Abstract
Objectives: Maintaining undifferentiated stem cells in defined conditions is of critical importance to improve their in vitro culture. We have evaluated the effects of culturing mouse stem (mES) cells under physiological oxygen concentration as well as by replacing fibroblast feeder layer (mEF) with gelatin or glycosaminoglycan hyaluronan (HA), on cell proliferation and differentiation.
Materials and methods: After 3 days culture or after long‐term cell culture under different conditions, levels of apoptotic cell death were determined by cell cycle and TUNEL (TdT‐mediated dUTP nick end labelling) assays and levels of cell proliferation by CFSE (5‐(and‐6)‐carboxyfluorescein diacetate succinimidyl ester) labelling. We assessed spontaneous differentiation into cardiomyocytes and mRNA expression of pluripotency and differentiation biomarkers.
Results: After 3 days culture under hypoxic conditions, levels of proliferation and apoptosis of mES cells were higher, in correlation with increase in intracellular reactive oxygen species. However, when cells were continuously grown for 1 month under those conditions, the level of apoptosis was, in all cases, under 4%. Hypoxia reduced spontaneous differentiation of mES into cardiomyocytes. Long‐term culture on HA was more effective in maintaining the pluripotent state of the mES cells when compared to that on gelatin. Level of terminal differentiation was highest on mEF, intermediate on HA and lowest on gelatin.
Conclusions: Our data suggest that hypoxia is not necessary for maintaining pluripotency of mES cells and appeared to be detrimental during ES differentiation. Moreover, HA may offer a valuable alternative for long‐term culture of mES cells in vitro.
Introduction
Potential use of stem cells in regenerative medicine relies on their removal from their natural habitat, their propagation in culture and their re‐introduction into a foreign tissue environment. To be able to do this, it is essential to understand how stem cells interact with different components of their culture environment in order to establish and maintain their properties [1]. Two major areas of study in current ES cell research include analyses of conditions to maintain their pluripotency and undifferentiated state, under continued in vitro culture, as well as development of in vitro culture conditions to create differentiation strategies for production of different cell types of interest [2]. Two essential factors of in vitro culture environments are oxygen concentration and use of defined substrata.
Oxygen tension in the mammalian reproductive tract has been reported to be much less than half of that of atmospheric oxygen, ranging from high values of around 60 mmHg (8.7% atmospheric O2) in the oviduct and uterus of hamsters and rabbits, to as low as 11 mmHg (1.5% O2) in the uterus of rhesus monkeys [3]. In hamsters and rabbits, intrauterine oxygen concentrations further decrease during blastulation and implantation, to 37 mmHg (5.3% O2) and 24 mmHg (3.5% O2) respectively [4]. Low oxygen environment found in the uterus is mandatory for early embryos to express oxygen‐regulated genes in correct temporal order, thereby ultimately driving their development [5]. Mouse embryonic stem (mES) cells are generally derived from the inner cell mass of blastocysts that grow in a low oxygen environment in vivo; however, in vitro mES cells are generally cultured in an atmosphere containing 20% O2 (normoxia). For human embryonic stem (hES) cells, it has been reported that hypoxic culture does not affect their growth but reduces their level of spontaneous cell differentiation [6]. These authors suggested that hypoxic culture would be necessary to maintain their full pluripotency. However, controversy remains as a recent report has suggested that there are no significant advantages in culturing hES cells under reduced oxygen tension [7].
In addition, generation of fresh supplies of murine embryonic stem cells without the need to grow on potentially contaminated and undefined mouse feeder layers is of high priority for stem cell research [8]. Several attempts have been made to develop feeder‐free systems and conditioned medium‐free culture [9, 10, 11] for mES cells [12]. Unconditioned medium supplemented with stem cell factor, foetal liver tyrosine kinase‐3 ligand, thrombopoietin and leukaemia inhibitory factor, but without basic fibroblast growth factor, was shown to be insufficient to maintain population expansion of undifferentiated hES cells [11, 13], emphasizing that autocrine and paracrine factors produced by h‐ and mES cells are not sufficient to maintain proliferation of undifferentiated stem cells in the long term. However, bioactive components present in the extracellular matrix may play a key role in mediating signals that recapitulate developmental processes in tissue‐specific differentiation and morphogenesis of both h‐ and mES cells [14, 15].
The extracellular matrix (ECM) is a uniquely assembled three‐dimensional molecular complex with diversity of composition. It is composed, with among others, of fibronectin, collagens, elastins and hyaluronan (HA) glycoprotein [16, 17]. Hyaluronan is a large glycosaminoglycan (average molecular mass of 106) composed of a repeating unit of [d‐glucuronic‐acid (1‐β‐3) N‐acetyl‐d‐glucosamine (1‐β‐4)]. HA is capable of binding huge amounts of water as well as receptor proteins (hyaladherins), resulting in stable pericellular matrices [18]. Hyaluronan also harbours many hormones and growth factors such as transforming growth factor β1, leukaemia inhibitory factor, basic fibroblast growth factor, stem cell factor, foetal liver tyrosine kinase‐3 ligand, thrombopoietin, ciliary neurotrophic factor, oncostatin M interleukin‐6 [IL‐6] family members, and more [19]. In particular, some of these factors (for example, IL‐6 and ciliary neurotrophic factor) have demonstrated effects in maintaining pluripotency of mES cells [20, 21].
Hyaluronan is detectable in the early vertebrate embryo as soon as formation of the blastocoelic cavity begins [22, 23]. It is a physiological component of mammalian follicular, oviductal and uterine fluids, and ECM. It has been previously reported that HA improves developmental potential of bovine oocytes and embryos [24, 25, 26]. Later, during foetal development, extensive synthesis of this polysaccharide occurs in the ECM of many tissues.
Recently, Gerecht et al. reported that murine embryonic fibroblasts (mEF) produce 8‐fold higher levels of HA (840 ng/ml) compared to initial levels in the media (105 ng/ml) and that abundant HA‐binding sites are located intracellularly on undifferentiated hES cells [10]. Success of mEF feeder layers for culture of hES cells is related to their ability to secrete HA [10]. HA hydrogels can support long‐term self‐renewal of hES cells, maintaining their undifferentiated state, and preserving their normal karyotype and their full differentiation capacity, as indicated by embryoid body formation [10]. However, little remains known about HA substrates for mES cell culture.
Currently, pluripotent mES cells are maintained on plastic or gelatin‐coated surfaces for cytotoxicity studies, while pluripotent mES cells for terminal differentiation studies are grown on mouse fibroblast feeder layers [12]. The present study was undertaken to test whether mouse stem cells, cultured in oxygen tensions similar to those found in the uterine environment, would prove advantageous over conventional culture conditions, in terms of population growth and differentiation. In addition, we have tested the potential of HA in maintaining a homogeneous population of undifferentiated mES cells eliminating the need to use fibroblast feeder layers.
Materials and methods
Reagents and media
All chemicals and culture media were purchased from Sigma Chemical Co. (Madrid, Spain) unless otherwise stated.
Cell cultures
Two mES cell lines (R1 129/Sv from the laboratory of Dr A. Nagy and MAR B6D2 F1 generated in our laboratory) were cultured at 37 °C under two different oxygen tensions (5% and 20%) or on three different substrates, mitomycin‐C treated mouse embryonic fibroblast (mEF): 0.1% gelatin and HA (0.12 mg/ml). Incubators were gassed with 5% CO2/95% air (containing therefore approximately 20% oxygen) or with a gas mixture containing 5% O2/5% CO2/90% N2. Ten to 20 early cell passages were used in all experiments.
Culture media
In all experiments, both cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 20% FBS (PAA Laboratories, Cölbe, Germany), 2 mm glutamine, 1 mm MEM non‐essential amino acids, 1 mmβ‐mercaptoethanol, 1000 U/ml recombinant mouse leukaemia inhibitory factor (LIF) (Chemicon International, Billerica, MA, USA) and an antibiotic mixture containing 100 U/ml penicillin and 100 μg/ml streptomycin. Culture medium were replaced at atmospheric conditions every day. Cells were detached with trypsin every other day.
Preparation of hyaluronan and gelatin
Gelatin‐ and hyaluronan‐(HA) [high molecular weight HA (600 ± 100 kDa) (MAP‐5; Bioniche Inc., Belleville, Ontario, Canada] coated wells were prepared by adding 2.0 ml aliquots of either 0.12 mg/ml HA in H2O or 0.1% gelatin to Corning 35 mm tissue culture wells. Hyaluronan and gelatin were aspirated after 30 min. Culture medium was immediately added to prevent desiccation and plates were used the same day.
Cell cycle and apoptosis analysis
For cell cycle analysis, DNA content was labelled with propidium iodide. Cells were detached with trypsin and washed with phosphate‐buffered saline (PBS). After fixing in 70% ethanol for 2 h and washing twice in PBS, samples were stained with propidium iodide (20 μg/ml)/Triton X‐100 (0.1%)/ribonuclease A (0.2 mg/ml) for flow cytometric analysis (Current Protocols in Cytometry, Cap. 7, 2001). Samples were analysed using a FACSCalibur® cytometer (Becton, Franklin Lakes, NJ, USA) and cellquest software. Numbers of apoptotic cells form a peak below that of G1 (often called sub‐G1 peak).
Measurement of apoptosis by TUNEL labelling
For detection of DNA strand breaks in apoptotic cells by fluorescence microscopy, cells were fixed in 70% ethanol and incubated in TUNEL (TdT‐mediated dUTP nick end labelling) reaction mixture (In Situ Cell Death Detection Kit, Fluorescein; Roche, Madrid, Spain), containing TdT and fluorescein dUTP, according to the manufacturer’s instructions. Incorporated fluorescein was visualized using a fluorescence microscope.
Cell proliferation measurement by CFSE labelling
To analyse proliferative activity of the cells (R1 and MAR ES cells) under different culture conditions, we have used CFSE labelling. CFSE (5‐(and‐6)‐carboxyfluorescein diacetate succinimidyl ester) is a fluorescent intracellular probe that subdivides equally into daughter cells at each mitosis. Thus, fluorescence intensity of cells decreases 2‐fold at each cell division.
R1 and MAR ES cells were centrifuged at 200 g for 5 min, and cell pellets were re‐suspended in pre‐warmed PBS/0.1% BSA at final concentration of 5 × 105 cells/ml. CFSE (Cell TraceTM CFSE Cell Proliferation Kit; Molecular Probes, Carlsbad, CA, USA) was then added at final concentration of 15 μm and incubated for 10 min at 37 °C. Staining was quenched by addition of five volumes of ice‐cold culture medium. After 5 min on ice, cells were washed three times in fresh media and cultured for up to three more days. Cells were then harvested for flow cytometric analysis every 24 h; this was performed using FACSCalibur® cytometer and cellquest software.
Intracellular ROS detection
For measurement of intracellular reactive oxygen species (ROS) levels, cells were detached with trypsin, resuspended in PBS, loaded with 5‐(and‐6)‐chloromethyl‐2′,7′‐dichlorodihydrofluorescein diacetate, acetyl ester (CM‐H2DCFDA) 20 μm; (Molecular Probes) and incubated for 30 min at 37 °C. As positive control, oxidative activity was stimulated with H2O2 to final concentration of 100 μm. Samples were analysed using FACSCalibur® cytometer and cellquest software.
Differentiation into cardiomyocytes
Differentiation into cardiomyocytes was performed as described by Ramirez et al. [27]. Briefly, ES cells were trypsinized and back‐plated for 15 min to deplete fibroblasts and then plated in non‐adherent 10 cm bacterial‐grade Petri dishes (5 × 105 cells per dish) in ES medium without LIF. Embryoid bodies (EBs) were collected after 4 days using a yellow pipette tip and individually transferred to 96‐well tissue plates coated with the different substrata without LIF to allow differentiation of EBs. Cultures were maintained under 20% or 5% oxygen and presence of beating cardiomyocytes in each well was monitored for another 28 days.
Analysis of marker gene expression by RT‐PCR
Total RNA was extracted from ES cell pellets using UltraspectTM RNA Isolation System (Biotecx Lab. Inc., Houston, TX, USA) according to the manufacturer’s instructions. Precipitated RNA was dissolved in DEPC‐treated water and digested with 1 U of RQ DNase I (Promega, Madison, WI) at 37 °C for 20 min. RNA was extracted by phenol purification and ethanol precipitation, reconstituted in 50 μl of DEPC‐treated water, and stored at −70 °C until RT‐PCR. RT reaction was performed according to the manufacturer’s instructions (Gibco‐BRL, Grand Island, NY, USA). Five micrograms of RNA was dissolved in water, heat‐denatured (65 °C, 2 min) and reverse‐transcribed at 37 °C for 60 min in final volume of 20 μl containing 0.5 mm of each dNTP, 0.2 μm oligo (dT), 0.5 μm of random primers, MMLV‐RT (0.5 μl), RNasin (0.2 μl) and 1× MMLV‐RT buffer with 8 mm DTT. After reverse transcription, different genes were PCR amplified by adding a 1.5 μl aliquot of each sample to the PCR mix containing the specific primers. PCR products were subjected to electrophoresis on 2% agarose gel. Primers used for RT‐PCR are listed in [27]. GAPDH was used as positive control and experiments were run in the absence of template RNA as negative control. Generation of anticipated fragments was strictly dependent on presence of RNA in the RT reaction.
Results
Culture of mES cells under physiological oxygen tension increased apoptosis and proliferation levels over short time points
Analysis of whether conditions that mimic oxygen tensions found in vivo in mouse reproductive tracts are advantageous for in vitro culture of mES cells, we plated two different mES cell lines (R1 129/Sv from the laboratory of Dr A. Nagy and MAR B6D2 F1 generated in our laboratory) on mEF feeder layers. After 3 days culture under two different oxygen concentrations (20% and 5%), we analysed cell cycle progression. Day 3 was chosen for analysis as it corresponds to time when ES colonies would be fully developed, and changes in proliferation/apoptosis or differentiation occurring in early phases would be revealed at that time. By propidium iodide staining, fluorescence intensity in each tested cell varies linearly with DNA content, ranged from 2n (Go‐G1) to 4n (G2) with an intermediate plateau that corresponds to S phase. The sub‐G1 peak in DNA histograms corresponds to the apoptotic population. DNA histograms from R1 and MAR ES cells showed higher numbers of apoptotic cells for both R1 and MAR ES cells under hypoxic conditions (Fig. 1a).
Figure 1.
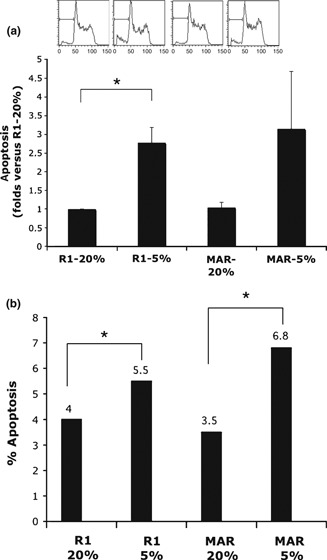
Apoptosis measurements from R1 and MAR ES cells grown on feeder layers. (a) Apoptosis measurements from R1 and MAR ES cells on feeder layer by cell cycle analysis. The sub‐G1 peak in DNA histograms was higher under hypoxic conditions, indicating higher numbers of apoptotic cells. Mean ± SE of two independent experiments is shown. *P < 0.05, Student’s t‐test. (b) Apoptosis measurements from R1 and MAR ES cells by TUNEL labelling. Mean values of two experiments are shown. After 3 days culture in different conditions, level of apoptotic cells for R1 and MAR was significantly higher under hypoxic conditions than under normoxic conditions (P < 0.05).
These data were corroborated by TUNEL labelling. After 3 days culture, the level of apoptotic cells for R1 and MAR was slightly but significantly higher (P < 0.05) under hypoxic conditions than under normoxic conditions (Fig. 1b).
Next we analysed kinetics of expansion of these cultures by CFSE labelling. Fluorescence profiles of CFSE‐labelled mouse R1 and MAR ES cells on labelling day (day 0) are shown in Fig. 2a, left panel. The right panel shows fluorescence profiles of cells recovered 3 days later. Under hypoxic conditions (5% O2), proliferation of both types of mES cells was greater than under normoxic conditions (20% O2).
Figure 2.
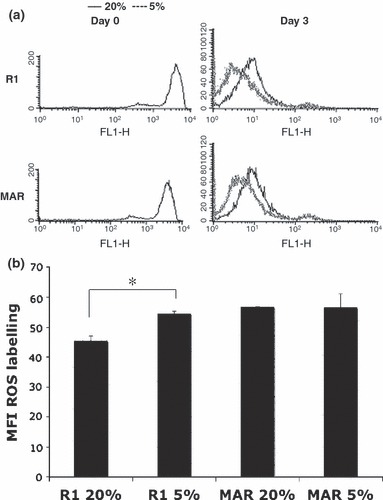
Proliferation and ROS measurements in mES cell cultures at different oxygen tensions. (a) Mouse R1 and MAR ES cells were labelled with CFSE. Profiles shown in the left panels correspond to labelled populations at day 0 (day of labelling). Right panel shows fluorescence profile of cells recovered 3 days later. Fluorescence intensity of cells decreased 2‐fold at each cell division. Under hypoxic conditions, proliferation of both types of mES cell was greater than under normoxic conditions. Culture of R1 and MAR ES cells on feeder layers and at 5% oxygen stimulated cells in this experiment to complete an extra cell cycle. (b) Mouse R1 and MAR ES cells were labelled with intracellular ROS probe CM‐H2DCFDA after 3 days culture under different oxygen tensions. Chart depicts the mean fluorescence intensity in two independent experiments. *P < 0.05 in Student’s t‐test.
As we were subjecting mES cell cultures to different oxygen tensions, we needed to determine whether intracellular redox states of cells was affected. The redox state of the cell is a consequence of precise balance between levels of oxidizing and reducing equivalents, such as ROS and endogenous antioxidants. Metabolically active cells can oxidize or reduce a variety of probes, providing a measure of cell viability and overall cell health. Therefore, cells were labelled with a ROS‐sensitive fluorescent probe and were cultured in 5% or 20% O2 atmosphere for 3 days. As shown in Fig. 2b, R1 cells exhibited significantly higher ROS production under hypoxic conditions, while there was no significant difference in MAR cell cultures.
However, if the cells were continuously grown for 1 month under the two different oxygen tensions, level of apoptosis, measured by TUNEL labelling, was undetectable at 20% oxygen and below 4% when cells were grown in 5% oxygen atmospheres (Fig. 3).
Figure 3.
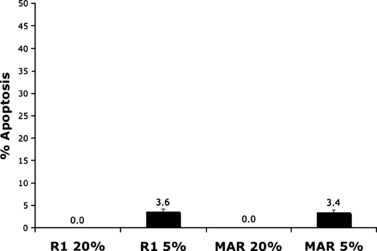
Apoptosis measurement from R1 and MAR ES cells after 1 month of culture under different oxygen tensions, by TUNEL labelling. Mean values of two experiments are shown. Level of apoptosis was under 4% in all conditions.
Low oxygen tension culture reduced terminal differentiation capacity of mES cell cultures
Next, we analysed cell pluripotency as well as capacity to differentiate, in the different cultures. By PCR, we analysed a series of genes previously reported as markers of early cell differentiation into cells of the three germ layers or into tissue‐specific precursors, as sensitive indicators of differentiation. We selected genes previously reported to be associated with the pluripotent state, and genes characteristic of blastocysts or other stem cell populations, as sensitive indicators of pluripotency. As shown in 1, 2 and Fig. 4, R1 cells demonstrated similar pluripotent and differentiation states at 5% or 20% O2. Similar results were found for MAR ES cells (data not shown). In contrast, when embryoid bodies were cultured under hypoxic conditions, levels of differentiation into beating cardiomyocytes were significantly lower than under normoxic conditions (11.9% versus 28.1% at 28 days, P = 0.014) (see also Fig. 8).
Table 1.
Expression of markers associated with pluripotent phenotypes in R1 ES cells
| Genes/cells | 5% O2 | 20% O2 | |
|---|---|---|---|
| R1 Day 0 | R1 Day 10 | R1 Day 10 | |
| Nanog | + | + | + |
| Oct3/4 | + | + | + |
| Rex1 | + | + | + |
| FoxD3 | + | + | + |
| Fgfr4 | + | + | + |
| Terf1 | + | + | + |
| Cx43 | + | + | + |
| Glut1 | + | + | + |
Table 2.
Expression of markers associated with differentiation phenotypes in R1 ES cells
| Genes/cells | 5% O2 | 20% O2 | |
|---|---|---|---|
| R1 Day 0 | R1 Day 10 | R1 Day 10 | |
| Gata‐4 | − | − | − |
| Gata‐2 | + | + | + |
| Afp | − | − | − |
| Foxa2 | + | + | + |
| Msx1 | − | − | − |
| T | − | − | − |
| Myf5 | − | − | − |
| Krt15 | + | + | + |
| Nes | +/− | + | + |
| Vim | + | + | + |
| Tubb3 | +/− | + | + |
Figure 4.
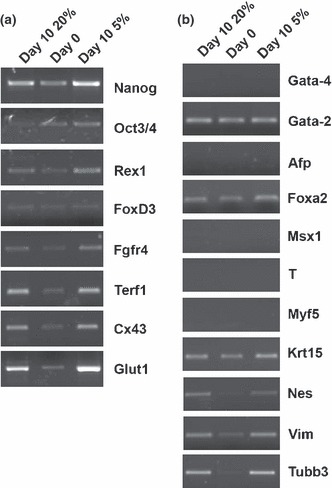
Expression of markers associated with pluripotent or differentiation phenotypes under different oxygen tensions. PCR detection of expression of markers associated with pluripotent (a) or differentiation phenotypes (b) in R1 ES cells cultured under different oxygen tensions.
Figure 8.
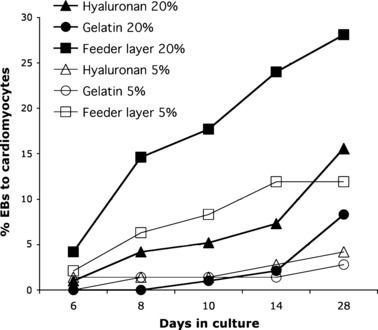
Beating cardiomyocyte formation over 30 days. EBs from mES cells were cultured under different conditions of oxygen concentrations (20%, filled symbols, or 5%, open symbols) and substrata – 120 μg/μl hyaluronan (triangles), 0.1% gelatin (circles) and mEF (squares) – and rates of beating cardiomyocytes counted at indicated times. Under hypoxic conditions, levels of differentiation into beating cardiomyocytes were significantly lower than under normoxic conditions on feeder layer (P = 0.014) and on HA (P = 0.035). Under normoxic conditions, feeder layers supported significantly higher cardiomyocyte formation when compared to 0.1% gelatin (P < 0.001) or to HA (P = 0.05).
Therefore, culture under low oxygen tension, although increasing proliferation level of the mEF cultures, caused an increment in oxidative stress and apoptosis and reduced their terminal differentiation capacity.
Comparison of cell cultures on different substrata
We needed to analyse use of different adhesive substrata for mES cells, to avoid use of feeder layers. We thus compared cultures on mEF with plates coated either with gelatin or hyaluronan. To determine optimal concentration of hyaluronan for culture, R1 ES cells were cultured for 10 days on Corning 35 mm tissue culture wells coated with different concentrations of hyaluronan (2, 1, 0.5, 0.25 and 0.12 mg/ml). Morphology of ES cell colonies and expression of biomarkers associated with pluripotent or differentiated phenotypes were evaluated (not shown). Thereafter, all experiments were performed at 0.12 mg/ml. Under such conditions, mES cells attached to HA‐coated plates and presented non‐differentiated morphology in compact colonies, similar to those grown on feeder layers (Fig. 5).
Figure 5.
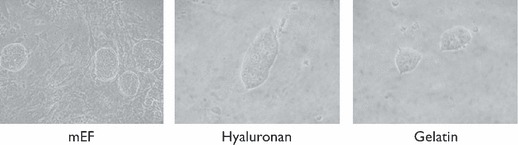
Morphology of ES cell colonies on different substrates after 2 days culture. Micrographs show mES cell colonies on the different substrata. Magnification: 200×.
First apoptotic level was analysed after 1 month culture on three different substrates in both R1 and MAR cells, by TUNEL labelling. As shown in Fig. 6, numbers of apoptotic cells were much reduced for both R1 and MAR ES cells under all conditions.
Figure 6.
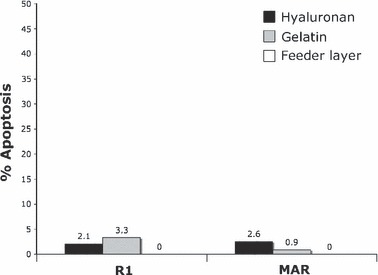
Apoptosis measurement by TUNEL labelling after 1 month continuous culture, on different substrata. Mouse R1 and MAR ES cells were grown on three different substrates for 1 month. Data shown are mean values of two independent experiments. Level of apoptotic cells for R1 and MAR ES cells was not significantly different.
However, when grown for 1 month on 0.1% gelatine, they lost expression of some pluripotency markers (Nanog and FoxD3 for R1 and Oct3/4, Rex1 and Nanog for MAR ES cells: Table 3 and Fig. 7a). On the other hand, mES cells grown on HA showed similar pluripotent states as mES cells cultured on mEF. Table 3 and Fig. 7a.
Table 3.
Expression of markers associated with pluripotent phenotypes in R1 and MAR ES cells continuously grown for 1 month
| Genes/cells | R1 MEF | R1 G | R1 HA | MAR MEF | MAR G | MAR HA |
|---|---|---|---|---|---|---|
| Nanog | + | − | + | + | − | + |
| Oct3/4 | + | + | + | + | + | + |
| Rex1 | + | + | + | + | + | + |
| FoxD3 | + | − | + | + | + | + |
| Fgfr4 | + | + | + | + | + | + |
| Terf1 | + | + | + | + | + | + |
| Cx43 | + | + | + | + | + | + |
Figure 7.
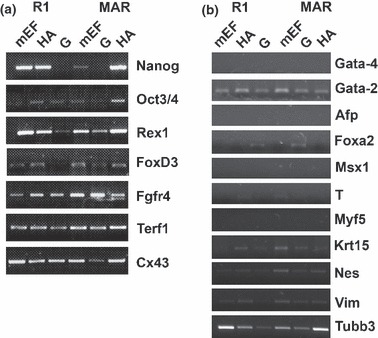
Expression of markers associated with pluripotent or differentiation phenotypes after one month on different substrates. PCR detection of expression of markers associated with pluripotent (a) or differentiation phenotypes (b) in R1 and MAR ES cells cultured for 1 month on different substrata.
When differentiation markers were analysed by RT‐PCR in different cultures, cells on HA showed a similar pattern to those grown on mEF, with the exception of Gata‐2 expression in endoderm layer genes (Table 4 and Fig. 7b). In contrast, when mES cells were grown on gelatin, both Gata‐2 and Foxa2 were detected, suggesting a more differentiated phenotype. When mesoderm and ectoderm genes were assessed, no significant differences were observed between the different substrata (Table 4 and Fig. 7b).
Table 4.
Expression of markers associated with differentiation phenotypes in R1 and MAR ES cells continuously grown for 1 month
| Genes/cells | R1 MEF | R1 G | R1 HA | MAR MEF | MAR G | MAR HA |
|---|---|---|---|---|---|---|
| Gata‐4 | − | − | − | − | − | − |
| Gata‐2 | + | + | + | + | + | + |
| Afp | − | − | − | − | − | − |
| Foxa2 | − | + | − | − | + | − |
| Msx1 | − | − | − | − | − | − |
| T | − | − | +/− | +/− | − | − |
| Myf5 | − | − | − | − | − | − |
| Krt15 | +/− | + | + | + | + | + |
| Nes | + | + | + | + | + | + |
| Vim | + | + | +/− | + | + | + |
| Tubb3 | + | + | + | + | + | + |
When differentiation into cardiomiocytes was assessed, levels of differentiation of cells grown on HA were comparable to those observed on feeder layers at hypoxic conditions, and lower than those grown under normoxic conditions on feeder layers, but significantly higher than levels observed on gelatin (Fig. 8).
Thus, all our data support that HA might be a valuable alternative to the use of feeder layers for expansion of mES cells.
Discussion
Stem cells can be defined as cells having high proliferative potential and the ability to self‐renew. They are undifferentiated, but can generate daughter cells of more than one distinct phenotype [28], and they can also migrate to areas of injury [29]. All such features are fundamental to stem cell function and can be dramatically altered by oxygen concentration during culture. However, beneficial effects of physiological oxygen environment of ES cells or culture on different substrata have not been previously reported. We hypothesized that low oxygen concentration, which is physiological for the early embryo, would reduce cell oxidant status, compared to culture of ES cells in supraphysiological oxygen. There have been several reports that show that cell proliferation and viability in culture, by many adult stem cells, is improved by culture in physiological oxygen [28]. Consistent with our data, virtually all stem cells cultured under lower physiological oxygen levels proliferate more than in traditional 20% O2 environments. Former studies on enhanced proliferation in lower oxygen levels have been reported for rat CNS‐derived multipotent stem cells [30], and foetus‐derived neural crest stem cells [31], adult murine skeletal muscle satellite cells [32, 33], and rat bone marrow‐derived mesenchymal stem cells and CD34+ bone marrow progenitor populations [34]. The practical benefit of low oxygen culture is that relatively rare cell populations are more easily expanded in vitro. Proliferation of first‐trimester trophoblast cells is also higher in 2% oxygen (versus 20%) in a bioreactor system [35]. Our results demonstrate that level of proliferation of mES cells on mEF is higher under hypoxic conditions.
However, our data also indicate that low oxygen tension induced oxidative stress at early time points that increased apoptosis. Stem cells have the potential to be used in cell therapy; however, tissue regeneration is limited by death of transplanted cells. One of the main mechanisms of stem cell death in transplanted organs is through ischaemia [36]. Hypoxic/ischaemic conditions have been long recognized as important mediators or modulators of apoptosis as these conditions bring about excessive production of ROS [37, 38, 39]. ROS directly damage cell membranes, DNA and protein, leading to alteration or loss of cell functions causing induction of apoptosis [40]. Our results demonstrate that mES cells after only 3 days of culture under hypoxic conditions showed higher numbers of apoptotic cells. Koyanagi‐Katsuta et al. found that apoptosis of mES cells was induced when they were dispersed as single cells, whereas this process was suppressed when they proliferated in aggregates, suggesting that direct interaction between ES cells and EF was required for suppression of apoptosis [41]. Those authors suggested that some protein factor(s) on cytoplasmic membranes of fibroblasts might have been responsible for blocking ES cell apoptosis. However, in our study, after 1 month of culture on the three different substrates, only occasional apoptotic events could be observed. Many ROS‐mediated responses protect cells against oxidative stress and re‐establish ‘redox homeostasis’ [40]. In mouse ES cells, both hypoxia/inducible factor 1 (HIF‐1) and HIF‐2α are expressed but HIF‐1α appears to be central to regulating hypoxic responses, as it targets many oxygen‐dependent genes that are not regulated by HIF‐2α [42]. However, HIF‐2α has been found to be a direct upstream regulator of Oct4 in mouse ES cells, suggesting that HIF‐2α is involved in regulation of stem cell maintenance [43]. In hES cells, HIF‐1α protein is only transiently expressed for approximately 48 h after exposure to low oxygen tension [44]. A direct role of HIF‐1 in regulating sensitivity to oxygen deprivation‐induced apoptosis has been explained in genetic studies using ES cells knocked out for HIF‐1α. HIF‐1α null cells showed lower apoptosis compared to wild‐type cells, during oxygen deprivation [45]. However, HIF‐1α can also trigger autophagy via BNIP3 and BNIP3L as a survival mechanism in cancer and stem cells [46, 47].
In vitro culture of ES cells must maintain not only proliferative capacity of the cells but also their pluripotential capacity. We explored expression of a set of markers characteristic of pluripotent cells, previously reported to be associated with the pluripotent state: Nanog [48, 49], Oct3/4 [50] and Rex1 [51]. We also determined markers expressed in undifferentiated cells controlled by Oct3/4 and SOX‐2 genes: FoxD3 [52], FGFR‐4 [53] and telomerase‐associated factor TERF1 [54]. In addition, expression of other markers present on blastocysts or other stem cell populations such as gap junction proteins, connexins‐43 (Cx43) [55] and glucose transporter GLUT1, was also examined [56]. Thus, to analyse expression of markers characteristic of differentiated phenotypes, we used published RT‐PCR primers that amplify genes characteristic of endoderm (Gata‐4, Gata‐2, Afp, Foxa2), mesoderm (Msx‐1, T, Myf5, Krt15) and ectoderm (Nes, Vim, Tubb3) lineages. Bands of appropriate size were observed for all these genes, using species‐specific primers.
Roberts et al. demonstrated that appearance of differentiated regions in human ES cell cultures, as assessed by morphology and loss of stem cell markers such us Oct4, was substantially reduced under hypoxic conditions. These authors concluded that hypoxic conditions would be required to maintain full pluripotency of mammalian ES cells [6]. However, for mouse ES cells, our data showed similar pluripotent and differentiation states in R1 and MAR mES cells at 5% or 20% O2.
Regarding differentiation, our data are in agreement with those reported for spontaneous differentiation of established hES cells, that is, suppressed by culture in hypoxia conditions [6]. Under hypoxia, level of differentiation into beating cardiomyocytes was significantly lower than under normoxic conditions. Thus, stem cell‐mediated events analogous to those in embryonic development may be masked or underestimated in vitro when non‐physiologically high oxygen tension is used for stem cell culture.
In summary of this part of our experiments, our data seemed to support the notion that low O2 tension was not necessary to prevent differentiation of mES cell colonies or to maintain them in a fully pluripotent state.
In addition, our findings have strongly suggested that use of hyaluronan, without feeder cells, may offer a valuable alternative for unifying and standardizing conditions for long‐term defined culture conditions of undifferentiated mES cells. The role of feeders is to facilitate cell proliferation and to inhibit their differentiation [57]. In our study, based on marker gene expression of pluripotency, mES cells on hyaluronan showed similar pluripotent states to those of mES cells cultured on mEF.
Concerning expression of differentiation markers, all cultures on mEF expressed Gata‐2, as it is expressed in fibroblasts [58]. Cultures on both gelatin and HA were also positive for expression of this gene. However, long‐term culture on gelatin also induced expression of Foxa2, which was neither observed on mEF nor on HA. Regarding mesoderm or ectoderm markers, there were no significant differences between the three culture conditions, all being positive for Krt15 as well as for all ectoderm layer markers. These data support the idea that mES cells cultured on HA for 1 month show similar pluripotency and differentiation states to cells cultured on mEF.
Therefore, all our data suggest that culture of mES cells at physiological oxygen tension might not be necessary for maintaining their pluripotential capacity, and that HA might offer a valuable alternative to feeder layers in long‐term culture of undifferentiated mES cell populations.
Acknowledgements
This study was supported by the Grant AGL2009‐11358 from the Spanish Ministry of Education and Science.
References
- 1. Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778. [DOI] [PubMed] [Google Scholar]
- 2. Rao RR, Stice SL (2004) Gene expression profiling of embryonic stem cells leads to greater understanding of pluripotency and early developmental events. Biol. Reprod. 71, 1772–1778. [DOI] [PubMed] [Google Scholar]
- 3. Feil D (2006) Effect of culturing mouse embryos under different oxygen concentrations on subsequent fetal and placental development. J. Physiol., 10, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gassmann M, Fandrey J, Bichet S, Wartenberg M, Marti HH, Bauer C et al. (1996) Oxygen supply and oxygen‐dependent gene expression in differentiating embryonic stem cells. Proc. Natl. Acad. Sci. USA 93, 2867–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bichet S, Wenger RH, Camenisch G, Rolfs A, Ehleben W, Porwol T et al. (1999) Oxygen tension modulates beta‐globin switching in embryoid bodies. FASEB J. 13, 285–295. [DOI] [PubMed] [Google Scholar]
- 6. Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 102, 4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H‐F, Kuo H‐C, Chen W, Wu F‐C, Yang Y‐S, Ho H‐N (2009) A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Hum. Reprod. 24, 71–80. [DOI] [PubMed] [Google Scholar]
- 8. Moore H (2006) The medium is the message. Nat. Biotechnol. 24, 160–161. [DOI] [PubMed] [Google Scholar]
- 9. Amit M, Shariki C, Margulets V, Itskovitz‐Eldor J (2004) Feeder layer‐ and serum‐free culture of human embryonic stem cells. Biol. Reprod. 70, 837–845. [DOI] [PubMed] [Google Scholar]
- 10. Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak‐Novakovic G (2007) Hyaluronic acid hydrogel for controlled self‐renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O’Sullivan C et al. (2005a) Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 23, 315–323. [DOI] [PubMed] [Google Scholar]
- 12. Greenlee AR, Kronenwetter‐Koepel TA, Kaiser SJ, Liu K (2005) Comparison of Matrigel and gelatin substrata for feeder‐free culture of undifferentiated mouse embryonic stem cells for toxicity testing. Toxicol. In Vitro 19, 389–397. [DOI] [PubMed] [Google Scholar]
- 13. Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA (2005b) Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2, 185–190. [DOI] [PubMed] [Google Scholar]
- 14. Lapidot T, Dar A, Kollet O (2005) How do stem cells find their way home? Blood 106, 1901–1910. [DOI] [PubMed] [Google Scholar]
- 15. Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55. [DOI] [PubMed] [Google Scholar]
- 16. Salustri A, Camaioni A, Di Giacomo M, Fulop C, Hascall VC (1999) Hyaluronan and proteoglycans in ovarian follicles. Hum. Reprod. Update 5, 293–301. [DOI] [PubMed] [Google Scholar]
- 17. Toole BP (1997) Hyaluronan in morphogenesis. J. Intern. Med. 242, 35–40. [DOI] [PubMed] [Google Scholar]
- 18. Mullegger J, Lepperdinger G (2002) Hyaluronan is an abundant constituent of the extracellular matrix of Xenopus embryos. Mol. Reprod. Dev. 61, 312–316. [DOI] [PubMed] [Google Scholar]
- 19. Mannello F, Tonti GA (2007) Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder‐free; medium with fetal calf serum, human serum, or enriched plasma; serum‐free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells 25, 1603–1609. [DOI] [PubMed] [Google Scholar]
- 20. Conover JC, Ip NY, Poueymirou WT, Bates B, Goldfarb MP, DeChiara TM et al. (1993) Ciliary neurotrophic factor maintains the pluripotentiality of embryonic stem cells. Development 119, 559–565. [DOI] [PubMed] [Google Scholar]
- 21. Niwa H, Burdon T, Chambers I, Smith A (1998) Self‐renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown JJ, Papaioannou VE (1993) Ontogeny of hyaluronan secretion during early mouse development. Development 117, 483–492. [DOI] [PubMed] [Google Scholar]
- 23. Fenderson BA, Stamenkovic I, Aruffo A (1993) Localization of hyaluronan in mouse embryos during implantation, gastrulation and organogenesis. Differentiation 54, 85–98. [DOI] [PubMed] [Google Scholar]
- 24. Palasz AT, Brena PB, Martinez MF, Perez‐Garnelo SS, Ramirez MA, Gutierrez‐Adan A et al. (2008) Development, molecular composition and freeze tolerance of bovine embryos cultured in TCM‐199 supplemented with hyaluronan. Zygote 16, 39–47. [DOI] [PubMed] [Google Scholar]
- 25. Palasz AT, Rodriguez‐Martinez H, Beltran‐Brena P, Perez‐Garnelo S, Martinez MF, Gutierrez‐Adan A et al. (2006) Effects of hyaluronan, BSA, and serum on bovine embryo in vitro development, ultrastructure, and gene expression patterns. Mol. Reprod. Dev. 73, 1503–1511. [DOI] [PubMed] [Google Scholar]
- 26. Stojkovic M, Kolle S, Peinl S, Stojkovic P, Zakhartchenko V, Thompson JG et al. (2002) Effects of high concentrations of hyaluronan in culture medium on development and survival rates of fresh and frozen‐thawed bovine embryos produced in vitro. Reproduction 124, 141–153. [PubMed] [Google Scholar]
- 27. Ramirez MA, Pericuesta E, Fernandez‐Gonzalez R, Pintado B, Gutierrez‐Adan A (2007) Inadvertent presence of pluripotent cells in monolayers derived from differentiated embryoid bodies. Int. J. Dev. Biol. 51, 397–408. [DOI] [PubMed] [Google Scholar]
- 28. Csete M (2005) Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 1049, 1–8. [DOI] [PubMed] [Google Scholar]
- 29. Blau HM, Brazelton TR, Weimann JM (2001) The evolving concept of a stem cell: entity or function? Cell 105, 829–841. [DOI] [PubMed] [Google Scholar]
- 30. Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B et al. (2000) Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 20, 7377–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ (2000) Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 20, 7370–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC et al. (2001) Oxygen‐mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 189, 189–196. [DOI] [PubMed] [Google Scholar]
- 33. Lennon DP, Edmison JM, Caplan AI (2001) Cultivation of rat marrow‐derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J. Cell. Physiol. 187, 345–355. [DOI] [PubMed] [Google Scholar]
- 34. Reykdal S, Abboud C, Liesveld J (1999) Effect of nitric oxide production and oxygen tension on progenitor preservation in ex vivo culture. Exp. Hematol. 27, 441–450. [DOI] [PubMed] [Google Scholar]
- 35. Ma T, Yang ST, Kniss DA (2001) Oxygen tension influences proliferation and differentiation in a tissue‐engineered model of placental trophoblast‐like cells. Tissue Eng. 7, 495–506. [DOI] [PubMed] [Google Scholar]
- 36. Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X et al. (2009) Berberine protects mesenchymal stem cells against hypoxia‐induced apoptosis in vitro. Biol. Pharm. Bull. 32, 1335–1342. [DOI] [PubMed] [Google Scholar]
- 37. Cao YJ, Shibata T, Rainov NG (2001) Hypoxia‐inducible transgene expression in differentiated human NT2N neurons – a cell culture model for gene therapy of postischemic neuronal loss. Gene Ther. 8, 1357–1362. [DOI] [PubMed] [Google Scholar]
- 38. Robey TE, Saiget MK, Reinecke H, Murry CE (2008) Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 45, 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang G, Hazra TK, Mitra S, Lee HM, Englander EW (2000) Mitochondrial DNA damage and a hypoxic response are induced by CoCl(2) in rat neuronal PC12 cells. Nucleic Acids Res. 28, 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dröge W (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. [DOI] [PubMed] [Google Scholar]
- 41. Koyanagi‐Katsuta R, Akimitsu N, Arimitsu N, Hatano T, Sekimizu K (2000) Apoptosis of mouse embryonic stem cells induced by single cell suspension. Tissue Cell 32, 66–70. [DOI] [PubMed] [Google Scholar]
- 42. Hu C‐J, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC (2006) Differential regulation of the transcriptional activities of hypoxia‐inducible factor 1 alpha (HIF‐1alpha) and HIF‐2alpha in stem cells. Mol. Cell. Biol. 26, 3514–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu C‐J et al. (2006) HIF‐2alpha regulates Oct‐4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cameron CM, Harding F, Hu W‐S, Kaufman DS (2008) Activation of hypoxic response in human embryonic stem cell‐derived embryoid bodies. Exp. Biol. Med. (Maywood) 233, 1044–1057. [DOI] [PubMed] [Google Scholar]
- 45. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M et al. (1998) Role of HIF‐1alpha in hypoxia‐mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490. [DOI] [PubMed] [Google Scholar]
- 46. Abaci HE, Truitt R, Luong E, Drazer G, Gerecht S (2010) Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 298, C1527–C1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bellot G, Garcia‐Medina R, Gounon P, Chiche J, Roux D, Pouysségur J et al. (2009) Hypoxia‐induced autophagy is mediated through hypoxia‐inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 29, 2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S et al. (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655. [DOI] [PubMed] [Google Scholar]
- 49. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K et al. (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642. [DOI] [PubMed] [Google Scholar]
- 50. Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H (1990) A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell 60, 461–472. [DOI] [PubMed] [Google Scholar]
- 51. Ben‐Shushan E, Thompson JR, Gudas LJ, Bergman Y (1998) Rex‐1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct‐3/4 and Oct‐6 binding to an octamer site and a novel protein, Rox‐1, binding to an adjacent site. Mol. Cell. Biol. 18, 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA et al. (1996) Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J. Biol. Chem. 271, 23126–23133. [DOI] [PubMed] [Google Scholar]
- 53. McDonald FJ, Heath JK (1994) Developmentally regulated expression of fibroblast growth factor receptor genes and splice variants by murine embryonic stem and embryonal carcinoma cells. Dev. Genet. 15, 148–154. [DOI] [PubMed] [Google Scholar]
- 54. Broccoli D, Smogorzewska A, Chong L, de Lange T (1997) Human telomeres contain two distinct Myb‐related proteins, TRF1 and TRF2. Nat. Genet. 17, 231–235. [DOI] [PubMed] [Google Scholar]
- 55. Rizos D, Lonergan P, Boland MP, Arroyo‐Garcia R, Pintado B, de la Fuente J et al. (2002) Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol. Reprod. 66, 589–595. [DOI] [PubMed] [Google Scholar]
- 56. Morita Y, Tsutsumi O, Oka Y, Taketani Y (1994) Glucose transporter GLUT1 mRNA expression in the ontogeny of glucose incorporation in mouse preimplantation embryos. Biochem. Biophys. Res. Commun. 199, 1525–1531. [DOI] [PubMed] [Google Scholar]
- 57. Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- 58. Minegishi N, Ohta J, Suwabe N, Nakauchi H, Ishihara H, Hayashi N et al. (1998) Alternative promoters regulate transcription of the mouse GATA‐2 gene. J. Biol. Chem. 273, 3625–3634. [DOI] [PubMed] [Google Scholar]


