Summary
The human fetal cerebral cortex develops through a series of partially overlapping histogenetic events which occur in transient cellular compartments, such as the subplate zone. The subplate serves as waiting compartment for cortical afferent fibers, the major site of early synaptogenesis and neuronal differentiation and the hub of the transient fetal cortical circuitry. Thus, the subplate has an important but hitherto neglected role in the human fetal cortical connectome. The subplate is also an important compartment for radial and tangential migration of future cortical neurons. We review the diversity of subplate neuronal phenotypes and their involvement in cortical circuitry and discuss the complexity of late neuronal migration through the subplate as well as its potential relevance for pathogenesis of migration disorders and cortical dysplasia. While migratory neurons may become misplaced within the subplate, they can easily survive by being involved in early subplate circuitry; this can enhance their subsequent survival even if they have immature or abnormal physiological activity and misrouted connections and thus survive into adulthood. Thus, better understanding of subplate developmental history and various subsets of its neurons may help to elucidate certain types of neuronal disorders, including those accompanied by epilepsy.
Keywords: Cortical dysplasia, Disorders of neuronal migration, Human fetal connectome, Radial migration, Tangential migration
Introduction
Recent advances in studying structural, physiological, and molecular features of the developing cortical circuitry clearly suggest that there are major organizational differences between fetal, perinatal, and postnatal human cerebral cortex and that the cortex develops through a series of complex reorganizational events 1, 2, 3, 4, 5, 6, 7, 8, 9. These reorganizational events may underlie complex changes in motor, sensory, behavioral, and cognitive functions of human premature infants, newborns, children, and adolescents 2, 3, 4, 10, 11, 12, 13, 14. The disturbances of these processes, especially when they occur during the fetal period, are likely to be involved in etiopathogenesis of many developmental brain disorders, such as cerebral palsy 15, autism 16, 17, schizophrenia 18, 19, and epilepsy 20, 21.
The prenatal development of the human cerebral cortex is characterized by both sequential and overlapping histogenetic events 22, 23 accompanied by significant changes in gene expression patterns 6, 9. In addition, the late fetal period (from 22 postconceptional weeks, PCW, onwards), which corresponds to the period of prematurely born infants, is characterized by major changes in development of cortical circuitry and connectivity, synaptogenesis and physiological features 1, 2, 3, including the emergence of resting state activity. Various developmental disorders, including cortical dysplasia, probably emerge during this clinically important period 1, 2, 3, 20, 21, 24, 25, 26.
The aim of this study is to review major histogenetic events and processes in the human fetal brain to provide a neurobiological framework for interpreting and analyzing developmental brain disorders frequently accompanied by epilepsy, such as disorders of neuronal migration and cortical dysplasias. We focus on the transient fetal subplate zone for the following reasons: (1) the subplate is the site of early synaptogenesis and synaptic interactions with thalamocortical and other cortical afferent systems 27, 28, 29, 30, 31, 32 as well as the site of early endogenous oscillatory activity, as demonstrated experimentally in rodents 33, 34, 35 and in the human subplate 36; (2) the transient subplate circuitry co‐exists with early developing permanent cortical circuitry during the late fetal period 2, when long corticocortical and commissural pathways continue to grow, and (3) the subplate remnant exists even in the early postnatal period, when short corticocortical connections develop 7. Finally, the subplate also serves as a prominent compartment involved in neuronal migration and thus may be involved in pathogenesis of various migration disorders 37, 38, 39.
Sequential Development and Transient Cellular Compartments of the Human Fetal Cerebral Wall
The complex cellular, modular, laminar, areal, and regional organization of the adult human cortical map and connectome develops through a long series of sequential (but partially overlapping) histogenetic events which begin during the 4 PCW 23 and terminate during the late adolescence and early adulthood 11, 12. As there are already extensive reviews of fetal cerebral wall lamination and development 1, 2, 3, 22, 23, 29, 40, including the major role of subplate in cortical development 23, 34, 41, 42, 43 and the history of the subplate discovery 43, here we mention only the most relevant facts.
The cortical histogenesis begins with proliferation of progenitor neuroepithelial cells in the ventricular zone (VZ) of paired telencephalic vesicles 23. Already at 5 PCW, the telencephalic wall consists of thin dorsal (pallium) and thick basal portion (subpallium). During the 6 and 7 PCW, first postmitotic neurons migrate from VZ towards the pial surface and form the so‐called mantle layer 45, also described as the primordial plexiform layer 46 or the preplate 23, 47. The first preplate cells are Cajal‐Retzius cells and early generated subplate neurons, which have no synapses but communicate through non‐synaptic junctions.
During the 7 PCW, the new proliferative zone (subventricular zone, SVZ) is formed, and during the 8 PCW the cell‐dense cortical plate appears, consisting of postmigratory neurons which migrate from the VZ along radial glial guides and finally settle in the cortical plate arranged in vertical ontogenetic columns 48. Thus, the neocortical anlage consists of three transient fetal zones: the superficial and cell‐poor marginal zone (MZ), the cell‐dense cortical plate (CP), and the plexiform pre‐subplate 29. This neocortical anlage is also characterized by early bilaminar synaptogenesis, with synapses present in MZ and presubplate, but absent from the CP 23, 27, 29. Between the neocortical anlage and periventricular proliferative zones (VZ‐SVZ), the intermediate zone (IZ) appears and contains early growing afferent fibers originating from brain stem (monoaminergic axons 49, 50, 51, 52), basal forebrain (cholinergic axons 53) and thalamus (glutamatergic axons 29, 54, 55).
Between 12 and 15 PCW, the deep portion of the neocortical CP gradually transforms into new, prominent and synapse‐ and fiber‐rich subplate zone 29, 56, 57. The subplate becomes the thickest and most voluminous transient compartment of the human fetal cerebral wall between 15 and 35 PCW and represents the major site of synaptogenesis and neuronal maturation and differentiation. The subplate also contains a large amount of hydrophyllic extracellular matrix and thus can be easily visualized in both in vitro 58, 59 and in vivo MRI studies 60, 61, 62. From 15 to 24 PCW, the subplate serves as the waiting compartment for ingrowing cortical afferents 28, 29, 40. From 24 to 28 PCW, there is gradual relocation of thalamocortical and basal forebrain afferents from the subplate into the cortical plate 1, 3, 29, 40, 53, 54, 55 with concomitant onset of synaptogenesis within the cortical plate 27, 29. This event represents a milestone in fetal cortical physiology because peripheral stimulation is for the first time able to synaptically activate cortical plate neurons 63, 64. Before that period, afferent axons predominantly activated subplate neurons and cortical activity was endogenous 1, 2, 29, 34, 41. Between 28 and 34 PCW, the subplate remains at the peak of its development, because there is continuous growth and relocation of massive corticocortical pathways; this period is also characterized by extensive growth of fetal white matter, further formation of cortical convolutions, and exponentially increasing synaptogenesis in the cortical plate which also begins to develop its six‐layered “Grundtypus” of cortical lamination 65. In addition, dendritic differentiation of cortical plate neurons also intensifies during this period 66, 67, 68. After 34 PCW, the subplate gradually diminishes in size, beginning at the depth of cortical sulci, but remains present even in the newborn and early postnatal brain as the subplate remnant 7. It should be noted that most of the human fetal subplate neurons not only survive the perinatal period, but continue to develop postnatally and remain very numerous in the adult gyral white matter 44, 69, 70.
Structural and Functional Organization of the Human Subplate
The human fetal subplate has a complex structure and consists of various cellular, fibrillar and extracellular elements 23, 29. Its composition is continuously changing throughout the midfetal, late fetal, perinatal, and early postnatal period 1, 2, 3. However, in all these periods, the subplate consists of migratory and postmigratory neurons, glial cells, significant amount of extracellular matrix, and various contingents of afferent and efferent axons involved in intense synaptogenesis. This intense synaptogenesis is clearly demonstrated by E.M. studies, but it is possible that not all of these early synapses are functionally active, as suggested in a recent study on acute slices of the postmortem human fetal brain tissue 36. Thus, to delineate the subplate in various developmental periods, one has to use a combination of various E.M., histological, histochemical, and immunocytochemical techniques 7, 29, 42, 58.
Morphological and Molecular Phenotypes of Subplate Neurons
The subplate contains early differentiated postmigratory and polymorphic (multipolar) neurons 44, 66, 67, 68, 69, 70. On the basis of Golgi method (Fig. 1), these neurons can be described as fusiform, inverted pyramidal, polymorphous and large multipolar 66, 67, 68. Even if we limit the review just to the studies of human and rhesus monkey brain, it is clear that these neurons also express a large variety of molecular markers, such as different neurotransmitters (Table 1), various receptors (Table 2), calcium‐binding proteins (Table 3) and synaptic and cytoskeletal markers as well as growth factors and axon guidance molecules (Table 4). Along with numerous neuropeptides 34, 41, 42, two major neurotransmitters, glutamate and GABA, are also present in subplate pyramidal neurons and interneurons 33, 101, 102, 103, 104. However, their respective roles and exact distribution in specific types of subplate neurons (especially in the human brain) are far from being satisfactorily elucidated.
Figure 1.
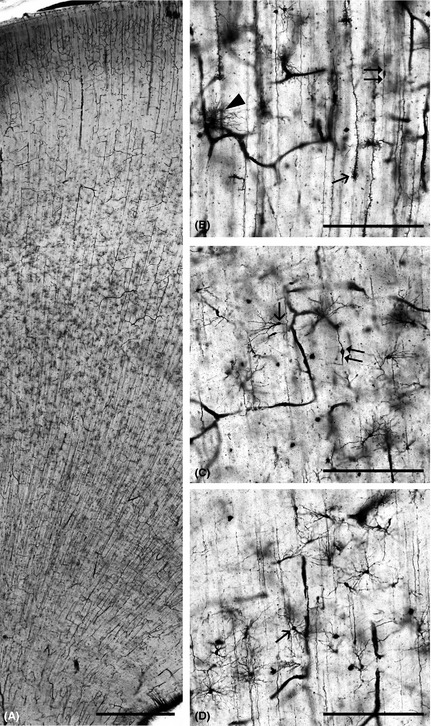
Microphotographs of Golgi stained human fetal somatosensory cortex (Stensaas’ modification of Del Rio Hortega method) in 23 PCW‐old preterm infant. Note the radial orientation of cellular elements in the telencephalic wall (A) due to the presence of radial glia and vertical arrangement of blood vessels; the subplate is recognized as a wide pale zone below the cortical plate (A). The subplate contains postmigratory cortical neurons (B, double arrows), radial glial cells starting to transform into astrocytes (B, arrow) as well as already formed fibrillar astrocytes in contact with blood vessels (B, arrowhead). The subplate contains several types of neurons: polymorphic (C, arrow), fusiform (C, double arrow), and inverted pyramidal (D, arrow). Bar = 1 cm (A) and 200 μm (B, C, D).
Table 1.
Expression of neurotransmitters in subplate neurons
| Marker | Species | Age | Source |
|---|---|---|---|
| GABA | Human | 14–32 GW | Yan et al. 71 |
| 7–13 PCW | Zecevic and Milosevic 72 | ||
| Monkey | Huntley et al. 73 | ||
| E70–E141 | Meinecke and Rakic 74 | ||
| NO & NADPH | Human | 15–32 GW | Yan et al. 75 |
| 15–28 PCW | Yan and Ribak 76 | ||
| 18–Newborn | Downen et al. 77 | ||
| 15–37 PCW | Judaš et al. 42 | ||
| 25–35 PCW | deAzevedo et al. 78 | ||
| NPY | Human | 14 PCW–34 years | Delalle et al. 79 |
| 14 PCW–34 years | Uylings and Delalle 80 | ||
| 11 PCW–Newborn | Wai et al. 81 | ||
| 13–16 PCW | Bayatti et al. 82 | ||
| 16 PCW | Wang et al. 83 | ||
| Monkey | Huntley et al. 73 | ||
| E60–160 | Mehra and Hendrickson 84 | ||
| Somatostatin | Human | 22–34 PCW | Kostović et al. 85 |
| Monkey | Huntley et al. 73 | ||
| Substance P | Monkey | E90–E160 | Mehra and Hendrickson 84 |
Table 2.
Expression of various receptors in subplate neurons
| Marker | Species | Age | Source |
|---|---|---|---|
| GABA A receptor | Monkey | E121–E155 | Huntley et al. 86 |
| E70–E141 | Meinecke and Rakic 74 | ||
| α1 and α2 adrenergic receptor | Monkey | E65–E143 | Lidow and Rakic 87 |
| β adrenergic receptor | Monkey | E90–128 | Lidow and Rakic 87 |
| α4 nAChR | Human | 17–24 GW and 34–42 GW | Schroder et al. 88 |
| p75NGFR | Human | 16–40 PCW | Kordower and Mufson 89 |
| 14–34 GW | Chen et al. 90 | ||
| Monkey | E56–E121 | Meinecke and Rakic 91 | |
| EphA3, 6, 7 | Monkey | E65–E95 | Donoghue and Rakic 92 |
| Trk | Human | 14–34 GW | Chen et al. 90 |
Table 3.
Expression of Ca2+ binding proteins in subplate neurons
| Marker | Species | Age | Source |
|---|---|---|---|
| Calbindin | Human | 20 PCW> | Ulfig 93 |
| Calretinin | Human | 20 PCW> | Ulfig 93 |
| 13–16 PCW | Bayatti et al. 82 | ||
| 16 PCW | Wang et al. 83 | ||
| Parvalbumin | Human | 26 PCW–Newborn | Honig et al. 94 |
| S100A4 | Human | 12–32 GW | Chan et al. 95 |
| S100A5 | Human | 12–32 GW | Chan et al. 95 |
| S100A13 | Human | 12–32 GW | Chan et al. 95 |
Table 4.
Expression of other markers in subplate neurons
| Marker | Species | Age | Source |
|---|---|---|---|
| GAD (67/65) | Human | 26 GW – 2 years | Xu et al. 96 |
| GAP43 | Human | 14–Newborn PCW | Honig et al. 94 |
| 13–17 PCW | Bayatti et al. 82 | ||
| vGAT | Human | 10 PCW | Bayatti et al. 82 |
| KCC2 | Human | 16 PCW | Bayatti et al. 82 |
| 16 PCW | Wang et al. 83 | ||
| MAP2 | Human | 16–22 GW | Sims et al. 97 |
| 14 PCW–Newborn | Honig et al. 94 | ||
| 16 PCW | Bayatti et al. 82 | ||
| Monkey | E75–160 | Mehra and Hendrickson 84 | |
| Synaptojanin | Human | 15–37 GW | Arai et al. 98 |
| Synaptophysin | Human | 10 PCW | Bayatti et al. 82 |
| NURR1 | Human | 15–22 PCW | Wang et al. 83 |
| TBR1 | Human | 9–12 PCW | Bayatti et al. 82 |
| α2zinc‐binding globulin | Human | 14 PCW | Wang et al. 83 |
| CTGF | Human | 22 PCW | Wang et al. 83 |
| Fetuin | Human | 14 PCW | Wang et al. 83 |
| 24–40 PCW | Elsas et al. 99 | ||
| Nogo‐A | Human | 16–36 PCW | Haybaeck et al. 100 |
Subplate Represents a Waiting Compartment for Cortical Afferent Fibers and Subplate Neurons Serve as Postsynaptic Targets for Various Inputs
The first afferent fibers to reach subplate neurons, already at the presubplate stage, originate from modulatory monoaminergic pathways ascending from the brain stem 29, 49, 50, 51, 52. In the rodent brain, early monoaminergic axons also make synapses below the cortical plate in the zone which corresponds to the human subplate 105. During the expansion of the deep cortical plate and the formation of the proper subplate (13–15 PCW), two new and massive afferent systems enter the subplate: basal forebrain cholinergic fibers 53, and thalamocortical fibers 29, 54, 55. A subset of human subplate neurons display strong AChE‐reactivity 69 and express M2 muscarinic receptors 106. The cholinergic activation of subplate neurons has been also demonstrated in the rodent brain 107. Activation of subplate neurons by glutamatergic thalamocortical axons has been demonstrated in fetal brains of cats and rodents 101, 103 (for review see 34, 41). While there is no convincing evidence on the activation of subplate neurons by glutamatergic corticocortical fibers, it stands to reason to assume its existence because corticocortical fibers represent the most massive component of axons waiting in the subplate 1, 3, 29, 40.
The first experimental evidence for the existence of “waiting” thalamocortical axons in the subplate was provided in the visual cortex of fetal rhesus monkeys 28, and it was since extensively documented that all afferent axons wait in the subplate (and eventually establish temporary synapses with subplate neurons) for a prolonged period of time, at least in humans and nonhuman primates 1, 3, 29, 34, 40, 41. The ingrowth of different cortical afferent systems (thalamocortical, basal forebrain, corticocortical) in the subplate is sequential (but partly overlapping) and enfolds throughout the entire fetal period. The same holds for the relocation of these afferents from the subplate into the cortical plate after 24 PCW. For example, thalamocortical axons in the human fetal brain invade the presubplate already at 13 PCW 29, 57, form extensive axonal plexuses in the subplate throughout the midfetal period (15–20 PCW), accumulate in the superficial subplate around 22 PCW, and penetrate the cortical plate after 24 PCW 29, 54, 55. The example of thalamocortical afferents suggests that the subplate serves as a substrate for a special geometry and directionality of fiber growth 23, 29: fibers are first directed from basal to dorsal pallial segments, and later grow radially through the subplate into the cortical plate. During their basal‐to‐dorsal growth, fibers are forming large axonal bundles or strata in the intermediate zone, that is, the fetal white matter, then turn obliquely to enter the subplate, and after a prolonged waiting period they finally radially relocate from the subplate into the cortical plate.
During their waiting period within the subplate, afferent axons are loosely arranged 29 and embedded in a voluminous and hydrophyllic extracellular matrix 58, 60, which contains a variety of axon guidance molecules. Some of these axons make early synapses with subplate neurons and thus make the subplate the major site of early synaptogenesis in the fetal human cortex.
The damage of the subplate during the waiting period and relocation of thalamocortical afferents can damage not only the development of thalamocortical circuitry but also the columnar development of the cortical plate 30, 31 (for review see 34, 41). Accordingly, the damage of thalamocortical system in the human preterm infant may lead to abnormal cerebral development 108, 109. Our long‐term studies suggest that the most critical period is during the accumulation of thalamocortical fibers in the superficial subplate (around 22 PCW) and during their relocation into the cortical plate at 24–28 PCW 1, 2, 3, 29, 40, 110. However, it should be noted that subplate continues to function as waiting compartment for growing long corticocortical afferents until birth 3 and it may continue to serve as mini‐waiting compartment for growth of short cortico‐cortical connections even after birth 7.
Subplate Neurons Serve as Presynaptic Elements in the Fetal Cortical Circuitry
The axons of subplate neurons establish synapses with (1) other subplate neurons, (2) thalamus, and (3) cortical plate neurons. It seems that glutamatergic presynaptic axons originate from subplate inverted pyramidal neurons which represent up to 50% of subplate neuronal population 68, 111. However, glutamatergic NMDA receptors in the subplate are different from those in the adult cortex and are active at −70 mV 33. Some subplate cells also project to the thalamus 31, 112 but it is not clear whether their neurotransmitter is really glutamate.
Various subplate interneurons contain GABA and neuropeptides and seem to contact other subplate neurons. However, GABA receptors on subplate neurons are also functionally different from those in the adult brain 113 and activation of subplate GABA neurons in rodents leads to the activation of depolarizing GABA receptors on other subplate neurons 33, 114.
The subplate neurons also send ascending axons to the overlying cortical plate 34, 41. Such projections were not directly demonstrated in the human fetal cortex, but if they are present their synaptic action in the cortical plate should occur after 23 or 24 PCW, because there are no synapses in the cortical plate before that time 27.
Subplate Neuronal Circuitry and its Functions
The physiological properties of subplate neurons and their local and extrinsic (input–output) circuitry were first described in the cat 41, 101, 102, 111. This was subsequently thoroughly elaborated in neurophysiological studies using rodents 33, 34, 35, 104, 107, 115, 116. The early subplate circuitry displays oscillatory features 33, 116 and has been described as having an endogenous activity which does not depend on extrinsic input 34.
In the human fetal brain, synapses may be found as deep as 4–6 mm below the cortical plate, on cell bodies and dendrites of subplate neurons 29. This shows that human subplate neurons also serve as postsynaptic elements for early cortical circuitry. While most of these synapses are asymmetric (excitatory?), some symmetrical (inhibitory?) synapses are located on cell bodies of subplate neurons 29. Similar findings were reported in fetal cats 103.
As already mentioned, before 24 PCW, the subplate is the major site of synaptogenesis in the human fetal brain, whereas there are no synapses in the cortical plate; but, cortical plate neurons seem to communicate through gap junctions 29, 33, 34, 104, 117, 118. However, synapses are also present on apical dendritic branches of cortical plate pyramidal neurons situated in the marginal zone, which serves as another early site of synaptogenesis in the fetal cortex 27, 29. The fact that the subplate serves as the major site of synaptic activity in midfetal and preterm brain has obvious functional and clinical implications: (1) first external stimuli (such as tactile or pain stimuli), travelling along thalamocortical axons, reach the subplate circuitry and extend to the cortical plate only after 24 PCW; (2) early influences of monoaminergic and cholinergic modulatory systems are also centered on transient subplate circuitry; (3) initial corticocortical connections remain centered on the subplate circuitry even after 28 PCW (when thalamocortical and basal forebrain afferents are already settled and active in the cortical plate); (4) the prolonged co‐existence of transient (subplate‐centered) and permanent (cortical plate‐centered) cortical circuitry represents a salient feature of human cortical development 1, 2 during at least 6 months (i.e., three last prenatal and three‐first postnatal months); (5) the transient subplate circuitry probably represents an important component of the emerging resting state activity during the perinatal period 119, 120, 121, 122.
In conclusion, the transient subplate‐centered cortical circuitry consists of elaborated local (modular?) circuits (Fig. 2) which receive specific inputs and send specific outputs, and continues to exist during the initial formation of the equivalent cortical plate‐based (i.e., adult‐like) cortical circuitry. Thus, the subplate represents vital but hitherto neglected component of the human fetal cortical connectome.
Figure 2.
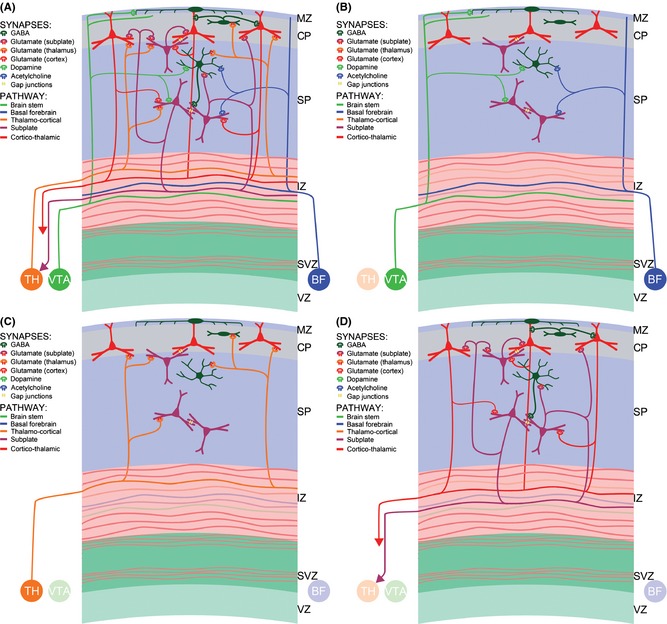
Simplified diagram (A) of transient subplate circuitry during the late midfetal period (24–26 PCW). Different presynaptic axons and postsynaptic receptors are represented by different colors (see legends along the diagrams). To enhance the understanding and visibility, three major circuitry systems are displayed separately: monoaminergic (B), thalamocortical (C) and intrinsic subplate plus corticothalamic (D). VZ and SVZ, ventricular and subventricular zone; IZ, intermediate zone; SP, subplate; CP, cortical plate; MZ, marginal zone.
Subplate Involvement in Neuronal Migration and Developmental Brain Disorders
Subplate as the Zone of Neuronal Migration
After its formation (13–15 PCW), the subplate becomes the thickest and the most voluminous transient compartment of the human fetal cerebral wall, reaching its developmental peak (6–10 mm in thickness!) between 28 and 32 PCW. It should be noted that during this entire period postmitotic cortical neurons migrate through the subplate on their way to the cortical plate. This also means that radial glial guides (along which these neurons migrate) are continuously present in the subplate. Thus, the subplate not only represents a large portion of the total migratory route, but may in fact represent a sort of “mine‐field” for travelling last‐generated migratory neurons (destined to corticocortical layers II and III); namely, these neurons continue to migrate after 24 PCW, when many radial glial cells are already transforming into astrocytes within the subplate and thus may not be able to serve as radial guides to migratory neurons.
With respect to generation and migration of subplate neurons themselves in the human or primate brain, very little is known at present. While subplate pyramidal neurons probably use the same radial migratory route as pyramidal neurons of the cortical plate, it is not known why and how they detach from the radial glia already in the subplate instead of continuing their journey to the cortical plate. For example, the reelin produced by Cajal‐Retzius cells in the marginal zone has been suggested to act as a stop signal for pyramidal neuron migration, but there is at present no evidence that subplate cells produce reelin 123, 124. Moreover, a recent study suggested that massive loss of Cajal‐Retzius cells does not disrupt neocortical layer order 125. On the other hand, migrating GABA interneurons seem to rely on mechanisms independent of reelin signaling 126 and use predominantly or exclusively tangential routes of migration. But, the exact migratory route is still unexplored for many subsets of GABA interneurons even in the rodent brain. With respect to human and nonhuman primate brain, it is known that a significant subset of GABA interneurons is generated in the VZ‐SVZ and uses tangential migratory route on their way to the cortical plate 127, 128. However, which (if any) of these interneurons are destined to the human subplate and how they settle there remains unknown. In distinction to the cortical plate, the subplate does not show clear laminar organization. Thus, it is difficult to analyze how and why different types of subplate neurons become settled at different subpial depths within the subplate.
Subplate may have a Key Role in Pathogenesis of Migration Disorders and Cortical Dysplasias
At present, there are several classifications of malformations of cortical development, which rely on combination of developmental, genetic and neuroimaging criteria 37, 38, 39. For example, cortical malformations may be broadly divided in disorders of neuronal position, disorders of axonal projection and assembly, and syndromes of cortical disorganization, that is, cortical dysplasias 37. As genetic studies have identified several genes associated with malformations of cortical development, and some of these genes are involved in pathogenesis of the largest malformation groups such as focal cortical dysplasia, heterotopia and polymicrogyria 39, molecular and genetic approaches opened new vistas for classifying and studying functional consequences and treatment options of various cortical abnormalities, for example, those associated with drug‐resistant epilepsy 20, 21, 39.
Another approach is to classify cortical malformations based on the stage of development at which cortical development was first affected and to use genotype, rather than phenotype, as the basis for classifying disorders 38. This revised classification 38 proposed that there are four major groups of cortical malformations: (1) Malformations due to abnormal proliferation/apoptosis; (2) Malformations due to abnormal migration; (3) Malformations due to abnormal late neuronal migration and cortical organization; and (4) Malformations of cortical development, not otherwise classified. The first three groups are useful for describing disorders of neurogenesis of all cortical neurons, the radial migration of projection (pyramidal) cortical neurons and tangential migration of cortical interneurons. These three groups can equally apply to the analysis of disorders in neurogenesis and migration of subplate neurons; unfortunately, that kind of analysis has not been applied to subplate neither in humans nor in experimental animals.
If subplate pyramidal neurons indeed use the radial glial cells as their migratory routes, any change in signaling properties and contact guidance with glial cells may lead to significant over‐ or underpopulation of subplate with putative excitatory (glutamatergic) and projection neurons. For example, this may cause the presence of supernumerary and immature subplate‐like neurons in cortical dysplasia, as recently suggested 20, 21. However, it should be noted that this pathology cannot be explained by abnormal survival of fetal subplate neurons normally programmed to undergo developmental cell death, because in the human brain the large majority of subplate neurons survive into adulthood as interstitial neurons of the gyral white matter 44, 70.
As already described, the subplate also contains a complex contingent of various axons distributed in a plexiform arrangement. If some subplate GABA‐ and neuropeptide‐containing interneurons use the neurophillic mode of migration (i.e., migration along axonal fascicles), this may explain why they finish scattered within the subplate in a seemingly haphazard manner—and, perhaps, in inappropriate numbers. On the other hand, the subplate extracellular matrix contains all kinds of contact guidance molecules, which may help guide not only growing axons but also migratory neurons to their proper targets. But this also means that any disturbance of this extracellular matrix (e.g., by hypoxic‐ischemic lesion in preterm infants) may cause serious disturbances in proper laminar and modular distribution of migratory neurons as well as various types of mis‐connection or dis‐connection of ingrowing cortical afferents. Therefore, subplate GABA interneurons (especially those of large multipolar type) may become not only supernumerary, but also misplaced at wrong positions and mis‐connected with wrong postsynaptic targets as well as being themselves wrong targets for presynaptic afferent axons. This may be another cause for abnormal physiological features of these cells in various types of cortical dysplasia and epilepsy syndromes.
All this shows that, for growing axons and travelling neurons, the subplate may represent not just the “enchanted loom” (to use the well‐known expression of Sherrington) for properly constructing adult cerebral cortex, but also the impenetrable and confusing jungle in which the weary travelers remain forever lost and thus contribute to all kinds of improperly designed and abnormal cortical arrangements. We are just becoming to be aware of numerous potential and important roles that subplate neurons may play in the pathogenesis of developmental brain disorders. The developmental neuropathology of the subplate is obviously in its infancy, but, thanks to the availability of modern molecular, genomic, and neuroimaging techniques, it may be steadily and prosperously advanced in the near future.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work has been supported by Croatian Science Foundation (grant no. 09.01/414 to MJ) and University of Zagreb (grant no. 1101275 to MV).
The first two authors contributed equally to this work.
References
- 1. Kostović I, Judaš M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol 2006;48:388–393. [DOI] [PubMed] [Google Scholar]
- 2. Kostović I, Judaš M. Transient patterns of cortical lamination during prenatal life: Do they have implications for treatment? Neurosci Biobehav Rev 2007;31:1157–1168. [DOI] [PubMed] [Google Scholar]
- 3. Kostović I, Judaš M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 2010;99:1119–1127. [DOI] [PubMed] [Google Scholar]
- 4. Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn, and pediatric brains. Neuroimage 2006;33:27–38. [DOI] [PubMed] [Google Scholar]
- 5. Huang H, Xue R, Zhang J, et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 2009;29:4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang HJ, Kawasawa YI, Cheng F, et al. Spatio‐temporal transcriptome of the human brain. Nature 2011;478:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostović I, Jovanov‐Milošević N, Radoš M. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct 2014;219:231–253. [DOI] [PubMed] [Google Scholar]
- 8. Fransson P, Metsäranta M, Blennow M, Aden U, Lagercrantz H, Vanhatalo S. Early development of spatial patterns of power‐law frequency scaling in fMRI resting‐state and EEG data in newborn brain. Cereb Cortex 2013;23:638–646. [DOI] [PubMed] [Google Scholar]
- 9. Pletikos M, Sousa AM, Sedmak G, et al. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron 2014;81:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Evans A, Hermoye L, et al. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 2007;38:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petanjek Z, Judaš M, Kostović I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer‐specific pattern. Cereb Cortex 2008;18:915–929. [DOI] [PubMed] [Google Scholar]
- 12. Petanjek Z, Judaš M, Šimić G, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 2011;108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colonnese M, Khazipov R. Spontaneous activity in developing sensory circuits: Implications for resting state fMRI. Neuroimage 2012;62:2212–2221. [DOI] [PubMed] [Google Scholar]
- 14. Omidvarnia A, Fransson P, Metsäranta M, Vanhatalo S. Functional bimodality in the brain networks of preterm and term human newborns. Cereb Cortex 2014; 24:2657–2668. [DOI] [PubMed] [Google Scholar]
- 15. Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McFadden K, Minshew NJ. Evidence for dysregulation of axonal growth and guidance in the etiology of ASD. Front Hum Neurosci 2013;7:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ebert DH, Greenberg ME. Activity‐dependent neuronal signaling and autism spectrum disorder. Nature 2013;493:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 19. Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci 2004;1021:64–76. [DOI] [PubMed] [Google Scholar]
- 20. Cepeda C, André VM, Levine MS, et al. Epileptogenesis in pediatric cortical dysplasia: The dysmature cerebral developmental hypothesis. Epilepsy Behav 2006;9:219–235. [DOI] [PubMed] [Google Scholar]
- 21. Cepeda C, André VM, Wu N, et al. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia 2007;48(Suppl 5):79–85. [DOI] [PubMed] [Google Scholar]
- 22. Kostović I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res 1990;85:223–239. [DOI] [PubMed] [Google Scholar]
- 23. Bystron I, Blackemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 2008;9:110–122. [DOI] [PubMed] [Google Scholar]
- 24. Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging 2002;16:621–632. [DOI] [PubMed] [Google Scholar]
- 25. Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005;147:609–616. [DOI] [PubMed] [Google Scholar]
- 26. Volpe JJ. Encephalopaty of prematurity includes neuronal abnormalities. Pediatrics 2005;116:221–225. [DOI] [PubMed] [Google Scholar]
- 27. Molliver ME, Kostović I, van der Loos H. The development of synapses in cerebral cortex of human fetus. Brain Res 1973;50:403–407. [DOI] [PubMed] [Google Scholar]
- 28. Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci 1977;278:245–260. [DOI] [PubMed] [Google Scholar]
- 29. Kostović I, Rakic P. Development history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 1990;297:441–470. [DOI] [PubMed] [Google Scholar]
- 30. Gosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature 1990;347:179–181. [DOI] [PubMed] [Google Scholar]
- 31. Gosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development 1993;117:1031–1047. [DOI] [PubMed] [Google Scholar]
- 32. Catalano SM, Shatz CJ. Activity‐dependent cortical target selection by thalamic axons. Science 1998;281:559–562. [DOI] [PubMed] [Google Scholar]
- 33. Hanganu IL, Kilb W, Luhmann HJ. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci 2002;22:7165–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci 2010;33:23–48. [DOI] [PubMed] [Google Scholar]
- 35. Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early γ oscillations synchronize developing thalamus and cortex. Science 2011;334:226–229. [DOI] [PubMed] [Google Scholar]
- 36. Moore AR, Zhou WL, Jakocevski I, Zecevic N, Antic SD. Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci 2011;31:2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross ME, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu Rev Neurosci 2001;24:1041–1070. [DOI] [PubMed] [Google Scholar]
- 38. Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology 2005;65:1873–1887. [DOI] [PubMed] [Google Scholar]
- 39. Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: Genetics, functional consequences and treatment options. Trends Neurosci 2008;31:154–162. [DOI] [PubMed] [Google Scholar]
- 40. Kostović I, Judaš M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 2002;267:1–6. [DOI] [PubMed] [Google Scholar]
- 41. Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 1994;17:185–218. [DOI] [PubMed] [Google Scholar]
- 42. Judaš M, Šestan N, Kostović I. Nitrinergic neurons in the developing and adult human telencephalon: Transient and permanent patterns of expression in comparison to other mammals. Microsc Res Tech 1999;45:401–419. [DOI] [PubMed] [Google Scholar]
- 43. Aboitiz F. Evolution of isocortical organization. A tentative scenario including roles of reelin, p35/cdk5 and the subplate role. Cereb Cortex 1999;9:655–661. [DOI] [PubMed] [Google Scholar]
- 44. Judaš M, Sedmak G, Pletikos M. Early history of subplate and interstitital neurons: From Theodor Meynert (1867) to the discovery of the subplate zone (1974). J Anat 2010;217:344–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. His W. Die Entwickelung des menschlichen Gehirns während der ersten Monate. Leipzig, Hirzel: Untersuchungsergebnisse, 1904. [Google Scholar]
- 46. Marin‐Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol 1978;152:109–126. [DOI] [PubMed] [Google Scholar]
- 47. Meyer G. Genetic control of neuronal migrations in human cortical development. Adv Anat Embryol Cell Biol 2007;189:1–111. [PubMed] [Google Scholar]
- 48. Rakic P. Specification of cerebral cortical areas. Science 1988;241:170–176. [DOI] [PubMed] [Google Scholar]
- 49. Nobin A, Björklund A. Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand 1973;388(Suppl):1–40. [PubMed] [Google Scholar]
- 50. Olson L, Boreus LO, Seiger A. Histochemical demonstration and mapping of 5‐hydroxytryptamine‐ and catecholamine‐containing neuron systems in the human fetal brain. Z Anat Entwicklungsgesch 1973;139:259–282. [DOI] [PubMed] [Google Scholar]
- 51. Zecevic N, Verney C. Development of the catecholamine neurons in human embryos and fetuses with special emphasis on the innervation of the cerebral cortex. J Comp Neurol 1995;351:509–535. [DOI] [PubMed] [Google Scholar]
- 52. Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec 2002;267:87–93. [DOI] [PubMed] [Google Scholar]
- 53. Kostović I. Prenatal development of nucleus basal is complex and related fiber systems in man: A histochemical study. Neuroscience 1986;17:1047–1077. [DOI] [PubMed] [Google Scholar]
- 54. Kostović I, Goldman‐Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 1983;219:431–437. [DOI] [PubMed] [Google Scholar]
- 55. Kostović I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci 1984;4:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kostović I, Molliver ME. A new interpretation of the laminar development of cerebral cortex: Synaptogenesis in different layers of neopallium in the human fetus. Anat Rec 1974;178:395. [Google Scholar]
- 57. Duque A, Krsnik Ž, Kostović I, Rakic P. Origin and secondary expansion of the transient subplate zone in the developing cerebrum of human and nonhuman primates. Abstract. Washington DC: Society for Neuroscience, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kostović I, Judaš M, Radoš M, Hrabač P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 2002;12:536–544. [DOI] [PubMed] [Google Scholar]
- 59. Widjaja E, Geibprasert S, Mahmoodabadi SZ, Blaser S, Brown NE, Shannon P. Alteration of human fetal subplate layer and intermediate zone during normal development on MR and diffusion tensor imaging. AJNR Am J Neuroradiol 2010;31:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Judaš M, Radoš M, Jovanov‐Milošević N, Hrabač P, Štern‐Padovan R, Kostović I. Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 2005;26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- 61. Prayer D, Kasprian G, Krampl E, et al. MRI of normal fetal brain development. Eur J Radiol 2006;57:199–216. [DOI] [PubMed] [Google Scholar]
- 62. Corbett‐Detig J, Habas PA, Scott JA, et al. 3D global and regional patterns of human fetal subplate growth determined in utero. Brain Struct Funct 2011;215:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vanhatalo S, Palva JM, Andersson S, Rivera C, Voipio J, Kaila K. Slow endogenous activity transients and developmental expression of K+‐Cl− cotransporter 2 in the immature human cortex. Eur J Neurosci 2005;22:2799–2804. [DOI] [PubMed] [Google Scholar]
- 64. Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci 2006;26:3662–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Johann Ambrosius Barth, 1909. [Google Scholar]
- 66. Mrzljak L, Uylings HB, Kostović I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol 1988;271:355–386. [DOI] [PubMed] [Google Scholar]
- 67. Mrzljak L, Uylings HB, Van Eden CG, Judaš M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res 1990;85:182–222. [DOI] [PubMed] [Google Scholar]
- 68. Mrzljak L, Uylings HB, Kostović I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol 1992;316:485–496. [DOI] [PubMed] [Google Scholar]
- 69. Kostović I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol 1980;9:219–242. [DOI] [PubMed] [Google Scholar]
- 70. Judaš M, Sedmak G, Pletikos M, Jovanov‐Milošević N. Populations of subplate and interstitital neurons in fetal and adult human telencephalon. J Anat 2010;217:381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yan XX, Zeng DS, Garey LJ. Prenatal development of GABA‐immunoreactive neurons in the human striate cortex. Dev Brain Res 1992;65:191–204. [DOI] [PubMed] [Google Scholar]
- 72. Zecevic N, Milosevic A. Initial development of γ‐Aminobutyric acid immunoreactivity in the human cerebral cortex. J Comp Neurol 1997;380:495–506. [DOI] [PubMed] [Google Scholar]
- 73. Huntley GW, Hendry SH, Killackey HP, Chalupa LM, Jones EG. Temporal sequence of neurotransmitter expression by developing neurons of fetal monkey visual cortex. Brain Res 1988;471:69–96. [DOI] [PubMed] [Google Scholar]
- 74. Meinecke DL, Rakic P. Expression of GABA and GABAA receptors by neurons of the subplate zone in developing primate occipital cortex: Evidence for transient local circuits. J Comp Neurol 1992;317:91–101. [DOI] [PubMed] [Google Scholar]
- 75. Yan XX, Garey LJ, Jen LS. Prenatal development of NADPH‐diaphorase‐reactive neurons in human frontal cortex. Cereb Cortex 1996;6:737–745. [DOI] [PubMed] [Google Scholar]
- 76. Yan XX, Ribak CE. Prenatal development of nicotinamide adenine dinucleotide phosphate‐diaphorase activity in the human hippocampal formation. Hippocampus 1997;7:215–231. [DOI] [PubMed] [Google Scholar]
- 77. Downen M, Zhao ML, Lee P, Weidenheim KM, Dickson DW, Lee SC. Neuronal nitric oxide synthase expression in developing and adult human CNS. J Neuropathol Exp Neurol 1999;58:12–21. [DOI] [PubMed] [Google Scholar]
- 78. deAzevedo LC, Hedin‐Pereira C, Lent R. Diaphorase‐positive neurons in the cingulate cortex of human fetuses during second half of gestation. Anat Embryol 2002;205:29–35. [DOI] [PubMed] [Google Scholar]
- 79. Delalle I, Evers P, Kostović I, Uylings HBM. Laminar distribution of neuropeptide Y‐immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol 1997;379:515–522. [DOI] [PubMed] [Google Scholar]
- 80. Uylings HBM, Delalle I. Morphology of neuropeptide Y‐immunoreactive neurons and fibers in human prefrontal cortex during prenatal and postnatal development. J Comp Neurol 1997;379:523–540. [DOI] [PubMed] [Google Scholar]
- 81. Wai SM, Kindler PM, Lam ETK, Zhang A, Yew DT. Distribution of neuropeptide Y‐immunoreactive neurons in the human brainstem, cerebellum, and cortex during development. Cell Mol Neurobiol 2004;24:667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bayatti N, Moss JA, Sun L, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex 2008;18:1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang WZ, Hoerder‐Subedissen A, Oeschger FM, et al. Subplate in the developing cortex of mouse and human. J Anat 2010;217:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mehra RD, Hendrickson AE. A comparison of the development of neuropeptide and MAP2 immunocytochemical labeling in the macaque visual cortex during pre and postnatal development. J Neurobiol 1993;24:104–124. [DOI] [PubMed] [Google Scholar]
- 85. Kostović I, Štefulj‐Fučić A, Mrzljak L, Jukić S, Delalle I. Prenatal and perinatal development of the somatostatin‐immunoreactive neurons in the human prefrontal cortex. Neurosci Lett 1991;124:153–156. [DOI] [PubMed] [Google Scholar]
- 86. Huntley GW, de Blas AL, Jones EG. GABAA receptor immunoreactivity in audult and developing monkey sensory‐motor cortex. Exp Brain Res 1990;82:519–535. [DOI] [PubMed] [Google Scholar]
- 87. Lidow MS, Rakic P. Unique profiles of the alpha 1‐, alpha 2‐, and beta‐adrenergic receptors in the developing cortical plate and transient embryonic zones of the rhesus monkey. J Neurosci 1994;14:4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schröder H, Schütz U, Burghaus L, et al. Expressionof the alpha4 isoform of the nicotinic acetylcholine receptor in the fetal human cerebral cortex. Dev Brain Res 2001;132:33–45. [DOI] [PubMed] [Google Scholar]
- 89. Kordower JH, Mufson EJ. Nerve growth factor receptor‐immunoreactive neurons within the developing human cortex. J Comp Neurol 1992;323:25–41. [DOI] [PubMed] [Google Scholar]
- 90. Chen EY, Mufson EJ, Kordower JH. TRK and p75 neurotrophin receptor systems in the developing human brain. J Comp Neurol 1996;369:591–618. [DOI] [PubMed] [Google Scholar]
- 91. Meinecke DL, Rakic P. Low‐affinity p75 nerve growth factor receptor expression in the embryonic monkey telencephalon: Timing and localization in diverse cellular elements. Neuroscience 1993;54:105–116. [DOI] [PubMed] [Google Scholar]
- 92. Donoghue MJ, Rakic P. Molecular evidence for the early specification of presumptive functional domains in the embryonic primate cerebral cortex. J Neurosci 1999;19:5967–5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ulfig N. Calcium‐binding proteins in the human developing brain. Adv Anat Embryol Cell Biol 2002;165:1–92. [PubMed] [Google Scholar]
- 94. Honig LS, Hermann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex 1996;6:794–806. [DOI] [PubMed] [Google Scholar]
- 95. Chan WJ, Xia CL, Dong DC, Heizmann CW, Yew DT. Differential expression of S100 proteins in the developing human hippocampus and temporal cortex. Microsc Res Tech 2003;60:600–613. [DOI] [PubMed] [Google Scholar]
- 96. Xu G, Broadbelt KG, Haynes RL, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol 2011;70:841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sims KB, Crandall JE, Kosik KS, Williams RS. Microtubule‐associated protein 2 (MAP2) immunoreactivity in human fetal neocortex. Brain Res 1988;449:192–200. [DOI] [PubMed] [Google Scholar]
- 98. Arai Y, Ijuin T, Itoh M, Takenawa T, Takashima S, Becker LE. Developmental changes of synaptojanin expression in the human cerebrum and cerebellum. Dev Brain Res 2001;129:1–9. [DOI] [PubMed] [Google Scholar]
- 99. Elsas J, Sellhaus B, Herrmann M, et al. Fetuin‐A in the developing brain. Dev Neurobiol 2012;73:354–369. [DOI] [PubMed] [Google Scholar]
- 100. Haybaeck J, Llenos IC, Dulay RJ, et al. Expression of Nogo‐A is decreased with increasing gestational age in the human fetal brain. Dev Neurosci 2012;34:402–416. [DOI] [PubMed] [Google Scholar]
- 101. Antonini A, Shatz CJ. Relation between putative transmitter phenotypes and connectivity of subplate neurons during cerebral cortical development. Eur J Neurosci 1990;2:744–761. [DOI] [PubMed] [Google Scholar]
- 102. Friauf E, Shatz CJ. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol 1991;66:2059–2071. [DOI] [PubMed] [Google Scholar]
- 103. Herrmann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur J Neurosci 1994;6:1729–1742. [DOI] [PubMed] [Google Scholar]
- 104. Hanganu IL, Kilb W, Luhmann HJ. Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex 2001;11:400–410. [DOI] [PubMed] [Google Scholar]
- 105. Molliver ME, Kristt DA. The fine structural demonstration of monoaminergic synapses in immature rat neocortex. Neurosci Lett 1975;1:305–310. [DOI] [PubMed] [Google Scholar]
- 106. Smiley JF, Levey AI, Mesulam MM. Infracortical interstitial cells concurrently expressing m2‐muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate‐diaphorase in the human and monkey cerebral cortex. Neuroscience 1998;84:755–769. [DOI] [PubMed] [Google Scholar]
- 107. Hanganu IL, Luhmann HJ. Functional nicotinic acetylcholine receptors on subplate neurons in neonatal rat somatosensory cortex. J Neuorphysiol 2004;92:189–198. [DOI] [PubMed] [Google Scholar]
- 108. Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion‐weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 2013;112:1–7. [DOI] [PubMed] [Google Scholar]
- 109. Ball G, Boardman JP, Aljabar P, et al. The influence of preterm birth on the developing thalamocortical connectome. Cortex 2013;49:1711–1721. [DOI] [PubMed] [Google Scholar]
- 110. Kostovic I, Judaš M. Early development of neuronal circuitry of the human prefrontal cortex In: Gazzaniga MS, editors. The cognitive neuroscience, 4th edn A Bradford Book. Cambridge/London: The MIT Press, 2009; 29–47 [Google Scholar]
- 111. Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci 1990;10:2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Molnár Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci 1998;18:5723–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rivera C, Li H, Thomas‐Crusells J, et al. BDNF‐induced TrkB activation down‐regulates the K+‐Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 2002;159:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben‐Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 2004;432:758–761. [DOI] [PubMed] [Google Scholar]
- 115. Hanganu IL, Okabe A, Lessmann V, Luhmann HJ. Cellular mechanisms of subplate‐driven and cholinergic input‐dependent. Network activity in the neonatal rat somatosensory cortex. Cereb Cortex 2009;19:89–105. [DOI] [PubMed] [Google Scholar]
- 116. Luhmann HJ, Kilb W, Hanganu‐Opatz IL. Subplate cells: Amplifiers of neuronal activity in the developing cerebral cortex. Front Neuroanat 2009;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zecevic N. Cellular composition of the telencephalic wall in human embryos. Early Hum Dev 1993;32:131–149. [DOI] [PubMed] [Google Scholar]
- 118. Zecevic N, Milosevic A, Rakic S, Marin‐Padilla M. Early development and composition of the human primordial plexiform layer: An immunohistochemical study. J Comp Neurol 1999;412:241–254. [PubMed] [Google Scholar]
- 119. Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA 2010;107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting‐state fMRI. Cereb Cortex 2011;21:145–154. [DOI] [PubMed] [Google Scholar]
- 122. Hoff GE, Van den Heuvel MP, Benders MJ, Kersbergen KJ, De Vries LS. On development of functional brain connectivity in the young brain. Front Hum Neurosci 2013;7:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pla R, Borrell V, Flames N, Marin O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci 2006;26:6924–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Morin SM, Gehlert DR. Distribution of NPY Y5‐like immunoreactivity in the rat brain. J Mol Neurosci 2006;29:109–114. [DOI] [PubMed] [Google Scholar]
- 125. Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal‐Retzius cells does not disrupt neocortical layer order. Development 2006;133:537–545. [DOI] [PubMed] [Google Scholar]
- 126. Tabata H, Nakajima K. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci 2003;23:9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci 2001;4:931–936. [DOI] [PubMed] [Google Scholar]
- 128. Letinic K, Zoncu R, Rakic P. Origin of GABAergic neuron sin the human neocortex. Nature 2002;417:645–649. [DOI] [PubMed] [Google Scholar]


