Abstract
The envelope proteins (env) of simian immunodeficiency virus (SIV) and HIV type 1 assemble to form noncovalently associated oligomers in the endoplasmic reticulum. After cleavage in a Golgi compartment, oligomeric env complexes are transported to the surface of infected cells, where incorporation into budding virions can occur. Difficulties in obtaining adequate quantities of virions retaining env, as well as the unstable nature and hydrophobicity of the oligomer, may account for the absence of previous biophysical studies to determine the oligomeric valency of membrane-associated env. The aim of this study was to evaluate the oligomeric state of SIV env before membrane-fusion activation. Virion-associated env, obtained by crosslinking and detergent extraction, and non-crosslinked secreted env ectodomain (recombinant gp140) were purified by lentil-lectin chromatography and gel filtration as single predominant species. Sedimentation equilibrium-derived mass values for both forms of SIV env were close to those predicted for trimeric assemblies. Determination of the mass of individual molecules by scanning transmission electron microscopy confirmed that SIV virion-associated env and gp140 formed largely homogeneous populations of trimers. Furthermore, a triangular or tri-lobed morphology was clearly visualized in a subset of the trimers.
The envelope proteins (env) of the primate lentiviruses simian immunodeficiency virus (SIV) and HIV type 1 (HIV-1) are synthesized as precursor membrane proteins, the ectodomains of which are cotranslationally translocated into the lumen of the endoplasmic reticulum. Here, modifications, including glycosylation, disulfide bonding, and oligomerization, take place (1). Folded, oligomeric env is transported to the Golgi complex, where cleavage by a cellular protease produces the external (SU) and transmembrane (TM) subunits. A transmembrane domain within the TM subunit anchors the noncovalently associated SU/TM complex to the cell membrane, where incorporation into budding virions can occur. env is the only virally encoded component to protrude beyond the viral membrane. Before virion entry, an interaction between SU and the cell surface receptor CD4 and one of the members of the chemokine receptor family (usually CC chemokine receptor 5 or CXC chemokine receptor 4), is believed to elicit conformational changes in the SU/TM complex that culminate in membrane fusion between the infected cell or virion and target cell (reviewed in refs. 2 and 3). Drawing from models derived from crystallographic analyses of native and low-pH (fusion activated) influenza hemagglutinin (4, 5), it has been proposed that fusion activation of env involves a conformation change from a metastable native (prefusion activated) form to a lower free energy postfusion conformation (6, 7).
The nature of the quaternary structure of env is of considerable interest because the oligomeric SU/TM complex functions in viral entry and forms the main target of the neutralizing humoral immune response of the host. Biochemical methods have been used to study the oligomeric structure of prefusion activated SIV env. SIV-infected cells have been reported to produce dimers of full-length precursor and TM subunit env (8). Similarly, a soluble uncleaved recombinant SIV env lacking the transmembrane domain and cytoplasmic tail (gp140) was reported to form tetramers and dimers (9). Chemical crosslinking and sucrose gradient sedimentation suggested that full-length or soluble uncleaved HIV-1 precursor occurred as dimers and higher-order oligomers (10). In contrast to these studies, SIV and HIV-1 gp140 expressed in the absence of complex carbohydrate groups was found to adopt a trimeric structure (11, 12). Virion-derived HIV-1 SU trimers have also been reported (13).
SIV strains isolated from macaques and sooty mangabeys are closely related to HIV type 2 (14). A predicted N-terminal α-helix of the TM subunit is required for efficient HIV type 2 env precursor oligomer formation and/or stability (15). The functional conservation of this domain within the primate lentiviruses is suggested by the formation of hetero-oligomers between the env precursors of HIV-1 and HIV type 2 or SIV (15, 16). Mixing peptides corresponding to the N-terminal α-helical domain and a predicted C-terminal α-helical domain of the SIV TM subunit resulted in the formation of a complex consisting of a six-helix bundle of three N- and three C-terminal helix peptides (17). High-resolution x-ray crystallographic analysis revealed a triple-stranded coiled-coil core of N-terminal helix peptides with three C-terminal helix peptides packed into grooves on the outer surface of the core in an antiparallel orientation (18, 19). This structure implies close proximity of the N-terminally located fusion peptides and the C-terminal transmembrane domains, leading to the suggestion that it represents the conformation adopted after the fusion process (postfusion conformation). The essential features of this structure, including the trimeric coiled-coil, have also been demonstrated in env of other viruses, including HIV-1 (6, 7), other retroviruses such as human T cell leukemia virus type 1 (20) and visna virus (21), and less closely related viral systems including GP2 of the filovirus Ebola (22), F protein of the paramyxovirus simian virus 5 (23), and hemagglutinin of the orthomyxovirus influenza virus (5). These results have widely been interpreted to indicate that trimeric prefusion activated structures for SIV and HIV-1 env are likely; however, definitive evidence for this is lacking.
In this study, we have applied biochemical and biophysical techniques to determine the oligomeric state of both soluble SIV env expressed by means of a recombinant vaccinia virus and SIV virion-associated env. Analysis of gel-filtration fractions by chemical crosslinking, sedimentation equilibrium, and scanning transmission electron microscopy (STEM) established that both soluble and virion-associated forms of env were trimeric.
Materials and Methods
Recombinant and Virion-Associated SIV env.
The recombinant vaccinia virus vAE1 (24) was used to express gp140 derived from SIVCPMAC under the control of a synthetic early/late vaccinia virus promoter. env expression, purification and gel filtration were performed as described (25). SIV gp140 was purified from the tissue culture supernatant by lentil-lectin affinity chromatography using 0.5 M methyl α-d-mannopyranoside for elution. Concentrated eluate was subjected to gel-filtration chromatography using a 16/60 Superdex 200 column (Amersham Pharmacia) with PBS/0.02% sodium azide as the buffer. The flow rate was 0.5 ml/min, and 1-ml fractions were collected.
The source of virion-associated env was sucrose density gradient banded SIVMNE virions that had been treated with 2,2′-dithiodipyridine, which blocks infectivity but does not affect the conformation or functional integrity of env (26). The SIVMNE env gene has an in-frame stop codon resulting in a truncated cytoplasmic domain. Oligomeric SU/TM complexes on the virion surface were stabilized by incubating virions (5.7 mg of total protein in a 4-ml vol of PBS) with 1 mM 3,3′-dithiobis(sulfosuccinimidyl propionate) (DTSSP, a thiol-cleavable, amine-reactive crosslinker; Pierce) at 37°C for 30 min and quenched by adjusting the samples to 100 mM glycine. Virions were then lysed by the addition of hydrogenated Triton X-100 (TX-100h; Calbiochem) at a final concentration of 1% with incubation at room temperature for 15 min. Virion-derived env was purified by lentil-lectin affinity chromatography and subjected to gel filtration as described above for gp140, except that all solutions were supplemented to a final concentration of 0.1% TX-100h to maintain protein solubility.
Immunoblotting.
SIV gp140 within individual gel-filtration fractions was subjected to SDS/8% PAGE under reducing conditions and transferred to nitrocellulose membranes. After blocking with 4% BSA, membranes were sequentially probed with sera from an SIV-positive macaque and iodinated protein A. Signal was detected and quantified by phosphor-screen autoradiography using a scanner and IMAGEQUANT software (Molecular Dynamics). To crosslink gp140, samples were incubated in the presence of ethylene glycol bis(succinimidyl succinate) (EGS; Pierce) at a final concentration of 5 mM for 30 min at room temperature before SDS/5% PAGE under reducing conditions and immunoblotting. Virion-derived env (DTSSP-crosslinked) was analyzed in the crosslinked or non-crosslinked state by performing SDS/PAGE in the absence or presence of reducing agent, respectively. For virion-derived SIV env, membranes were sequentially probed with the murine anti-SU (V3) monoclonal antibody KK46 and iodinated polyclonal rabbit anti-mouse Ig before detection as described above.
Sedimentation Equilibrium.
Sedimentation equilibrium analysis was performed using a Beckman Optima XL-A/I analytical ultracentrifuge. Cells were loaded with volumes of 120–135 μl of sample at a concentration of ≈0.1–0.4 mg/ml. Sedimentation equilibrium absorbance data were acquired at 280 nm. Three different rotor speeds (6,000, 7,000, and 8,000 rpm for gp140 or 6,000, 7,500, and 9,000 rpm for virion-derived env) were used for each sample, at a rotor temperature of 10°C. A global nonlinear regression analysis was performed using the data analysis software package provided by Beckman–Coulter (Version 4.0 and origin Version 4.1, Microcal, Amherst, MA). The partial specific volume for a glycoprotein (v̄gp) is equal to wp(v̄p) + wc(v̄c) where wp and wc and v̄p and v̄c are the protein and carbohydrate weight fractions and protein and carbohydrate partial specific volumes, respectively. Mass spectral analysis of SIV SU (cleaved from gp140 or shed from the virion surface after incubation at 60°C for 1 h) allowed for the determination of the average carbohydrate molar mass for each potential N-linked glycosylation site (2.06 kDa for SIV gp140 and 1.95 kDa for the virion-associated SU/TM complex), because the protein molar mass was known from sequence data. A partial specific volume value of 0.622 ml/g was used for the average glycosylation based on the analysis of glycoproteins by Lewis and Junghans (27). This value is in agreement with molar mass determinations of HIV-1 gp120 by sedimentation equilibrium and mass spectral analysis (25). For gp140 the wp, wc values and the protein v̄p are 0.619, 0.381, and 0.724 ml/g, respectively. For the SU/TM complex, the wp, wc values and the protein v̄p are 0.618, 0.382, and 0.726 ml/g, respectively. At 10°C the estimated v̄gp values were 0.685 and 0.686 ml/g for gp140 and the SU/TM complex, respectively.
For virion-derived env, the contribution of both protein-bound and free TX-100h micelles to mass were eliminated by using sucrose to match the solvent density to the detergent density (28). The density of sucrose at 10°C in 0.1% TX-100h/PBS was determined by using an Anton Paar DMA 5000 density meter (Graza, Austria). The buoyant molecular weight of 0.1% TX-100h micelles was measured as a function of solution density at 40,000 rpm at 10°C. A linear relationship between buoyant molecular mass and density was found with a zero value at a density of 1.040 g/ml, corresponding to a sucrose percentage of 8.43% (wt/vol), in agreement with the published value (28). A 50% (wt/vol) sucrose/PBS solution was used to adjust the sample and reference solutions to the matching density. Potential effects of preferential solvation on the buoyant molar mass of the protein/detergent complex were estimated to be small relative to a subunit buoyant molar mass.
STEM Measurements.
Thin (≈3 nm) carbon films were floated onto copper grids. A 5-μl sample of env (concentration ≈30–80 μg/ml) was applied to the grid and allowed to adsorb for 100 s, followed by a 5-μl sample of tobacco mosaic virus at a concentration of 0.4 mg/ml with absorbing for 200 s. The grid was washed five times in deionized water and then mounted in a KF80 plunge-freezing machine (Leica). The grid was immediately plunge-frozen into liquid ethane at −180°C, cryotransferred at −170°C to a HB501 STEM (VG Scientific), and then freeze-dried by warming slowly to −100°C (29). The sample was recooled to −170°C and annular dark-field images were acquired digitally at an accelerating voltage of 100 kV by using a Digiscan acquisition system (Gatan, Warrendale, PA). The STEM field-emission source provided a probe diameter of ≈1 nm, which was matched to the pixel size. The probe current was reduced to ≈2 pA. Dark-field STEM images measuring 1,024 × 1,024 pixels were recorded with a 100-μs counting time per pixel to give an electron dose of ≈103 e/mm2 and an acquisition time of 100 s. Images were processed and quantified using the image program (available from W. S. Rasband at the National Institutes of Health, http://rsb.info.nih.gov/nih-image/). Mass values were calibrated by using tobacco mosaic virus particles contained in the same field as the protein assemblies; 100-nm lengths of tobacco mosaic virus have a mass of 13.1 MDa.
Results
Purification and Gel-Filtration Analysis of SIV gp140.
SIV gp140 (lacking the transmembrane domain) was secreted mainly as uncleaved protein when synthesized in mammalian cells using a vaccinia virus expression vector. After lentil-lectin chromatography and gel filtration, SDS/PAGE and immunoblotting showed that gp140 was resolved as one major, relatively sharp gel-filtration peak (Fig. 1A). The absence of detectable contaminating protein in gel-filtration fractions used in subsequent analyses was demonstrated by SDS/PAGE and Coomassie blue staining (Fig. 1A Inset, showing fraction 52). Immunoblotting of EGS-crosslinked proteins (Fig. 1B) revealed that essentially all SIV gp140 had an electrophoretic mobility less than that expected for monomer, which migrated more rapidly than a 250-kDa mass standard (Fig. 1A Inset), suggesting that all gp140 was oligomeric. Allowing for the broadness of bands typically observed for crosslinked glycoproteins, most gp140 appeared to belong to a single oligomeric species. However, a shoulder on the earlier-eluting side of the gel-filtration peak (Fig. 1A) and the detection of more slowly SDS/PAGE-migrating env in the corresponding crosslinked fractions (Fig. 1B) suggested the presence of some env molecules with a higher number of subunits than the major oligomeric species. Immunoblotting of non-crosslinked sample revealed a small amount of protein with an apparent mass between 105 and 160 kDa within fractions 65–67 (data not shown), consistent with the presence of monomeric gp120 (25) derived from cleaved gp140.
Figure 1.
Gel-filtration analysis and sedimentation equilibrium concentration profiles of SIV gp140. (A) Aliquots of gel-filtration fractions were subjected to SDS/8% PAGE and detected by immunoblotting with SIV-positive sera and iodinated protein A. gp140 was quantified by phosphor screen autoradiography, and is shown as a percentage of total gp140-specific signal. Blue dextran 2000 (giving void volume) had an elution peak in fraction 43. (Inset) SDS/8% PAGE of fraction 52 followed by Coomassie blue staining. (B) Gel-filtration aliquots crosslinked with a final concentration of 5 mM EGS were analyzed by SDS/5% PAGE and immunoblotted as above. The bar in A Inset and B indicates the electrophoretic mobility of a 250-kDa marker protein. (C Upper) Sedimentation equilibrium concentration profiles of individual gel filtration fractions 51 (□), 52 (○), 53 (▵), and 54 (⋄). Solid lines show the best-fit distributions after global modeling of data obtained at three different rotor speeds. For clarity, only data at 7,000 rpm are shown. (Lower) Residuals of the fitted lines to the experimental data.
Mass Determination of SIV gp140.
For globular proteins, gel-filtration analysis can be used to determine mass by using proteins of known shape as calibration standards. Our previous experience with the fully glycosylated HIV-1 gp120 subunit suggested that env was nonglobular in shape, precluding the use of gel filtration alone to determine mass (25). Sedimentation equilibrium and STEM, both of which allow shape-independent mass determination, were used here to determine the number of subunits in the oligomeric species. Individual 1-ml fractions collected during gel filtration were concentrated ≈4- to 8-fold (final gp140 concentration ≈0.2–0.4 mg/ml) before analysis. The sedimentation equilibrium absorbance versus radial position data (Fig. 1C) were analyzed by global nonlinear regression. The masses determined by this analysis, and the calculated numbers of subunits (in parentheses) are shown in Table 1. The gp140 contained within fractions 53 and 54 gave average mass values closely approximating the expected values for a trimeric structure. The average masses of gp140 from fractions 51 and 52 (435.6 and 430.8 kDa, respectively) are somewhat higher than expected for trimer (3.48 and 3.45 subunits, respectively), and suggest the presence of some molecules with higher mass, which was also indicated by the presence of a shoulder on the earlier-eluting side of the gel-filtration peak.
Table 1.
Mass of SIV gp140
| Fraction no. | Mass, kDa (no. of subunits)
|
|
|---|---|---|
| Sedimentation equilibrium (weight average) | STEM (no. average) | |
| 51 | 435.6 (3.48) | 357 (2.86) |
| 52 | 430.8 (3.45) | |
| 53 | 408.7 (3.27) | 373 (2.98) |
| 54 | 380.3 (3.04) | |
The mass of SIV SU (cleaved from gp140) was determined by mass spectrometry (99 kDa; D. Sheeley and R.J.C., unpublished data), allowing the average mass for each potential N-linked glycosylation site to be calculated. This value was then multiplied by the number of potential N-linked glycosylation sites (23 sites) and added to the protein molar mass (known from the amino acid sequence data) of SIV gp140 to give an estimate of the monomer mass (125 kDa).
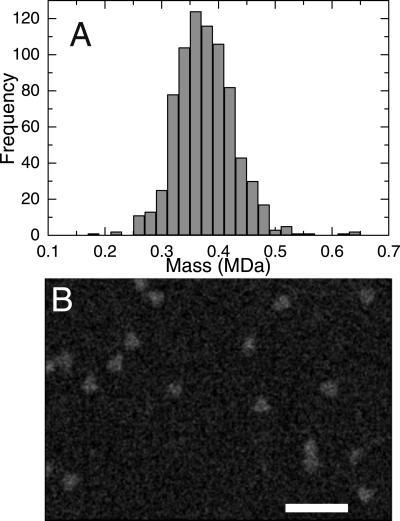
STEM provides a quantitative method for measuring the masses of individual macromolecular complexes (30). Examination of a histogram of the measured masses of 415 molecular assemblies within a pool of fractions 53 and 54 revealed a relatively symmetrical peak with a mean value of 373 kDa and a standard deviation of 63 kDa (Fig. 2A, Table 1). This observation is consistent with the predicted mass of a trimer (375 kDa). The presence of substantial numbers of molecules within a relatively wide mass range (250–450 kDa) can be attributed to the heterogeneity of glycosylation and random experimental error caused by counting statistics in the low-dose STEM images. The STEM mass determinations for a pool of fractions 51 and 52 are also consistent with a trimeric structure (Fig. 2B, Table 1). A total of 415 molecular assemblies were analyzed, giving a mean value of 357 kDa and a standard deviation of 73 kDa. In a subset of STEM images (10–20% of total) of both SIV gp140 pools, an apparent triangular or tri-lobed morphology was observed (Fig. 2C). This morphology is consistent both with that observed previously for virion-associated SIV env (31) and with trimeric structure. Overall, the data clearly indicate that the SIV gp140 analyzed here has a trimeric quaternary structure.
Figure 2.
Mass distribution of SIV gp140 determined by quantitative analysis of STEM images. (A) Analysis of pooled gel-filtration fractions 53 and 54. (B) Analysis of pooled gel-filtration fractions 51 and 52. A total of 415 individual molecular assemblies were analyzed for each sample pool. (C) Montage of SIV gp140 STEM images exhibiting triangular or tri-lobed morphology. Images from a pool of gel-filtration fractions 53 and 54 are depicted, with smoothing to reduce pixelation. (Scale bar = 50 nm.)
Purification and Gel-Filtration Analysis of SIV SU/TM Complexes.
To assess the quaternary structure of virion-associated SIV env, SU/TM complexes were stabilized by crosslinking before virion lysis, glycoprotein purification, and gel filtration. SDS/PAGE of a pool of fractions 51 and 52 in the presence of reducing agent followed by Coomassie blue staining (Fig. 3A Inset) revealed a dark staining band corresponding to SU and a faint, diffuse band corresponding to TM. This result indicated the absence of detectable levels of contaminating protein in gel-filtration fractions and therefore no significant crosslinking between env and any cellular proteins on the virion surface. Gel-filtration fractions were reduced then immunoblotted with an anti-SU monoclonal antibody, and the results were expressed as the percentage of total signal (Fig. 3A). Virion-derived SIV SU/TM complexes resolved as a relatively sharp and symmetrical peak, with most protein having eluted at 50–53 ml. Immunoblotting of gel filtration fractions under nonreducing conditions (DTSSP crosslinks retained; Fig. 3B) demonstrated that virion-derived SU/TM complexes had an electrophoretic mobility lower than that expected for an SU/TM heterodimer or SU and TM monomers, which have apparent masses of between 105 and 160 kDa, and slightly above 30 kDa, respectively. This result demonstrated that, under the experimental conditions used, virion-derived env retained oligomeric structure during purification. Purification by the above method without prior crosslinking led to partial dissociation of SU/TM oligomers (data not shown), indicating the lability of the interaction between subunits of the complex.
Figure 3.
Gel-filtration analysis and sedimentation equilibrium concentration profiles of virion-derived SIV env. (A) Aliquots of gel-filtration fractions were subjected to SDS/8% PAGE in the presence of reducing agent and immunoblotted with an SIV env-specific monoclonal antibody and iodinated antimouse Ig. env was quantified by phosphor screen autoradiography. (Inset) SDS/4–20% PAGE of a pool of fractions 51 and 52 followed by Coomassie blue staining. (B) Gel-filtration aliquots were analyzed by SDS/5% PAGE in the absence of reducing agent (crosslinks maintained) and immunoblotted as above. The bar in A Inset and B indicates the electrophoretic mobility of a 250-kDa marker protein. (C Upper) Sedimentation equilibrium concentration profiles of individual gel-filtration fractions 50 (○), 51 (▵), 52 (+), and 53 (□). Solid lines show the best-fit distributions after global modeling of data obtained at three different rotor speeds. For clarity, only data at 7500 rpm are shown. The profiles for fractions 50 and 53 are overlaid because of very similar starting concentrations. (Lower) Residuals of the fitted lines to the experimental data.
Mass Determination of Virion-Derived SIV SU/TM Complexes.
The similarity of the gel-filtration profiles for SIV gp140 and virion-derived SIV env suggested the possibility of a similar quaternary structure. Note that as the SIVMNE env gene has an in-frame stop codon resulting in a truncated cytoplasmic domain, the difference in the expected masses of gp140 and virion-derived SU/TM trimers is not great (375 versus 399 kDa, respectively). Individual gel-filtration fractions of virion-derived SIV env were concentrated ≈8-fold (final env concentration 0.1–0.4 mg/ml) before sedimentation equilibrium. The sedimentation equilibrium absorbance versus radial position data and the derived mass values with the calculated numbers of subunits (in parentheses) for individual fractions 50–53 are given in Fig. 3C and Table 2, respectively. The values for these fractions range from 396.9 to 430.2 kDa, which is consistent with trimeric status (range 2.98–3.23 subunits). For STEM analysis, SU/TM complexes from peak gel-filtration fractions 51 and 52 were pooled. Examination of a histogram of the measured masses of 765 molecular complexes revealed a symmetrical peak (Fig. 4A). The mean of this distribution was 387 kDa with a standard deviation of 52 kDa (Table 2). This mean is consistent with the expected mass of a trimer (399 kDa). Many of the STEM images (≈40–60%) showed a triangular or tri-lobed morphology (Fig. 4B) indistinguishable from that seen with gp140. The data presented here demonstrate that virion-derived SIVMNE SU/TM complexes are trimeric.
Table 2.
Mass of virion-associated SIV env
| Fraction no. | Mass, kDa (number of subunits)
|
|
|---|---|---|
| Sedimentation equilibrium (weight average) | STEM (no. average) | |
| 50 | 396.9 (2.98) | |
| 51 | 430.2 (3.23) | 387 (2.91) |
| 52 | 406.3 (3.05) | |
| 53 | 405.1 (3.05) | |
The mass of SIV SU (shed from the virion surface after incubation at 60°C for 1 h) was determined by mass spectrometry (103 kDa, D. Sheeley and R.J.C., unpublished data), allowing the average mass for each potential N-linked glycosylation site to be calculated. This value was then multiplied by the number of potential N-linked glycosylation sites (26 sites) and added to the protein molar mass (known from the amino acid sequence data) of virion-associated SIV env to give an estimate of the monomer mass (133 kDa).
Figure 4.
Mass distribution of virion-associated SIV env determined by quantitative analysis of STEM images. (A) Analysis of a pool of gel-filtration fractions 51 and 52. A total of 765 individual molecular assemblies were analyzed. (B) STEM field of virion-derived SIV env images exhibiting triangular or tri-lobed morphology, with smoothing to reduce pixelation. (Scale bar = 50 nm.)
Discussion
Difficulties in obtaining adequate quantities of purified virions retaining env, as well as the lability and hydrophobicity of the membrane-bound structure, may account for the absence of previous biophysical studies to determine the oligomeric valency of virion-associated env. We overcame these problems by large-scale virus isolation and crosslinking the env subunits before detergent extraction. We used two independent techniques, ultracentrifugation and STEM, and found that SU/TM complexes on the surface of virions form relatively homogeneous populations of trimers. A similar result was obtained by analysis of the soluble SIV env precursor analogue gp140. Furthermore, both forms of env were visualized as triangular or tri-lobed structures. These observations indicate that the determinants of trimeric structure reside within the ectodomain and are not negated by loss of membrane anchoring during folding and oligomerization in the endoplasmic reticulum. This oligomeric state is paralleled by that seen for the presumed postfusion structure of SIV TM, which has a trimeric coiled-coil core (18, 19). That structure was elucidated by crystallographic analysis of a complex of peptides corresponding to predicted N- and C-terminal helices of TM. Similar strategies have been used to demonstrate that postfusion conformation TMs of diverse enveloped viruses, including HIV-1 (6, 7), human T cell leukemia virus type 1 (20), visna virus (21), Ebola virus (22), simian virus 5 (23), human respiratory syncytial virus (32), Moloney murine leukemia virus (33), and influenza virus (5), all have trimeric cores composed of N-terminal helix elements. This structural similarity is consistent with a functionally conserved mechanism of env-mediated membrane fusion.
Biochemical evidence suggests that in some viral systems env activation involves a change in oligomeric state, including a homodimer to homotrimer transition in tick-borne encephalitis virus (34) and a heterodimer to homotrimer transition in Semliki Forest virus (35); activation is induced by low pH in these examples. In contrast, influenza virus hemagglutinin, which also undergoes marked conformational changes on low-pH activation, is maintained as a homotrimer throughout (4, 5). The results reported here and previously (18, 19) indicate that SIV env also maintains a homotrimeric conformation before and after fusion activation. Furthermore, fully glycosylated recombinant gp140 also forms a relatively homogeneous population of trimers and shows morphological similarity to virion-derived SU/TM complexes, based on low-resolution STEM images. This observation suggests that SIV gp140 may be a good model for env presented on the virion surface for future immunological and structural studies.
Acknowledgments
We thank Dr. R. Doms for providing recombinant vaccinia virus, Dr. G. Stubbs for providing tobacco mosaic virus, Dr. D. Sheeley for mass spectral analysis, and N. Cooper for cells. Monoclonal antibody KK46 was obtained from Dr. K. Kent and Ms. C. Arnold at the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. This work was supported in part by a National Institutes of Health Intramural AIDS Targeted Antiviral Program Grant. R.J.C. was supported by C. J. Martin Fellowship 987004 provided by the National Health and Medical Research Council (Australia).
Abbreviations
- HIV-1
human immunodeficiency virus type 1
- env
envelope protein
- SIV
simian immunodeficiency virus
- EGS
ethylene glycol bis(succinimidyl succinate)
- SU
external subunit
- TM
transmembrane subunit
- STEM
scanning transmission electron microscopy
- DTSSP
3,3′-dithiobis(sulfosuccinimidyl propionate)
- TX-100h
hydrogenated Triton X-100
References
- 1.Earl P L, Moss B, Doms R W. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt R, Sodroski J. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 3.Eckert D M, Kim P S. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 4.Wilson I A, Skehel J J, Wiley D C. Nature (London) 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 5.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Nature (London) 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 6.Chan D C, Fass D, Berger J M, Kim P S. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 7.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Nature (London) 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 8.Rey M-A, Laurent A G, McClure J, Krust B, Montagnier L, Hovanessian A G. J Virol. 1990;64:922–926. doi: 10.1128/jvi.64.2.922-926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes A D, Spitali M, Hutchinson G, Rud E W, Stephens P E. J Gen Virol. 1994;75:207–213. doi: 10.1099/0022-1317-75-1-207. [DOI] [PubMed] [Google Scholar]
- 10.Earl P L, Moss B. AIDS Res Hum Retroviruses. 1993;9:589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Zhou G, Kim M, Chishti Y, Hussey R E, Ely B, Skehel J J, Reinherz E L, Harrison S C, Wiley D C. J Biol Chem. 2000;275:34946–34953. doi: 10.1074/jbc.M004905200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C W-H, Chishti Y, Hussey R E, Reinherz E L. J Biol Chem. 2001;276:39577–39585. doi: 10.1074/jbc.M107147200. [DOI] [PubMed] [Google Scholar]
- 13.Weiss C D, Levy J A, White J M. J Virol. 1990;64:5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beer B E, Bailes E, Sharp P M, Hirsch V M. In: Human Retroviruses and AIDS. A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Kuiken C, Foley B, Hahn B, Marx P, McCutchan F, Mellors J W, Mullins J, Wolinsky S, Korber B, editors. Los Alamos, NM: Los Alamos National Laboratory; 1999. pp. 460–474. [Google Scholar]
- 15.Center R J, Kemp B E, Poumbourios P. J Virol. 1997;71:5706–5711. doi: 10.1128/jvi.71.7.5706-5711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doms R W, Earl P L, Chakrabarti S, Moss B. J Virol. 1990;64:3537–3540. doi: 10.1128/jvi.64.7.3537-3540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blacklow S C, Lu M, Kim P S. Biochemistry. 1995;34:14955–14962. doi: 10.1021/bi00046a001. [DOI] [PubMed] [Google Scholar]
- 18.Malashkevich V N, Chan D C, Chutkowski C T, Kim P S. Proc Natl Acad Sci USA. 1998;95:9134–9139. doi: 10.1073/pnas.95.16.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z-N, Mueser T C, Kaufman J, Stahl S J, Wingfield P T, Hyde C C. J Struct Biol. 1999;126:131–144. doi: 10.1006/jsbi.1999.4116. [DOI] [PubMed] [Google Scholar]
- 20.Kobe B, Center R J, Kemp B E, Poumbourios P. Proc Natl Acad Sci USA. 1999;96:4319–4324. doi: 10.1073/pnas.96.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malashkevich V N, Singh M, Kim P S. Proc Natl Acad Sci USA. 2001;98:8502–8506. doi: 10.1073/pnas.151254798. . (First Published July 10, 2001; 10.1073/pnas.151254798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissenhorn W, Carfí A, Lee K-H, Skehel J J, Wiley D C. Mol Cell. 1998;2:605–616. doi: 10.1016/s1097-2765(00)80159-8. [DOI] [PubMed] [Google Scholar]
- 23.Baker K A, Dutch R E, Lamb R A, Jardetzky T S. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- 24.Edinger A L, Ahuja M, Sung T, Baxter K C, Haggarty B, Doms R W, Hoxie J A. J Virol. 2000;74:7922–7935. doi: 10.1128/jvi.74.17.7922-7935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Center R J, Earl P L, Lebowitz J, Schuck P, Moss B. J Virol. 2000;74:4448–4455. doi: 10.1128/jvi.74.10.4448-4455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, et al. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis M S, Junghans R P. Methods Enzymol. 2000;321:136–149. doi: 10.1016/s0076-6879(00)21191-9. [DOI] [PubMed] [Google Scholar]
- 28.Mayer G, Ludwig B, Müller H-W, van den Broek J A, Friesen R H E, Schubert D. Progr Colloid Polymer Sci. 1999;113:176–181. [Google Scholar]
- 29.Leapman R D, Andrews S B. J Microsc. 1992;165:225–238. doi: 10.1111/j.1365-2818.1992.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 30.Wall J S, Hainfeld J F. Annu Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- 31.Grief C, Hockley D J, Fromholc C E, Kitchin P A. J Gen Virol. 1989;70:2215–2219. doi: 10.1099/0022-1317-70-8-2215. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Singh M, Malashkevich V N, Kim P S. Proc Natl Acad Sci USA. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. . (First Published December 5, 2000; 10.1073/pnas.260499197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fass D, Harrison S C, Kim P S. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 34.Allison S L, Schalich J, Stiasny K, Mandl C W, Kunz C, Heinz F X. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahlberg J M, Bron R, Wilschut J, Garoff H. J Virol. 1992;66:7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]