper respiratory infections.
Eligible patients from these two trials were enrolled in an open label extension study that allowed them to take clobazam for up to 6 years 38. The results showed that clobazam was indeed effective over a long time period, with 80% of patients “very much improved” or “much improved” after 3 years. A majority of patients who initially responded well to clobazam maintained that response after several years. For example, in patients who attained greater than a 50% decrease in drop seizure frequency within the first 3 months, 86% preserved that level of reduction at the 3‐year mark. This effect was achieved without significant increases in dosages over the years within the limitations of the open‐ended trial. Adverse effects were consistent with the previous two trials.
An analysis of several clinical studies revealed that high‐dose (1.0 mg/kg/day) and medium‐dose (0.5 mg/kg/day) clobazam were superior to rufinamide, felbamate, lamotrigine, and topiramate in reducing drop seizures 39, 40. Clobazam also caused a greater median reduction in total seizure frequency than these four drugs, and it is recommended that clobazam be considered as an adjunctive therapy before more invasive interventions 40.
Monotherapy
A recent Cochran review concludes that there is a shortage of randomized controlled trials in clobazam monotherapy 41. However, other types of studies show that children who have taken clobazam as monotherapy have generally seen positive results. In a Canadian study of 235 patients, clobazam demonstrated equivalent efficacy to carbamazepine and phenytoin for children with partial (focal) epilepsies or generalized tonic–clonic seizures (Table 1) 42, 43. As a whole, 56% of children remained on their original medication after 1 year, and there was no significant difference between patients on clobazam and those taking carbamazepine or phenytoin. In addition, Dulac et al. led a study in which 25 children were treated with only clobazam for up to 26 months, and 17 of 25 (68%) of them achieved satisfactory results or a 75% decrease in the baseline frequency of seizures 44. Clobazam has been recommended for consideration as “first line” monotherapy for all children with partial (focused) epilepsies and some with generalized epilepsies 42.
Table 1.
Clobazam, carbamazepine, and phenytoin as monotherapy in children
| Clobazam (N = 119) | Carbamazepine (N = 78) | Phenytoin (N = 38) | |
|---|---|---|---|
| Clinical Benefit | 55% | 57% | 57% |
| Seizure Free (12 months) | 23% | 25% | 11% |
| Tolerance | 7.5% | 4.2% | 6.7% |
| Withdrawn for Safety | 4% | 17% | 10% |
These data show outcomes of the Canadian Study Group for Childhood Epilepsy (1998). Clobazam demonstrated similar efficacy to carbamazepine and phenytoin. Clinical benefit is defined as patients remaining on initial therapy for 1 year.
Adults have not been as well studied as children in monotherapy trials, but results are still favorable. An open label trial completed by Mehndiratta et al. gave clobazam to 26 drug‐naïve epileptic patients over a 24‐week period 45. It found that 64% became seizure free, and another 20% had more than a 50% decrease in seizure frequency.
Antianxiety Properties
In addition to its anticonvulsant properties, clobazam has also been shown to reduce anxiety. Clobazam (30–80 mg/day) was superior to diazepam (15–40 mg/day) in a study of 159 anxious outpatients, although they both produced a similar rate of side effects 46. Another placebo‐controlled study between the two drugs found that they were approximately equal in treating anxiety, but dizziness was more common with diazepam 47. Lemoine et al. found that clobazam, lorazepam, and buspirone were all equally effective in 128 patients with generalized anxiety disorder 48. As patients with epilepsy tend to have higher rates of anxiety, this anxiolytic quality of clobazam is especially beneficial 2.
Tolerance
Like many other benzodiazepines, clobazam induces tolerance in some patients who take the drug. According to a review by Robertson, approximately 36% of patients develop tolerance, but the percentage ranges from 0 to 86% depending on the study 49. This is in part due to different definitions of tolerance between studies. The largest and most well‐controlled studies show that tolerance is not usually a problem. For example, a large‐scale Canadian study that followed 877 adult and pediatric patients over 7 years found that only 9% of patients developed tolerance to the point of discontinuing therapy 50. In addition, the clinical trials in Lennox–Gastaut found that most patients who initially responded to clobazam continued to respond over several years 38. There has been no reliable way to predict which patients will develop tolerance upon starting clobazam, but patients who respond well to long‐term therapy tend to have had epilepsy for a shorter period of time, have a known cause of epilepsy, and have increased clobazam blood levels when compared with patients who develop tolerance 51.
Clobazam performs similarly to other anticonvulsants in terms of tolerance. In a large multicenter monotherapy Canadian study, 7.5% of children developed tolerance to clobazam, compared to 4.2% of patients on carbamazepine and 6.7% of patients on phenytoin 42, 43. A Japanese study found that tolerance affected 48% of patients taking clorazepate for >4 weeks, but only 24% of patients treated with clobazam for more than 3 months 52. In fact, 70% of the patients who had developed “tolerance” to clobazam started responding to the drug again after the dosage was maintained or increased 52. Intermittent therapy may decrease tolerance, but further studies are needed to confirm this effect 53.
Tolerance may limit the benefits of clobazam for some people, but a sizable proportion of patients retain response to therapy. Approximately 28% of patients can expect long‐term benefits from clobazam 54. In fact, a majority of patients who experience a substantial decrease in seizure frequency for the first couple months of therapy tend to maintain these benefits for months and even years. In a study of 183 patients with intractable complex partial seizures, Shimizu et al. found that 61 patients initially became seizure free after taking clobazam 55. Approximately half of these patients developed tolerance within the first 3 months of therapy. Recurrence of seizures before 3 months may simply reflect regression to the mean because seizure frequency fluctuates. However, 74.2% of the patients who were seizure free at the 3‐month mark remained seizure free for the subsequent 3 months, indicating that clobazam still confers great benefit to many patients in the long term.
Safety
Clobazam is generally considered safe to use, with only mild side effects when compared to other AEDs. As with any benzodiazepine, there is a risk for dependence 18. In addition, data from 50 clinical studies collected from over 3000 epileptic adult and pediatric patients show that the most common side effects include sedation, dizziness, and ataxia 12. These adverse effects are dose‐dependent, and approximately 40% of patients experience them in mild to moderate severity. However, clobazam causes less sedation than other 1,4‐benzodiazepines 9. For example, healthy volunteers who took clobazam at 10 or 20 mg/day experienced less psychomotor and sedation side effects than those who took clonazepam at 0.5 and 1 mg/day 56. Mood and behavioral changes have also been noted 57. Severe adverse effects do exist, but they are exceedingly rare. In over 1.1 million patient‐years of clobazam exposure recorded from 1994 to 2004, hepatic failure, drug‐related status epilepticus, or death have been reported in only five patients 12.
Despite the overall safety of clobazam, there have been case reports linking the drug to the development of toxic epidermal necrosis (TEN) and Stevens–Johnson syndrome (SJS) 58, 59. Even though these cases are severe, they are very rare. The FDA reports 6/31,000 US patients and 15 patients outside the USA who developed SJS/TEN while taking clobazam 60. There appears to be a disconnection between worldwide and US experience, possibly indicating under‐reporting of these adverse effects in other countries. However, almost all cases involved concurrently taking one or more drugs associated with SJS/TEN, such as lamotrigine. Although most cases improved after withdrawing clobazam, it is unclear if this was spontaneous or a result of stopping clobazam 60.
Withdrawal Effects
Abrupt discontinuation of clobazam is often associated with withdrawal adverse effects. These symptoms include seizures, irritability, restlessness, difficulty in concentration, and insomnia, among many others 61.
Such adverse withdrawal reactions can be avoided if clobazam is gradually discontinued. In four Phase I studies where clobazam was discontinued abruptly, 68 of 207 patients suffered 193 withdrawal adverse events 61. In contrast, in three Phase II or Phase III studies where clobazam was gradually tapered off over 3 weeks, none of the 87 participants experienced withdrawal adverse events 61.
Dosage Regimens
Clobazam (Onfi) is available in the USA in both tablet and liquid oral form. Tablets are either 10 mg or 20 mg, but they may be cut in half on the score line. The oral solution contains 2.5 mg/mL. Prescribing information recommends doses between 5 mg and 40 mg, depending on patient weight, clinical efficacy, and tolerability 18. Doses >5 mg/day should be given in 2 divided doses, although the pharmacokinetics suggest once daily dosing should be adequate. For patients weighing 30 kg or less, clobazam should be initiated at 5 mg/day. Dosage may be increased to 10 mg/day after 1 week to a maximum of 20 mg/day after 2 weeks, as needed. Patients weighing more than 30 kg should be started at 10 mg/day. Prescribing information recommends that dosage may increase to 20 mg/day after 1 week to a maximum of 40 mg/day after 2 weeks, as needed 18. However, dosages up to 80 mg/day have been used and may be given to patients 38. Although most of these values are from the recommended dosing from the package insert, the senior author (RM) uses about half these doses and titrates over several weeks as tolerated.
Geriatric patients, CYP2C19 poor metabolizers, and people who suffer from mild or moderate hepatic impairment will metabolize clobazam slower, so they should be initiated at 5 mg/day at most, regardless of body weight. Clinical response is the best guide to dose escalation.
Conclusions
Clobazam is a unique AED that is highly effective and safe in the treatment of a broad spectrum of epilepsies. Although it is only approved for Lennox–Gastaut syndrome in the USA, the 1,5‐benzodiazepine has demonstrated remarkable efficacy as a monotherapeutic and as adjunctive therapy for refractory epilepsy in children and adults. In one of the author's experiences (RM), clobazam is highly effective in about 50% of patients, and it is poorly tolerated or not effective in the other half. This has been observed in patients with focal (partial onset) seizures uncontrolled despite trials of 6 or more AEDs. Clobazam causes less sedation than other benzodiazepines, and its anxiolytic properties are an added benefit. It also seems to develop less efficacy tolerance than other benzodiazepines. Clobazam should be considered early when first‐line drugs fail to provide control or are poorly tolerated, and evidence exists that it can even be used for monotherapy.
Disclosure
Lundbeck, the pharmaceutical firm marketing Onfi (clobazam) in the United States, did provide data that we specifically requested. The authors have not received any monetary support or compensation of any kind from Lundbeck. Lundbeck has not seen or commented on this review. There are no other relationships that might lead to a perceived conflict of interest. The content of this manuscript has not been published or submitted for publication elsewhere.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52(Suppl 7):2–26. [DOI] [PubMed] [Google Scholar]
- 2. Kwon O‐Y, Park S‐P. Depression and anxiety in people with epilepsy. J Clin Neurol 2014;10:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 4. Mattson R, Cramer J, Collins J, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic‐clonic seizures. N Engl J Med 1985;313:145–151. [DOI] [PubMed] [Google Scholar]
- 5. Glauser T, Ben‐Menachem E, Bourgeois B, et al. ILAE treatment guidelines: Evidence‐based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47:1094–1120. [DOI] [PubMed] [Google Scholar]
- 6. Sternbach LH. The Benzodiazepine Story In: Priest RG, Filho UV, Amrein R, et al., editors. Benzodiazepines today and tomorrow. Netherlands: Springer, 1980;5–17. [Google Scholar]
- 7. Baenninger A, Alberto Costa e Silva J, Hindmarch I, Moeller HJ, Rickels K. Good chemistry: the life and legacy of valium inventor Leo Sternbach. New York: McGraw‐Hill, 2004. [Google Scholar]
- 8. Shorvon SD. Antiepileptic drugs In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs, 4th edn New York: Raven Press, 1995;763–777. [Google Scholar]
- 9. Sankar R. GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5‐benzodiazepine clobazam. CNS Drugs 2012;26:229–244. [DOI] [PubMed] [Google Scholar]
- 10. Wheless JW, Phelps SJ. Clobazam: A newly approved but well‐established drug for the treatment of intractable epilepsy syndromes. J Child Neurol 2013;28:219–229. [DOI] [PubMed] [Google Scholar]
- 11. Giarratano M, Standley K, Benbadis SR. Clobazam for treatment of epilepsy. Expert Opin Pharmacother 2012;13:227–233. [DOI] [PubMed] [Google Scholar]
- 12. Ng Y, Collins SD. Clobazam. Neurotherapeutics 2007;4:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doi T, Ueda Y, Tokumaru J, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by clobazam during epileptogenesis in Fe(+++)‐induced epileptic rats. Brain Res Mol Brain Res 2005;142:91–96. [DOI] [PubMed] [Google Scholar]
- 14. Kasugai Y, Swinny JD, Roberts JDB, et al. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze‐fracture replica immunolabelling. Eur J Neurosci 2010;32:1868–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen HS, Nichol K, Lee D, Ebert B. Clobazam and its active metabolite N‐desmethylclobazam display significantly greater affinities for α₂‐ versus α₁‐GABA(A)‐receptor complexes. PLoS ONE 2014;9:e88456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rupp W, Badian M, Christ O, et al. Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol 1979;7(Suppl 1):51S–57S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volz M, Christ O, Kellner HM, et al. Kinetics and metabolism of clobazam in animals and man. Br J Clin Pharmacol 1979;7(Suppl 1):41S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lundbeck [Internet] . ONFI (clobazam) prescribing information; 2011 [cited 2014 August 6]. Available from: www.lundbeck.com.
- 19. Brogden RN, Heel RC, Speight TM, Avery GS. Clobazam: A review of its pharmacological properties and therapeutic use in anxiety. Drugs 1980;20:161–178. [DOI] [PubMed] [Google Scholar]
- 20. Giraud C, Tran A, Rey E, et al. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: Importance of CYP2C19. Drug Metab Dispos 2004;32:1279–1286. [PubMed] [Google Scholar]
- 21. Walzer M, Bekersky I, Blum RA, Tolbert D. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy 2012;32:340–353. [DOI] [PubMed] [Google Scholar]
- 22. Kosaki K, Tamura K, Sato R, et al. A major influence of CYP2C19 genotype on the steady‐state concentration of N‐desmethylclobazam. Brain Dev 2004;26:530–534. [DOI] [PubMed] [Google Scholar]
- 23. Sennoune S, Mesdjian E, Bonneton J, et al. Interactions between clobazam and standard antiepileptic drugs in patients with epilepsy. Ther Drug Monit 1992;14:269–274. [DOI] [PubMed] [Google Scholar]
- 24. Allen JW, Oxley J, Robertson MM, et al. Clobazam as adjunctive treatment in refractory epilepsy. Br Med J (Clin Res Ed) 1983;286:1246–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keene DL, Whiting S, Humphreys P. Clobazam as an add‐on drug in the treatment of refractory epilepsy of childhood. Can J Neurol Sci 1990;17:317–319. [DOI] [PubMed] [Google Scholar]
- 26. Renfroe J, Conry J, Ng Y, et al. Effects of concomitant lamotrigine or valproate therapy on clobazam for lennox‐gastaut syndrome: subanalyses of the CONTAIN Trial. Neurology 2012;78. P06.120. [Google Scholar]
- 27. Weintraub D, Buchsbaum R, Resor SR, Hirsch LJ. Effect of antiepileptic drug comedication on lamotrigine clearance. Arch Neurol 2005;62:1432–1436. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto Y, Takahashi Y, Imai K, et al. Impact of cytochrome P450 inducers with or without inhibitors on the serum clobazam level in patients with antiepileptic polypharmacy. Eur J Clin Pharmacol 2014;70:1203–1210. [DOI] [PubMed] [Google Scholar]
- 29. Bun H, Monjanel‐Mouterde S, Noel F, et al. Effects of age and antiepileptic drugs on plasma levels and kinetics of clobazam and N‐desmethylclobazam. Pharmacol Toxicol 1990;67:136–140. [DOI] [PubMed] [Google Scholar]
- 30. Koeppen D, Baruzzi A, Capozza M, et al. Clobazam in therapy‐resistant patients with partial epilepsy: A double‐blind placebo‐controlled crossover study. Epilepsia 1987;28:495–506. [DOI] [PubMed] [Google Scholar]
- 31. Montenegro MA, Cendes F, Noronha AL, et al. Efficacy of clobazam as add‐on therapy in patients with refractory partial epilepsy. Epilepsia 2001;42:539–542. [DOI] [PubMed] [Google Scholar]
- 32. Kalra V, Seth R, Mishra D, Saha NC. Clobazam in refractory childhood epilepsy. Indian J Pediatr 2010;77:263–266. [DOI] [PubMed] [Google Scholar]
- 33. Scott DF, Moffett A. The long‐term effect of clobazam as adjunctive therapy in epilepsy. Acta Neurol Scand 1988;77:498–502. [DOI] [PubMed] [Google Scholar]
- 34. Buchanan N. Clobazam in the treatment of epilepsy: Prospective follow‐up to 8 years. J R Soc Med 1993;86:378–380. [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt D. Benzodiazepines clinical efficacy and use in epilepsy In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic Drugs, 5th edn Philadelphia: Lippincott Williams & Wilkins, 2002;206–207. [Google Scholar]
- 36. Conry JA, Ng YT, Paolicchi JM, et al. Clobazam in the treatment of Lennox‐Gastaut syndrome. Epilepsia 2009;50:1158–1166. [DOI] [PubMed] [Google Scholar]
- 37. Ng YT, Conry JA, Drummond R, et al. Randomized, phase III study results of clobazam in Lennox‐Gastaut syndrome. Neurology 2011;77:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conry JA, Ng YT, Kernitsky L, et al. Stable dosages of clobazam for Lennox‐Gastaut syndrome are associated with sustained drop‐seizure and total‐seizure improvements over 3 years. Epilepsia 2014;55:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cramer JA, Sapin C, François C. Indirect comparison of clobazam and other therapies for Lennox‐Gastaut syndrome. Acta Neurol Scand 2013;128:91–99. [DOI] [PubMed] [Google Scholar]
- 40. VanStraten AF, Ng YT. Update on the management of Lennox‐Gastaut syndrome. Pediatr Neurol 2012;47:153–161. [DOI] [PubMed] [Google Scholar]
- 41. Arya R, Anand V, Garg SK, Michael BD. Clobazam monotherapy for partial‐onset or generalized‐onset seizures. Cochrane Database Syst Rev 2014;10:CD009258. [DOI] [PubMed] [Google Scholar]
- 42. Clobazam has equivalent efficacy to carbamazepine and phenytoin as monotherapy for childhood epilepsy. Canadian Study Group for Childhood Epilepsy. Epilepsia 1998;39:952–959. [DOI] [PubMed] [Google Scholar]
- 43. Bawden HN, Camfield CS, Camfield PR, et al. The cognitive and behavioural effects of clobazam and standard monotherapy are comparable. Canadian Study Group for Childhood Epilepsy. Epilepsy Res 1999;33:133–143. [DOI] [PubMed] [Google Scholar]
- 44. Dulac O, Figueroa D, Rey E, Arthuis M. Monotherapy with clobazam in epilepsies in children. Presse Med 1983;12:1067–1069. [PubMed] [Google Scholar]
- 45. Mehndiratta MM, Krishnamurthy M, Rajesh KN, Singh G. Clobazam monotherapy in drug naïve adult patients with epilepsy. Seizure 2003;12:226–228. [DOI] [PubMed] [Google Scholar]
- 46. Rickels K, Brown AS, Cohen D, et al. Clobazam and diazepam in anxiety. Clin Pharmacol Ther 1981;30:95–100. [DOI] [PubMed] [Google Scholar]
- 47. Jacobson AF, Goldstein BJ, Dominguez RA, Steinbook RM. A placebo‐controlled, double‐blind comparison of clobazam and diazepam in the treatment of anxiety. J Clin Psychiatry 1983;44:296–300. [PubMed] [Google Scholar]
- 48. Lemoine P, Rouillon F, Pouget D. Efficacy and withdrawal of clobazam, lorazepam and buspirone in the treatment of anxiety disorders. Encephale 1996;22:461–467. [PubMed] [Google Scholar]
- 49. Robertson MM. Current status of the 1,4‐ and 1,5‐benzodiazepines in the treatment of epilepsy: The place of clobazam. Epilepsia 1986;27(Suppl 1):S27–S41. [DOI] [PubMed] [Google Scholar]
- 50. Clobazam in treatment of refractory epilepsy: The Canadian experience. A retrospective study. Canadian Clobazam Cooperative Group. Epilepsia 1991;32:407–416. [PubMed] [Google Scholar]
- 51. Singh A, Guberman AH, Boisvert D. Clobazam in long‐term epilepsy treatment: Sustained responders versus those developing tolerance. Epilepsia 1995;36:798–803. [DOI] [PubMed] [Google Scholar]
- 52. Sugai K. Clobazam as a new antiepileptic drug and clorazepate dipotassium as an alternative antiepileptic drug in Japan. Epilepsia 2004;45(Suppl 8):20–25. [DOI] [PubMed] [Google Scholar]
- 53. Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: Tolerance avoided. J Neurol Neurosurg Psychiatry 1984;47:1279–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Remy C. Clobazam in the treatment of epilepsy: A review of the literature. Epilepsia 1994;35(Suppl 5):S88–S91. [DOI] [PubMed] [Google Scholar]
- 55. Shimizu H, Kawasaki J, Yuasa S, et al. Use of clobazam for the treatment of refractory complex partial seizures. Seizure 2003;12:282–286. [DOI] [PubMed] [Google Scholar]
- 56. Wildin JD, Pleuvry BJ, Mawer GE, et al. Respiratory and sedative effects of clobazam and clonazepam in volunteers. Br J Clin Pharmacol 1990;29:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klehm J, Thome‐Souza S, Sánchez Fernández I, et al. Clobazam: Effect on frequency of seizures and safety profile in different subgroups of children with epilepsy. Pediatr Neurol 2014;51:60–66. [DOI] [PubMed] [Google Scholar]
- 58. Redondo P, Vicente J, España A, et al. Photo‐induced toxic epidermal necrolysis caused by clobazam. Br J Dermatol 1996;135:999–1002. [DOI] [PubMed] [Google Scholar]
- 59. Ertam I, Sezgin AO, Unal I. A case with Stevens Johnson syndrome triggered by combination of clobazam, lamotrigine, and valproic acid treatment. Int J Dermatol 2009;48:98–99. [DOI] [PubMed] [Google Scholar]
- 60. FDA U.S. Food and Drug Administration [Internet] . FDA Drug Safety Communication: FDA warns of serious skin reactions with the anti‐seizure drug Onfi (clobazam) and has approved label changes; 2013 [cited 2014 October 18]. Available from: www.fda.gov.
- 61. Tolbert D, Harris SI, Bekersky I, et al. Withdrawal‐related adverse events from clinical trials of clobazam in Lennox‐Gastaut syndrome. Epilepsy Behav 2014;37:11–15. [DOI] [PubMed] [Google Scholar]


