Abstract
Objectives
Tongue squamous cell carcinoma (TSCC) is the most frequent type of oral malignancy. Increasing evidence has shown that miRNAs play key roles in many biological processes such as cell development, invasion, proliferation, differentiation, metabolism, apoptosis and migration.
Materials and methods
qRT‐PCR analysis was performed to measure miR‐137 expression. CCK‐8 analysis, cell colony formation, wound‐healing analysis and invasion were performed to detect resultant cell functions. The direct target of miR‐137 was labelled and measured by luciferase assay and Western blotting.
Results
We demonstrated that expression of miR‐137 was downregulated in TSCC tissues compared to matched normal ones. miR‐137 expression was downregulated in TSCC lines (SCC4, SCC1, UM1 and Cal27) compared to the immortalized NOK16B cell line and normal oral keratinocytes in culture (NHOK). In addition, we have shown that miR‐137 expression was epigenetically regulated in TSCCs. Overexpression of miR‐137 suppressed TSCC proliferation and colony formation. Ectopic expression of miR‐137 promoted expression of the epithelial biomarker, E‐cadherin, and inhibited the mesenchymal biomarker, N‐cadherin, as well as vimentin and Snail expression, indicating that miR‐137 suppressed TSCC epithelial‐mesenchymal transition (EMT). We also showed that ectopic expression of miR‐137 inhibited TSCC invasion and migration. In addition, we identified SP1 as a direct target gene of miR‐137 in SCC1 cells. SP1 overexpression rescued inhibitory effects exerted by miR‐137 on cell proliferation and EMT.
Conclusions
These results indicate that miR‐137 acted as a tumour suppressor in TSCC by targeting SP1.
1. Introduction
Tongue squamous cell carcinoma (TSCC) was the most frequent type of oral squamous cell carcinoma (OSCC) and was famous for its high ability of metastasis and proliferation.1, 2, 3, 4 TSCC usually leads to dysfunction of speech, mastication and deglutition.5, 6, 7 Although recent development has been achieved in the therapeutic managements of TSCC, the mortality of TSCC is still high.8, 9, 10 It is necessary to understand the molecular pathways involving in TSCC progression to find the novel therapeutic strategies for TSCC.
MicroRNAs (miRNAs), a class of small and non‐protein‐coding RNAs, regulate gene expression through binding to the 3′UTR of their target mRNAs.11, 12, 13 Increasing studies have demonstrated that miRNAs play a key role in many biological processes such as cell development, invasion, proliferation, differentiation, metabolism, apoptosis and migration.14, 15, 16, 17, 18, 19 Emerging evidences have showed that deregulated expression miRNA is correlated with cancer initiation, progression and promotion through modulating tumour suppressor gene or oncogene.20, 21, 22, 23, 24
Previous studies showed that miR‐137 played an important role in the tumour development.25, 26, 27, 28, 29, 30 For example, Dang et al.31 demonstrated that miR‐137 promoter methylation in oral lichen planus and oral squamous cell carcinoma. Wiklund et al.32 showed that aberrant miR‐375 expression can be detected, and distinguished oral squamous cell carcinoma (OSCC) patient oral rinse and saliva from healthy volunteers. Langevin et al.33 demonstrated that miR‐137 is methylated in pharyngeal and laryngeal squamous cancers tissues in addition to the oral squamous cell carcinoma. However, the role of miR‐137 in the TSCC was still uncovered. In this study, we demonstrated that the expression of miR‐137 was downregulated in TSCC tissues and cells. Overexpression of miR‐137 suppressed the TSCC cell proliferation, colony formation, invasion and migration. In addition, we identified SP1 as a direct target gene of miR‐137 in SCC1 cell. SP1 overexpression rescued the inhibitory effects exerted by miR‐137 on cell proliferation and EMT. These results suggested that miR‐137 exerted a suppressive role in TSCC by targeting SP1.
2. Materials and methods
2.1. Clinical tissues and cell lines cultured and oligonucleotide transfection
Human TSCC tissues and matched normal tissues (located >3 cm from the tumour tissues) were collected from the Stomatology Hospital of Jinan. Each patient was given written informed consent and the research was approved with Declaration of Helsinki and the ethics committee of the Stomatology Hospital of Jinan. Each tissue was diagnosed and confirmed by pathological examination. The TSCC cell lines (SCC4, SCC1, UM1 and Cal27) and immortalized NOK16B cell line and normal oral keratinocyte cell culture (NHOK) were purchased from ATCC (American Type Culture Collection) and were cultured in the DMEM/F12 medium supplemented with 10% FBS (foetal bovine serum; GIBCO, Grand Island, NY, USA). miR‐137 mimic and scramble mimic were obtained from the Genecopoeia (Guangzhou, China) and were transfected into the cell by using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's information.
2.2. MTT assay and colony formation assay
MTT assay (3‐[4, 5‐dimethylthiazol‐2‐yl]‐2, 5‐diphenyl‐tetrazoliumbromide) was performed to measure the cell proliferation at 24, 48 and 72 hours in the cells after transfection. MTT solution was put into the well and cells were continued to culture for 4 hours and then dimethylsulfoxide was added. The OD (optical density) was detected at 490 nm on the microplate reader. For cell colony formation analysis, cells were cultured in the six‐well plate and continued to culture for 2 weeks. Cell colony was fixed with the methanol and stained with crystal violet.
2.3. Migration and invasion assay
Wound‐healing analysis was performed to measure the cell migration. Cells were cultured in the six‐well plates and transfected with miR‐137 mimic or scramble. The cell was scratched by the sterile plastic and washed with medium and continued to culture for 24–48 hours. For invasion assay, cells were cultured in the upper transwell chamber (BD Biosciences, Franklin Lakes, NJ, USA) coated with Matrigel. Medium without serum was added in the upper chamber, where medium supplemented with 10% FBS in lower chamber was served as chemoattractant. After 48 hours, the cells which did not invade through the chamber were washed. Then the chamber was stained by methanol and crystal violet and counted with the microscope (Olympus, Tokyo, Japan).
2.4. Western blot
Protein was isolated from tissue or cells by using RIPA buffer. The total proteins were separated using 12% SDS PAGE gel and then transferred to the membrane (PVDF; Millipore, Boston, MA, USA). The membrane was blocked by milk and then probed with SP1 antibody (1:1000; Sigma‐Aldrich, St. Louis, MO, USA) and GAPDH antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The protein signal was measured by ECL (enhanced chemiluminescence reagents; Thermo Scientific, Waltham, MA, USA).
2.5. Quantitative RT‐PCR
Total RNA from cells or tissues was isolated using Trizol (Invitrogen) following the manufacturer's information. The relative expression of miR‐137 and SP1 was determined using qRT‐PCR according to the manufacturer's description. The expression level of miR‐137 and SP1 was measured by using the 2−ΔΔCt method, where GAPDH and U6 were used as an internal control for SP1 and miR‐137 respectively.
2.6. Dual‐luciferase reporter assay
One day before transfection, cell was cultured in the 24‐well plate for the dual‐luciferase reporter assay. The SP1 3′ UTR of SP1 cDNA containing putative site for the miR‐137 was synthesized and inserted into the Renilla lucifearse plasmid (Promega, Madison, WI, USA). Cell was co‐transfected with miR‐137 mimic or scramble and pGL3‐SP1‐3′UTR or MUT 3′UTR for 48 hours and measured by the Dual‐Luciferase Reporter System (Promega) according to the instruction.
2.7. Trichostatin A and 5‐Aza‐CdR treatment
Cells were treated with the 5‐aza‐2′‐deoxycytidine (5‐Aza‐CdR; Sigma‐Aldrich) at 0.5, 1.5 and 3 μmol/L or 300 nmol/L trichostatin A (TSA; Sigma‐Aldrich) for 24 hours. RNA of cells was purified with the TRIzol reagent according to the instructions, and the expression of miR‐137 was measured by qRT‐PCR.
2.8. Statistical analysis
Data were shown as mean ± SD. The differences between groups were detected by Student's t‐test. All statistical analyses used the spss 17.0 software. P<.05 was considered to be significant.
3. Results
3.1. The expression of miR‐137 was downregulated in TSCC tissues and cell lines
We measured miR‐137 expression in 25 TSCC tissues and the matched normal tissues. The expression of miR‐137 was downregulated in TSCC tissues compared to the matched normal tissues (Fig. 1). Moreover, we demonstrated that miR‐137 expression was downregulated in TSCC cell lines (SCC4, SCC1, UM1 and Cal27) compared to immortalized NOK16B cell line and normal oral keratinocyte cell (NHOK).
Figure 1.
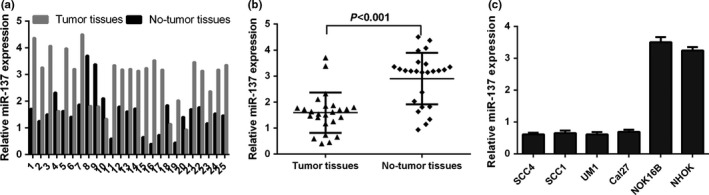
The expression of miR‐137 was downregulated in the TSCC tissues and cell lines. (a) The miR‐137 expression in the 25 TSCC tissues and matched normal tissues was measured by qRT‐PCR analysis. (b) The expression of miR‐137 was downregulated in the TSCC tissues compared to the matched normal tissues. (c) The expression of miR‐137 in the TSCC cell lines (SCC4, SCC1, UM1 and Cal27) and one immortalized NOK16B cell line and normal oral keratinocyte cell culture (NHOK) was detected by qRT‐PCR
3.2. miR‐137 expression was epigenetically regulated in TSCC cell
We treated SCC1 and UM1 cells with the DNA methylation inhibitor 5‐aza‐CdR (AZA) and histone deacetylase inhibitor trichostatin A (TSA). We found that miR‐137 expression was upregulated in both SCC1 and UM1 cells after TSA or AZA treatment (Fig. 2a,b).
Figure 2.
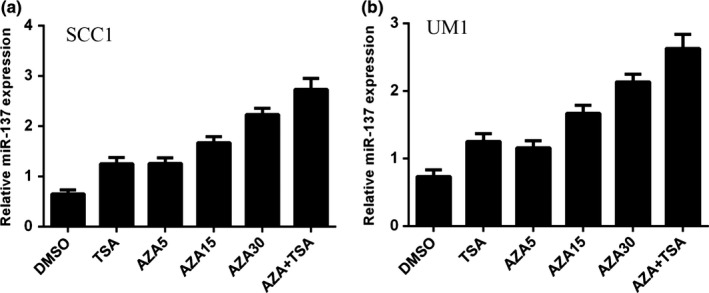
miR‐137 expression was epigenetically regulated in TSCC cell. (a) The expression of miR‐137 was measured in the SCC1 cell treated with TSA and 5‐Aza‐CdR by qRT‐PCR. (b) The expression of miR‐137 was measured in the UM1 cell treated with TSA and 5‐Aza‐CdR by qRT‐PCR
3.3. miR‐137 suppressed TSCC cell proliferation and colony formation
The expression of miR‐137 was upregulated after treatment with the miR‐137 mimic (Fig. 3a). Ectopic expression of miR‐137 suppressed SCC1 cell proliferation (Fig. 3b). Moreover, overexpression of miR‐137 inhibited SCC1 cell colony formation (Fig. 3c,d).
Figure 3.
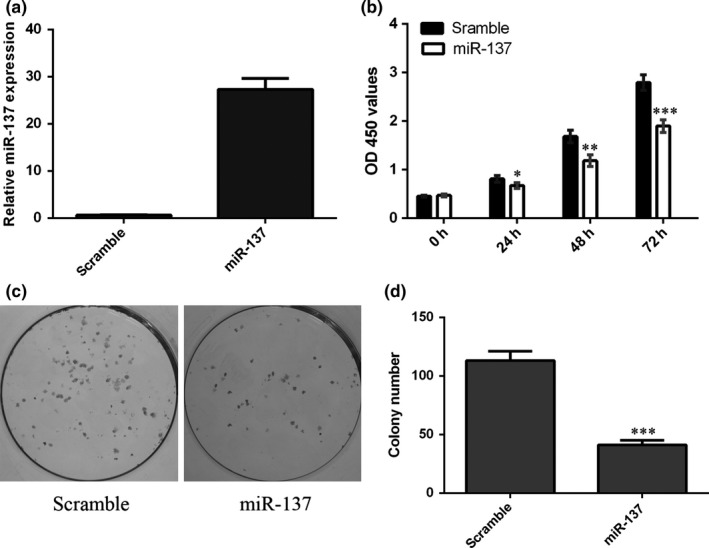
miR‐137 suppressed the TSCC cell proliferation and colony formation. (a) The expression of miR‐137 was detected in the SCC1 cell after treatment with the miR‐137 mimic. (b) Ectopic expression of miR‐137 suppressed the SCC1 cell proliferation. (c) Overexpression of miR‐137 inhibited the SCC1 cell colony formation. (d) The relative colony formation number was shown. *P<.05, **P<.01 and ***P<.001
3.4. miR‐137 suppressed TSCC cell epithelial‐mesenchymal transition (EMT)
Overexpression of miR‐137 promoted the expression of E‐cadherin in SCC1 cell (Fig. 4a). Moreover, ectopic expression of miR‐137 suppressed the expression of N‐cadherin, Snail and vimentin in SCC1 cell (Fig. 4b–d).
Figure 4.
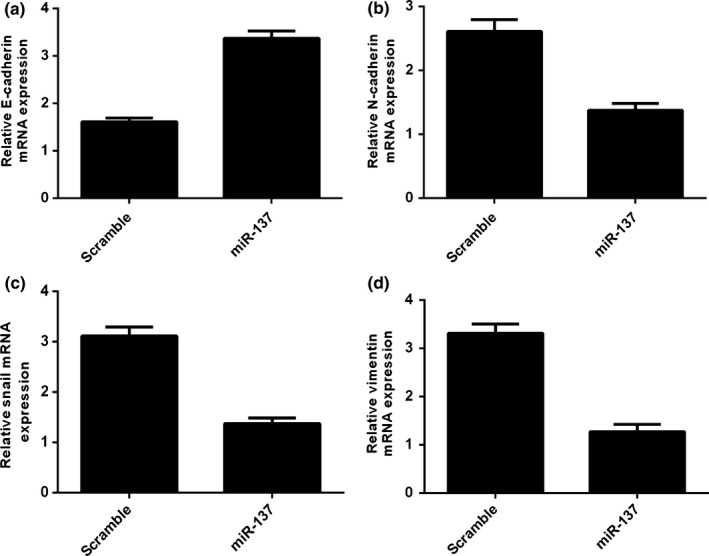
miR‐137 suppressed TSCC cell epithelial‐mesenchymal transition (EMT). (a) Overexpression of miR‐137 promoted the E‐cadherin expression in the SCC1 cell. (b) The mRNA expression of N‐cadherin was measured in the SCC1 cell using qRT‐PCR. (c) Ectopic expression of miR‐137 suppressed the N‐cadherin expression. (d) The mRNA expression of vimentin was measured in the SCC1 cell using qRT‐PCR
3.5. miR‐137 inhibited TSCC cell invasion and migration
We demonstrated that overexpression of miR‐137 inhibited SCC1 cell invasion (Fig. 5a). In addition, the wound‐healing analysis showed that ectopic expression of miR‐137 suppressed SCC1 cell migration (Fig. 5b).
Figure 5.
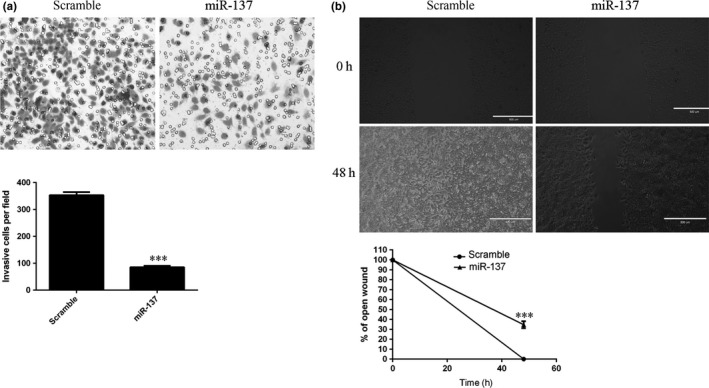
miR‐137 inhibited the TSCC cell invasion and migration. (a) We demonstrated that overexpression of miR‐137 inhibited the SCC1 cell invasion (Fig. 5a). In addition, we performed the wound‐healing analysis to measure the cell migration. We showed that ectopic expression of miR‐137 suppressed the SCC1 cell migration (Fig. 5b). ***P<.001
3.6. miR‐137 targeted SP1 in TSCC cell
TargetScan showed that SP1 was a potential target gene of miR‐137 (Fig. 6a). Ectopic expression of miR‐137 suppressed the luciferase activity of the wild‐type SP1 3′ UTR vector, whereas the mutated type was not changed in SCC1 cell (Fig. 6b). Overexpression of miR‐137 inhibited SP1 protein expression in SCC1 cell (Fig. 6c). Furthermore, the expression of SP1 was upregulated after treatment with SP1 vector (Fig. 6d). Moreover, restoration of SP1 could inhibit the expression of epithelial biomarker, E‐cadherin, and induce the expression of mesenchymal biomarker, N‐cadherin, vimentin and Snail in SCC1 cell (Fig. 6f–i).
Figure 6.
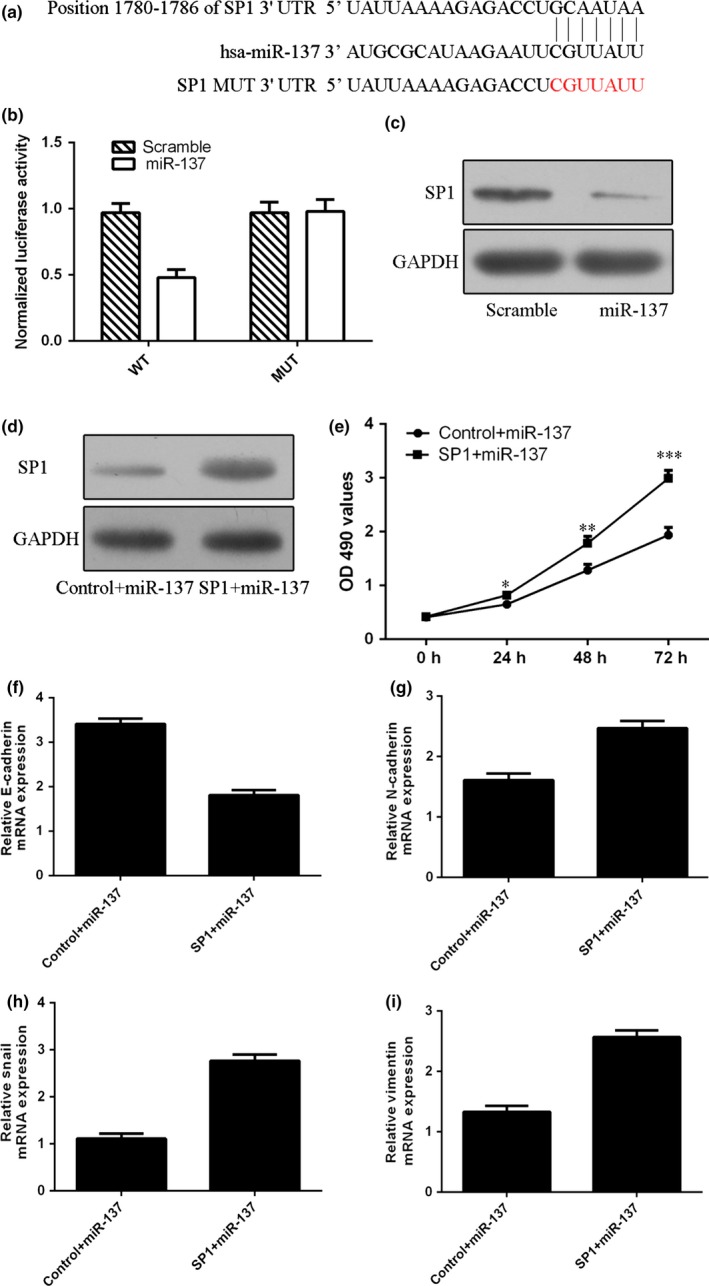
miR‐137 targets SP1 in TSCC cell. (a) The potential target gene of miR‐137, SP1, using TargetScan software. (b) Ectopic expression of miR‐137 suppressed the luciferase activity of wild‐type SP1 3′ UTR vector, whereas the luciferase activity of mutated type was not changed in the SCC1 cell. (c) The protein expression of SP1 was measured by Western blot. (d) The protein expression of SP1 was measured by Western blot. (e) The SCC1 cell proliferation was measured by CCK‐8 analysis. (f) The mRNA expression of E‐cadherin was detected by qRT‐PCR. (g) The mRNA expression of N‐cadherin was detected by qRT‐PCR. (h) The mRNA expression of Snail was detected by qRT‐PCR. (i) The mRNA expression of vimentin was detected by qRT‐PCR. *P<.05, **P<.01 and ***P<.001
4. Discussion
In our study, we demonstrated that the expression of miR‐137 was downregulated in TSCC tissues compared to the matched normal tissues. miR‐137 expression was downregulated in TSCC cell lines (SCC4, SCC1, UM1 and Cal27) compared to immortalized NOK16B cell line and normal oral keratinocyte cell culture (NHOK). We also showed that miR‐137 expression was epigenetically regulated in TSCC cell. Overexpression of miR‐137 suppressed TSCC cell proliferation and colony formation. Ectopic expression of miR‐137 promoted the expression of epithelial biomarker, E‐cadherin, and inhibited the expression of mesenchymal biomarker, including N‐cadherin, vimentin and Snail. These results suggested that miR‐137 suppressed TSCC cell epithelial‐mesenchymal transition (EMT). We also showed that ectopic expression of miR‐137 inhibited TSCC cell invasion and migration. In addition, we identified SP1 as a direct target gene of miR‐137 in SCC1 cell. SP1 overexpression rescued the inhibitory effects exerted by miR‐137 on cell proliferation and EMT. These results suggested that miR‐137 exerted a tumour suppressive role by targeting SP1 in TSCC.
Previous studies showed that miR‐137 played an important role in the tumour development.25, 26, 27, 28, 29, 30 For example, Shen et al.34 found that miR‐137 expression was downregulated in lung cancer tissues. They demonstrated that miR‐137 overexpression suppressed lung cancer cell proliferation, cell survival, migration and cell cycle by targeting nuclear casein kinase and cyclin‐dependent kinase substrate1(NUCKS1) expression. Liang et al.35 showed that miR‐137 expression was downregulated in the paediatric high‐grade glioma, and CENPE, NCAPG and KIF14 were the direct target genes of miR‐137. Dong et al.36 demonstrated that the expression of miR‐137 was downregulated in the papillary thyroid carcinoma tissues and was negatively correlated with lymph node metastasis and TNM (tumour‐node‐metastasis) stage. Overexpression of miR‐137 suppressed the papillary thyroid carcinoma cell colony formation, proliferation, invasion and migration through inhibiting CXCL12 (C‐X‐C motif chemokine 12) expression. Another study demonstrated that miR‐137 expression was downregulated in the colon cancer stem cell compared to normal colon stem cells.37 Forced expression of miR‐137 inhibited the development of colon cancer organoids by targeting double cortin‐like kinase 1(DCLK1) expression. Dang et al.31 demonstrated miR‐137 promoter methylation in oral lichen planus and oral squamous cell carcinoma. Wiklund et al.32 showed that aberrant miR‐375 expression can be detected, and distinguished oral squamous cell carcinoma (OSCC) patient oral rinse and saliva from healthy volunteers. Langevin et al.33 demonstrated that miR‐137 is methylated in pharyngeal and laryngeal squamous cancers tissues in addition to the oral squamous cell carcinoma. However, the role of miR‐137 in the TSCC was still uncovered. However, the role of miR‐137 in TSCC was still unknown. In our study, we showed that the expression of miR‐137 was also downregulated in the TSCC tissues compared to the matched normal tissues. MiR‐137 expression was downregulated in TSCC cell lines (SCC4, SCC1, UM1 and Cal27) compared to immortalized NOK16B cell line and normal oral keratinocyte cell culture (NHOK). Moreover, forced expression of miR‐137 suppressed TSCC cells cell proliferation, colony formation, migration and invasion. Furthermore, ectopic expression of miR‐137 promoted the expression of epithelial biomarker, E‐cadherin, and inhibited the expression of mesenchymal biomarker, N‐cadherin, vimentin and Snail. These data suggested that miR‐137 acted as a tumour suppressor gene in the development of TSCC.
SP1 was identified as the direct target gene of miR‐137 in TSCC. We used the TargetScan software to search the target genes of miR‐137 and found that Sp1 was a possible target gene of miR‐137. Furthermore, ectopic expression of miR‐137 suppressed the luciferase activity of wild‐type SP1 3′ UTR vector, whereas the luciferase activity of mutated type was not changed in SCC1 cell. Overexpression of miR‐137 inhibited the expression of SP1 protein in SCC1 cell. Sp1 is one zinc finger transcription factor which binds to many promoters that contains GC‐rich motifs.38, 39, 40 Previous studies showed that SP1 played an important role in a lot of cellular processes including cell apoptosis, proliferation, migration, response to DNA damage, immune responses and inflammation.41, 42, 43, 44, 45, 46, 47 Moreover, Jia et al.48 showed that miR‐375 was downregulated in TSCC tissues and miR‐375 overexpression suppressed TSCC cell proliferation and cell cycle by targeting SP1. In this study, we showed that Sp1 overexpression reversed the effects of miR‐137 on TSCC cell proliferation and EMT. Taken together, our data showed that miR‐137 regulated SP1 expression and acted as a tumour suppressor gene in TSCC.
In conclusion, miR‐137 was downregulated in TSCC tissues and cells and was a potential tumour suppressor miRNA that suppressed TSCC cell proliferation, migration and invasion. SP1 regulation by miR‐137 might play an important role in the regulation of TSCC malignant behaviour. This study showed that miR‐137 may be a novel therapeutic target for TSCC.
Lanying Sun and Jin Liang are co‐first author.
This work was supported by the Open Foundation of Shandong Provincial Key Laboratory of Dental Tissue Regeneration (SDKQ201504).
References
- 1. Qiu K, Huang Z, He Z, You S. miR‐22 regulates cell invasion, migration and proliferation in vitro through inhibiting CD147 expression in tongue squamous cell carcinoma. Arch Oral Biol. 2016;66:92–97. doi: 10.1016/j.archoralbio.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 2. Tsushima N, Sakashita T, Homma A, et al. The role of prophylactic neck dissection and tumor thickness evaluation for patients with cN0 tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016; doi: 10.1007/s00405-016-4077-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Zhang T, Liang L, Liu X, et al. TGFbeta1‐Smad3‐Jagged1‐Notch1‐Slug signaling pathway takes part in tumorigenesis and progress of tongue squamous cell carcinoma. J Oral Pathol Med. 2016; 45(7):486‐93.doi: 10.1111/jop.12406. [DOI] [PubMed] [Google Scholar]
- 4. Lao XM, Liang YJ, Su YX, Zhang SE, Zhou XI, Liao GQ. Distribution and significance of interstitial fibrosis and stroma‐infiltrating B cells in tongue squamous cell carcinoma. Oncol Lett. 2016;11:2027–2034. doi: 10.3892/ol.2016.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han J, Wang L, Wang X, Li K. Downregulation of microrna‐126 contributes to tumorigenesis of squamous tongue cell carcinoma via targeting KRAS. Med Sci Monit. 2016;22:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao L, Yu L, Li CM, Li Y, Jia BL, Zhang B. Karyopherin alpha2 induces apoptosis in tongue squamous cell carcinoma CAL‐27 cells through the p53 pathway. Oncol Rep. 2016;35:3357–3362. doi: 10.3892/or.2016.4750. [DOI] [PubMed] [Google Scholar]
- 7. Shegefti MS, Malekzadeh M, Malek‐Hosseini Z, Khademi B, Ghaderi A, Doroudchi M. Reduced serum levels of syndecan‐1 in patients with tongue squamous cell carcinoma. The Laryngoscope. 2016;126:E191–E195. doi: 10.1002/lary.25812. [DOI] [PubMed] [Google Scholar]
- 8. Adeel M, Suhail A. Squamous cell carcinoma of oral tongue in young patients – a 10 years tertiary care experience. J Pak Med Assoc. 2016;66:155–158. [PubMed] [Google Scholar]
- 9. Su X, Wang J, Chen W, Li Z, Fu X, Yang A. Overexpression of TRIM14 promotes tongue squamous cell carcinoma aggressiveness by activating the NF‐kappaB signaling pathway. Oncotarget. 2016;7:9939–9950. doi: 10.18632/oncotarget.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viveka TS, Shyamsundar V, Krishnamurthy A, Ramani P, Ramshankar V. p53 Expression helps identify high risk oral tongue pre‐ malignant lesions and correlates with patterns of invasive tumour front and tumour depth in oral tongue squamous cell carcinoma cases. Asian Pac J Cancer Prev. 2016;17:189–195. [DOI] [PubMed] [Google Scholar]
- 11. Zheng B, Liang L, Wang C, et al. MicroRNA‐148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432. [DOI] [PubMed] [Google Scholar]
- 12. Yu X, Li Z, Shen J, et al. MicroRNA‐10b promotes nucleus pulposus cell proliferation through RhoC‐Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS ONE. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Yu X, Li Z, Chen G, Wu WK. MicroRNA‐10b induces vascular muscle cell proliferation through Akt pathway by targeting TIP30. Curr Vasc Pharmacol. 2015;13:679–686. [DOI] [PubMed] [Google Scholar]
- 14. Liu G, Cao P, Chen H, Yuan W, Wang J, Tang X. MiR‐27a regulates apoptosis in nucleus pulposus cells by targeting PI3K. PLoS ONE. 2013;8:e75251. doi: 10.1371/journal.pone.0075251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ayaz L, Gorur A, Yaroglu HY, Ozcan C, Tamer L. Differential expression of microRNAs in plasma of patients with laryngeal squamous cell carcinoma: potential early‐detection markers for laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139:1499–1506. doi: 10.1007/s00432-013-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long XB, Sun GB, Hu S, et al. Let‐7a microRNA functions as a potential tumor suppressor in human laryngeal cancer. Oncol Rep. 2009;22:1189–1195. [DOI] [PubMed] [Google Scholar]
- 17. Huang J, Wang Y, Guo Y, Sun S. Down‐regulated microRNA‐152 induces aberrant DNA methylation in hepatitis B virus‐related hepatocellular carcinoma by targeting DNA methyltransferase 1. Hepatology. 2010;52:60–70. doi: 10.1002/hep.23660. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Tang J, Zhang B, et al. Epigenetic modification of MiR‐429 promotes liver tumour‐initiating cell properties by targeting Rb binding protein 4. Gut. 2014;64:156–167. doi: 10.1136/gutjnl-2013-305715. [DOI] [PubMed] [Google Scholar]
- 19. Zhang B, Liu T, Wu T, Wang Z, Rao Z, Gao J. microRNA‐137 functions as a tumor suppressor in human non‐small cell lung cancer by targeting SLC22A18. Int J Biol Macromol. 2015;74:111–118. doi: 10.1016/j.ijbiomac.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 20. Li Z, Yu X, Wang Y, et al. By downregulating TIAM1 expression, microRNA‐329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir‐10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 22. Chen D, Huang J, Zhang K, et al. MicroRNA‐451 induces epithelial‐mesenchymal transition in docetaxel‐resistant lung adenocarcinoma cells by targeting proto‐oncogene c‐Myc. Eur J Cancer. 2014;50:3050–3067. doi: 10.1016/j.ejca.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23. Yongchun Z, Linwei T, Xicai W, et al. MicroRNA‐195 inhibits non‐small cell lung cancer cell proliferation, migration and invasion by targeting MYB. Cancer Lett. 2014;347:65–74. doi: 10.1016/j.canlet.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 24. Yu T, Li J, Yan M, et al. MicroRNA‐193a‐3p and ‐5p suppress the metastasis of human non‐small‐cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34:413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 25. Deng D, Xue L, Shao N, et al. miR‐137 acts as a tumor suppressor in astrocytoma by targeting RASGRF1. Tumour Biol. 2016;37:3331–3340. doi: 10.1007/s13277-015-4110-y. [DOI] [PubMed] [Google Scholar]
- 26. Liu M, Lang N, Qiu M, et al. miR‐137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int J Cancer. 2011;128:1269–1279. doi: 10.1002/ijc.25452. [DOI] [PubMed] [Google Scholar]
- 27. Silber J, Lim DA, Petritsch C, et al. miR‐124 and miR‐137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L, Wang X, Wang H, et al. miR‐137 is frequently down‐regulated in glioblastoma and is a negative regulator of Cox‐2. Eur J Cancer. 2012;48:3104–3111. doi: 10.1016/j.ejca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 29. Luo C, Tetteh PW, Merz PR, et al. miR‐137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 30. Zhu X, Li Y, Shen H, et al. miR‐137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS. 2013;587:73–81. doi: 10.1016/j.febslet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31. Dang J, Bian YQ, Sun JY, et al. MicroRNA‐137 promoter methylation in oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med. 2013;42:315–321. doi: 10.1111/jop.12012. [DOI] [PubMed] [Google Scholar]
- 32. Wiklund ED, Gao S, Hulf T, et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS ONE. 2011;6:e27840. doi: 10.1371/journal.pone.0027840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langevin SM, Stone RA, Bunker CH, et al. MicroRNA‐137 promoter methylation is associated with poorer overall survival in patients with squamous cell carcinoma of the head and neck. Cancer. 2011;117:1454–1462. doi: 10.1002/cncr.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen H, Wang L, Ge X, et al. MicroRNA‐137 inhibits tumor growth and sensitizes chemosensitivity to paclitaxel and cisplatin in lung cancer. Oncotarget. 2016;7:20728–20742. doi: 10.18632/oncotarget.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liang ML, Hsieh TH, Ng KH, et al. Downregulation of miR‐137 and miR‐6500‐3p promotes cell proliferation in pediatric high‐grade gliomas. Oncotarget. 2016;7:19723–19737. doi: 10.18632/oncotarget.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong S, Jin M, Li Y, Ren P, Liu J. miR‐137 acts as a tumor suppressor in papillary thyroid carcinoma by targeting CXCL12. Oncol Rep. 2016;35:2151–2158. doi: 10.3892/or.2016.4604. [DOI] [PubMed] [Google Scholar]
- 37. Sakaguchi M, Hisamori S, Oshima N, Sato F, Shimono Y, Sakai Y. miR‐137 regulates the tumorigenicity of colon cancer stem cells through the inhibition of DCLK1. Mol Cancer Res. 2016;14:354–362. doi: 10.1158/1541-7786. [DOI] [PubMed] [Google Scholar]
- 38. Yen WH, Ke WS, Hung JJ, Chen TM, Chen JS, Sun HS. Sp1‐mediated ectopic expression of T‐cell lymphoma invasion and metastasis 2 in hepatocellular carcinoma. Cancer Med. 2016;5:465–477. doi: 10.1002/cam4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hay CW, Hunter I, MacKenzie A, McEwan IJ. An Sp1 modulated regulatory region unique to higher primates regulates human androgen receptor promoter activity in prostate cancer cells. PLoS ONE. 2015;10:e0139990. doi: 10.1371/journal.pone.0139990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y, Tang Q, Wu J, Zheng F, Yang L, Hann SS. Inactivation of PI3‐K/Akt and reduction of SP1 and p65 expression increase the effect of solamargine on suppressing EP4 expression in human lung cancer cells. J Exp Clin Cancer Res. 2015;34:154. doi: 10.1186/s13046-015-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. An HJ, Lee HJ, Jang S, et al. Transcription factor Sp1 prevents TRF2(DeltaBDeltaM)‐induced premature senescence in human diploid fibroblasts. Mol Cell Biochem. 2016;414:201–208. doi: 10.1007/s11010-016-2672-7. [DOI] [PubMed] [Google Scholar]
- 42. Zeng X, Xu Z, Gu J, et al. Induction of miR‐137 by isorhapontigenin (ISO) directly targets Sp1 protein translation and mediates its anticancer activity both in vitro and in vivo. Mol Cancer Ther. 2016;15:512–522. doi: 10.1158/1535-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang M, Xiao J, Wang J, et al. MiR‐24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016;5:1163–1173. doi: 10.1002/cam4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Yang W, Zhu X, Wei C. p53 inhibits the expression of p125 and the methylation of POLD1 gene promoter by downregulating the Sp1‐induced DNMT1 activities in breast cancer. OncoTargets Ther. 2016;9:1351–1360. doi: 10.2147/OTT.S98713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cui F, Wang S, Lao I, et al. miR‐375 inhibits the invasion and metastasis of colorectal cancer via targeting SP1 and regulating EMT‐associated genes. Oncol Rep. 2016;36:487–493. doi: 10.3892/or.2016.4834. [DOI] [PubMed] [Google Scholar]
- 46. Hu J, Shan Z, Hu K, et al. miRNA‐223 inhibits epithelial‐mesenchymal transition in gastric carcinoma cells via Sp1. Int J Oncol. 2016;49:325–335. doi: 10.3892/ijo.2016.3533. [DOI] [PubMed] [Google Scholar]
- 47. Zhao N, Li S, Wang R, et al. Expression of microRNA‐195 is transactivated by Sp1 but inhibited by histone deacetylase 3 in hepatocellular carcinoma cells. Biochim Biophys Acta. 2016;1859:933–942. doi: 10.1016/j.bbagrm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 48. Jia L, Huang Y, Zheng Y, et al. miR‐375 inhibits cell growth and correlates with clinical outcomes in tongue squamous cell carcinoma. Oncol Rep. 2015;33:2061–2071. doi: 10.3892/or.2015.3759. [DOI] [PubMed] [Google Scholar]


