Abstract
Objective
The metastatic ability of breast cancer cells with chemoresistant properties is higher when compared to that of their parental wild‐type cells. Expression of AnnexinA2 (Anxa2), a 36‐kDa calcium‐dependent phospholipid binding protein, is increased in metastatic tumours and has been found to be associated with the phenotype of drug resistance and metastasis.
Materials and Methods and Results
In the present study, we found that up‐regulation of Anxa2 correlates with enhanced migration and invasion ability of MCF‐7 breast cancer cells both in vitro and in vivo. Western blot analysis revealed that exposure to chemotherapeutic drugs may induce elevated expression of Anxa2. In addition, our data have shown that Anxa2 might influence proliferation, migration and invasion of MCF‐7 cells by increasing expression of c‐myc and cyclin D1 via activation of Erk1/2 signalling pathways.
Conclusion
Our findings suggest that up‐regulation of Anxa2 may play an important role in modulating proliferation and invasion of breast cancer MCF‐7 cells through regulation of many relevant downstream target genes.
Introduction
Surgical treatment, radiotherapy and chemotherapy are still the main treatment options for breast cancer at its different stages. One of the leading causes of treatment failure is that tumour cells are not sufficiently sensitive to chemotherapeutic agents, mostly due to multidrug resistance (MDR), the phenomenon in which tumour cells' resistance to a number of chemotherapeutic drugs, formed after exposure to a single cytotoxic drug 1, 2.
Recent studies have demonstrated that metastatic ability of breast cancer cells with chemoresistant properties is markedly higher compared to wild‐type cells and expression of AnnexinA2 (Anxa2), a 36‐kDa calcium‐dependent phospholipid binding protein, has been shown to be higher in metastatic cancer 3, 4, 5, 6, 7. In addition, increased expression of Anxa2 correlates with malignant changes in multiple human cancers, including mammary adenocarcinoma, lung cancer, colon carcinoma and primary renal carcinoma. Although drug sensitivity had no significant changes after knockdown of Anxa2, metastatic and invasion ability was significantly decreased 8. These findings suggest that Anxa2 may be linked to the phenotype of cells with drug resistance and metastatic potential.
One previous study has suggested that knockdown of Anxa2 did not reverse drug resistance in MCF‐7/ADR cells; however, cell proliferation, migration, and invasion activities were decreased after down‐regulation of Anxa2 in these cells 8. Thus, we hypothesize that Anxa2 may be up‐regulated along with acquisition of the MDR phenotype. In the present study, we have attempted to investigate the potential role of Anxa2 up‐regulation in proliferation and metastasis of MCF‐7 breast cancer cells. Our results suggest that up‐regulation of Anxa2 may play an important role in regulating their proliferation, migration and invasion by increasing expression of c‐myc and cyclin D1 via activation of Erk1/2 signalling pathways.
Materials and methods
Reagents and cell lines
Restriction enzymes of gene cloning (TaKaRa Biotechnology, Dalian, Liaoning, China), EndoFree Plasmidez Flow Miniprep kit (Biomiga, San Diego, CA) and G418 (geneticin) were purchased from Calbiochem (La Jolla, CA). Full length Anxa2 cDNA was cloned in a plasmid transfer vector pcDNA3.1(−) and then transfected into MCF‐7 cells along with null vector pcDNA3.1(−) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Stable clones with Anxa2 overexpression (MCF‐7/Anxa2) were screened. Human breast cancer MCF‐7 cells and the corresponding multidrug‐resistant variant (adriamycin‐resistant) MCF‐7/ADR cells were provided by Dr ZiZheng Hou of the Detroit Hospital, Detroit, MI. SiAnxa2/MCF7/ADR cells with down‐regulated Anxa2 expression by small interference RNA‐mediated gene silencing were originally generated by Dr Fei Zhang in our laboratory 8. Cells were cultured in RPMI‐1640 medium, supplemented with 10% foetal bovine serum (Hyclone, Logan, UT, USA) at 37 °C in humidified 5% CO2 atmosphere.
Construction of expression vector and screening stable transfectants
A 1033‐bp fragment of Anxa2 cDNA was synthesized by RT‐PCR from total RNA extracted from breast cancer MCF‐7 cells. The purified cDNA was introduced into a mammalian expression vector pcDNA3.1 (−) at the sites of XhoI and KpnI. Following the manufacturer's instructions, three independent MCF‐7 stable transfectants were screened for overexpression of Anxa2 after 21 days selection in 0.6 mg/ml G418. Transfectants were routinely cultured under selection.
Western blot analysis
A quantity of 25 μg of lysates per sample was separated by SDS–PAGE using 10% polyacrylamide gels and transferred to PVDF membrane which was subsequently incubated with mouse monoclonal antibodies to Anxa2 (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), STAT3, c‐myc, cyclin D1, cyclin E (1:1000; Santa Cruz Biotechnology) 9, 10, Erk1/2, p‐Erk, P38, p‐P38 (1:1000; Cell Signaling Technology, Beverly, MA, USA), for 2 h, and corresponding proteins were immunodetected by incubation with HRP (horseradish peroxidase)‐linked goat anti mouse IgG secondary antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA) using an ECL detection kit (Pierce Biotechnology, Rockford, IL, USA). Mouse monoclonal antibody to ß‐actin (1:5000; Santa Cruz Biotechnology) was used as gel loading control.
Cell proliferation assay
Both growth curve and colony formation assays were used to observe and compare cell proliferation ability. Briefly, 1 × 104 cells/well were cultured in 12‐well plates at 37 °C with 5% CO2. Surviving cells were counted using a cytometer every day for seven consecutive days. For colony formation assay, cell suspensions of MCF‐7 wild‐type cells and transfectants were seeded into six‐well plates, 3 × 102 cells/well. Cells were incubated for 10 days then fixed in methyl hydrate for 10 min. Colonies were then stained and counted using an optical microscope.
Flow cytometry
Flow cytometry was used to compare differences in cell cycle characteristics between MCF‐7 cells and transfectants. Cells were incubated with propidium iodide (PI, 50 μg/ml) for 30 min the following day. Flow cytometric analysis was performed on a Beckman Coulter EPICS analyzer (Krefeld, Germany).
Wound healing assay
Cell migration ability was also calculated, and compared by scratch injury assay. MCF‐7 cells and transfectants were seeded in six‐well plates until full confluence prior to the assay. Floating cells were removed with sterile PBS three times. Images were taken at 0, 9 and 24 h using an inverted microscope.
Cell migration and invasion assays in vitro
Cell motility and invasion of MCF‐7 or transfectants were measured in 12‐well Boyden chamber plates, pore size 8 μm, polycarbonate membrane filter inserts (Millipore Chemicon, Becton Dickinson, San Jose, California, USA). For cell invasion assay, interiors of transwell inserts were coated with diluted matrigel (Becton Dickinson) to imitate presence of a basement membrane. After trypsinization, cells were suspended to a concentration of 3 × 106 cell/ml in 0.1% bovine serum albumin (BSA) – RPMI1640 only 11, 12. After incubation for 36 h, filter membranes were mounted for bright field light microscopy.
In vivo tumourigenicity studies
Four to six‐week‐old female BALB C/nude mice (Vital Laboratory Animal Center, Beijing, China) were used as animal models. Mice were roomed five per cage, and provided with sterile food (Vital Laboratory Animal Center, Beijing, China) and sterile water. MCF‐7/ADR multidrug‐resistant variants and transfectants of siAnxa2/MCF‐7/ADR were injected at a concentration of 2 × 106 cells, in a single axillary flank region. Tumours, which formed 30 days after cell injection, were harvested. Then, 1 mm3 blocks of neoplastic tissues were aseptically incised and implanted into both flanks and groins of new mice. This re‐implantation into fresh mice was to improve population growth rate of tumour cells and to minimize chances of rejection. Measurements were initiated when tumours reached a diameter of approximately 2 mm, which was recorded as day 0. Maximum (a) and minimum (b) diameters of tumours were recorded every 3 days for up to 30 days, using a vernier calliper. Tumour volume (V) was calculated according to the formula: V = 1/2 × (a 2 × b).
Statistical analysis
All data quantification and statistical analysis were performed using spss 13.0 software (Chicago, IL, USA). Comparison between different experimental groups was completed by using one‐way ANOVA or Student's t‐test, when appropriate. Data are presented as mean ± SD and all experiments were independently repeated at least three times. P‐values of <0.05 (two‐sided) were considered statistically significant.
Results
Screening of stabile clones overexpressing Anxa2
Results of restriction enzyme digestion experiments indicated that we had successfully amplified Anxa2 cDNA fragment (1033 bp) and subcloned it into a pcDNA3.1 vector (~5.4 kb) (Fig. 1a). We chose the verified vector named pcDNA3.1‐Anxa2 to transfect MCF‐7 cells, while null vector pcDNA3.1 was applied as control. Western blot analysis was used to screen the stable clones with Anxa2 overexpression. Intensity analysis of Anxa2 expression levels by using image analysis software program Image J showed higher than 3‐fold increase (Fig. 1b) in transfectants as compared to control cells (Fig. 1c). In addition to overexpression assays, we employed siAnxa2/MCF‐7/ADR (Fig. 1d) to further study potential implications of the Anxa2 gene in breast cancer cells, from the perspective of gene silencing. Low‐dose adriamycin (0.01 μm) was also used in cultured MCF‐7 cells to simulate the condition and procedure of drug resistance. Four weeks later, western blotting showed that exposure to adriamycin could apparently up‐regulate expression of the Anxa2 gene (Fig. 1e). MDR1, encoding the P‐gp protein, has been widely accepted as a drug resistance gene and used for studying potential mechanisms underlying multidrug resistance, in human malignant cells 1. Expression of P‐gp remained unchanged after knockdown of Anxa2 (Fig. 1f), suggesting that Anxa2 may not be a necessary contributor to the drug‐resistant phenotype.
Figure 1.

Screening of stable clones that overexpress Anxa2. Chemotherapeutic drug treatment (0.01 μm adriamycin) significantly up‐regulated expression of Anxa2, whereas expression of multidrug resistance protein remained unchanged after knockdown of Anxa2. (a) Identification of the expression vector pcDNA3.1‐Anxa2 construct. M1: 1 kb DNA Ladder; 1‐4: The pcDNA3.1‐Anxa2 construct was digested with XhoI and KpnI. M2: 2000 DNA marker. (b) Histogram showing relative quantification of Anxa2 expression levels in siAnxa2/MCF/ADR clones compared to wild‐type and control cells. **P < 0.01 versus control. (c) Anxa2 gene expression markedly enhanced in screened stable transfectants compared to that of wild‐type cells. Control: MCF‐7 cells were transfected with null pcDNA3.1 vector. Clone1–3: Transfectants stably overexpressing Anxa2. (d) Western blotting analysis of Anxa2 expression in siAnxa2/MCF‐7/ADR cells. Control group MCF‐7/ADR cells transfected with siRNA plasmid containing a scrambled sequence. A, B: Transfectants of siAnxa2/MCF‐7/ADR. (e) Exposure of 0.01 μm adriamycin significantly increased expression of Anxa2. (f) Expression of multidrug resistance protein, p‐gp, remained unchanged after knockdown of Anxa2. β‐actin used as gel loading control. All experiments were repeated at least three times.
Up‐regulation of Anxa2 increased the proliferation of MCF‐7 cells
To observe the effects of Anxa2 up‐regulation on the MCF‐7 cells, cell proliferation in stable clones overexpressing Anxa2 was evaluated by cell population growth curves and colony formation assay. During 6‐day observations, we found that the proliferation rate of Anxa2‐overexpressing groups was apparently higher compared to that of wild‐type MCF‐7 cells. As shown in Fig. 2a, three Anax2 overexpressing clones grew significantly faster than wild‐type MCF‐7 cells and control cells (P < 0.05). To further evaluate proliferation ability, we performed colony formation assays and found that capacity for colony formation was significantly enhanced in Anxa2‐overexpressing cells compared to wild‐type and control cells (Fig. 2b). Number of colonies, which were defined as of more than 30 cells originally derived from a single cell, of Anxa2‐overexpressing groups was more than of control cells and differences in colony number were statistically significant (P < 0.01). Cell cycle analysis showed that up‐regulation of Anxa2 caused an increase in S‐phase cells (Table 1) and our data demonstrated that up‐regulation of Anxa2 also induced an increase in proportion of G2/M + S phase cells, compared to that of control cells (Fig. 2c).
Figure 2.
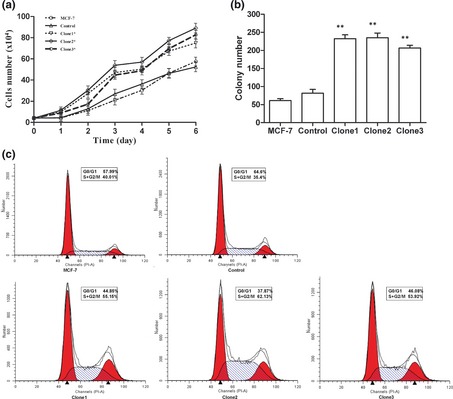
Up‐regulation of Anxa2 increased proliferation of MCF ‐7 cells. (a) Living cells were counted using a cytometer every day for 6 consecutive days. Transfectants with Anxa2 overexpression proliferated much faster than control cells. *P < 0.05 versus control. (b) Colony formation was significantly higher in Anxa2‐overexpressing transfectants of MCF‐7 cells compared to wild‐type and control cells. Bar charts show average number of colonies and comparison between MCF‐7/Control cells and MCF‐7/Anxa2 groups. Statistical significance was assessed by one‐way ANOVA, **P < 0.01 versus control. (c): Result of Beckman Coulter EPICS analyzer showed that up‐regulation of Anxa2 induced increase in proportion of G2/M + S phase cells compared to controls. Clone1–3: Transfectants stably overexpressing Anxa2. Data are mean ± SD. Experiments were repeated at least three times.
Table 1.
Up‐regulation of Anxa2 induced an increase of S‐phase cells compared to wild‐type MCF‐7 cells
| Cell type | G1 | S | G2/M |
|---|---|---|---|
| MCF‐7 | 55.34 ± 2.78 | 28.29 ± 2.25 | 16.37 ± 2.44 |
| Control | 62.32 ± 1.99 | 25.70 ± 1.43 | 11.99 ± 1.87 |
| Clone1 | 44.85 ± 2.39 | 36.77 ± 2.65* | 18.39 ± 2.13 |
| Clone2 | 36.01 ± 1.74 | 45.62 ± 1.76* | 18.37 ± 2.99 |
| Clone3 | 45.36 ± 0.96 | 35.88 ± 1.53* | 18.76 ± 0.74 |
All data are presented as the mean ± SD from at least three independent experiments performed in triplicate, *P < 0.05 versus control.
Up‐regulation of Anxa2 increased migration and invasion of MCF‐7 cells
It has been previously reported that Anxa2 was overexpressed in invasive tumours and it could contribute to cancer progression and invasion 6. Thus, next we employed the transwell chamber‐based assay to further unveil the role of Anxa2 in breast tumour cell migration and invasion. As shown in Fig. 3a,b, numbers of Anxa2‐overexpressing cells on the surface of the diaphragm membrane were higher than that of wild‐type MCF‐7 and control cells. Quantitative representation (Fig. 3c) showed ~4‐ to 6‐fold increase in cell migration ability as well as ~8‐ to 10‐fold increase in cell invasion of transfectants compared to those of parental wild‐type cells (P < 0.01). Wound healing assay confirmed the result of the migration assay, that overexpression of Anxa2 in MCF‐7 cells led to a significant increase in their migratory ability (Fig. 3d,e).
Figure 3.

Up‐regulation of Anxa2 increased migration and invasion of MCF ‐7 cells. (a, b) Up‐regulation of Anax2 expression resulted in increased cell migration and enhanced cell invasion through Matrigel. Our migration and invasion assay revealed number of up‐regulated Anxa2 cells attached to bottom of the membrane was markedly higher than that of control cells. (c) Number of invading cells was evaluated in three fields for each experimental group. Quantitative analysis showed 4‐ to 6‐fold increase in cell migration as well as 8‐ to 10‐fold increase in cell invasion of transfectants compared to that of parental cells. **P < 0.01 versus control. (d) Representative of wound healing assays at 0 and 24 h. (e) Wound healing assay demonstrated that MCF‐7/Anxa2 cells exhibited increased migration ability compared to the control group. Clone 1–3: Transfectants stably overexpressing Anxa2. All data are mean ± SD. Experiments were repeated at least three times, and statistical significance was assessed by one‐way ANOVA, *P < 0.05 versus control.
Decreased Anxa2 expression suppressed proliferation of MCF‐7/ADR cells in vivo
Four‐ to six‐week‐old female BALB C/nude mice were used as animal models. MCF‐7/ADR multidrug‐resistant variants and the transfectants of siAnxa2/MCF‐7/ADR were injected into single axillary flank regions of the mice. We measured maximum and minimum diameters of tumours, using a vernier calliper, after 30 days. Average tumour volume in siAnxa2 mice increased slowly and reached only 38 mm3 whereas average tumour volume in control mice was in the region of 289 mm3 (Fig. 4a,b). As expected, average tumour volume in siAnxa2 mice (71.81 ± 3.13 mm3) increased significantly more slowly than of the control group (521.92 ± 15.23 mm3). As shown in Fig. 4c,d, the Anxa2 siRNA group exhibited significantly reduced tumour growth in vivo compared to that of the MCF‐7/ADR/Control group. In summary, we showed that the MCF‐7/ADR/Control group had faster proliferation rates in vivo than Anxa2 siRNA ones, specially after 2 weeks.
Figure 4.

Proliferation of MCF ‐7/ ADR cells was suppressed by knockdown of Anxa2. (a) Reduced Anxa2 expression suppressed proliferation of MCF‐7/ADR cells in vivo. Proliferation of MCF‐7/ADR cells treated with Anxa2‐specific RNAi was significantly lower compared to control MCF‐7/ADR cells. (b) Quantitative representation of tumour size from our proliferation assays in vivo. *P < 0.05 versus control. (c) Representative of tumours from proliferation assays in vivo. (d) Tumour volume in siAnxa2‐treated group increased more slowly than that of the control group, indicating that proliferation of MCF‐7/ADR cells was suppressed by Anxa2 siRNA. Data are mean volume ± SD from three mice per group, *P < 0.05.
Anxa2 may potentially affect migration and invasion of tumour cells via the STAT3 signalling pathway
To further examine the role of Anxa2 in proliferation of MCF‐7 cells, expression of cell cycle‐related proteins was analyzed by western blotting. It has been reported that overexpression of Anxa2 can promote up‐regulation of c‐myc 13, 14, 15, 16, which is consistent with our results (Fig. 5a). C‐myc is a proto oncogene encoding a transcription factor necessary for growth and proliferation of cancer cells. Previous studies have also shown that overexpression of cyclin D1 correlates with cell cycle progression, which is required for transition from G1 to S phase 17, 18, 19. Our data demonstrated that expression of c‐myc and cyclin D1 was markedly lower in Anxa2 siRNA MCF‐7/ADR cells and higher in clones with Anxa2 overexpression; nevertheless, there was no significant difference in Cyclin E expression between transfectant and control cells (Fig. 5a–c). The change in c‐myc was not as obvious as of cyclin D1, while quantitative analysis for expression of c‐myc in MCF‐7/ADR cells and MCF‐7 cells, by image J (Fig. 5d,e) revealed statistical significance.
Figure 5.

Western blot analysis of cell cycle‐related proteins in both MCF ‐7 cells and MCF ‐7/ ADR cells. (a–c) Western blot analysis revealed lower expression of c‐myc and Cyclin D1 in transfectants of Anxa2 siRNA, whereas increased c‐myc and Cyclin D1 were detected in clones with up‐regulated Anxa2 expression. However, there was no significant difference in Cyclin E expression between transfectants and control cells. (d, e) Quantitative analysis of expression of c‐myc in MCF‐7/ADR cells and MCF‐7 cells, by image J, shown as a histogram. **P < 0.01 versus control. Clone1, Clone2: Transfectants of clones stably overexpressing Anxa2 gene. A, B: Transfectants of siAnxa2/MCF‐7/ADR. Experiments were repeated at least three times. **P < 0.01.
Anxa2 may activate ERK1/2 signalling pathways during tumour invasion and metastasis
Recent studies have demonstrated that Anxa2 can activate ERK1/2, p38MAPK signalling pathways to up‐regulate expression of MMPs during tumour invasion and metastasis 20, 21. To better understand whether Anxa2 is involved in metastatic cascades in Anxa2 expressing breast cancer cells, we compared activation of MAPK in response to foetal bovine serum (FBS) control, Anxa2 up‐regulated and Anxa2 siRNA MCF‐7 cells. All groups were treated with 10% FBS culture medium for various time points (0, 5, 10, 20, 40 min) after 48 h starvation, and kinetics of MAPK activation were observed. As shown in Fig. 6a,b, ERK was activated much more rapidly in cells overexpressing Anxa2 than in control cells. On the other hand, ERK was deactivated in cells with inhibited Anxa2 expression compared to corresponding control cells. We also examined the potential effects of Anxa2 on expression of phosphorylated P38. Interestingly, expression of p‐P38 appeared to be elevated in both MCF‐7/Anxa2 cells and MCF‐ 7/ADR/Anxa2 siRNA cells (Fig. 6c,d). We consider this phenomenon to be a result of a stress response to the FBS, whereas expressions of signal factors are changed. These results suggest that FBS‐induced activation of ERK may be regulated by Anxa2 in MCF‐7 cells and its multidrug‐resistant derivative, and that Anxa2 may play a potential role in activating MAPK signalling cascades.
Figure 6.

Anxa2 may play an important role in activating the MAPK signalling pathway to promote tumour invasion and metastasis. (a, b) Phosphorylation of ERK protein (p‐ERK) was lower in siAnxa2/MCF‐7/ADR cells and higher in MCF‐7/Anxa2 cells, but level of total ERK was not markedly altered with phosphorylation of p‐ERK. (c, d) Results showed elevated levels of p‐P38 expression as a result of either up‐regulation of Anxa2 or knockdown of Anxa2. MCF‐7/Anxa2: Clones with stable Anxa2 overexpression. Anxa2 siRNA: Transfectants of siAnxa2/MCF‐7/ADR.
Discussion
Annexins make up a large multigene family of water‐soluble proteins that can bind to negatively charged phospholipids and cell membranes in a Ca2+‐dependent fashion 22. Anxa2 is an important member of this family and has been suggested to be a potential marker for early cancer diagnosis and prognosis 23, 24, 25. Expression of Anxa2 is higher in metastatic tumours 26, 27, 28, and chemotherapeutic treatment can lead to enhanced expression of Anxa2 in various cancer cells.
A previous study by our group has demonstrated that down‐regulation of Anxa2 induced a decrease in proportion of G2/M + S phase cells compared to that of the appropriate controls, and cell proliferation was slowed after knockdown of Anxa2 in MCF‐7/ADR cells 8. C‐myc can modulate progression from G0 to G1 phase to promote DNA replication and previous research has identified c‐myc mRNA as a component of the ribonucleoprotein complex formed by Anxa2 in vivo. It was also shown that there was a direct interaction between Anxa2 and c‐myc mRNA 29, 30, suggesting that Anxa2 may act as a novel RNA‐binding protein that may regulate translation of c‐myc RNA. Overexpression of Anxa2 can promote up‐regulation of c‐myc 29, which is consistent with our results (Fig. 5). It is reported that overexpression of cyclin D1, also known as PRAD1, correlates with the cycle progression 16, 18, 19. C‐myc can regulate many downstream genes, such as cyclin D1, that are essential for cell proliferation and invasion in multiple tumour subtypes 16. However, there have been few studies reporting the direct link between Anxa2 and cyclin D1. It is suggested that Erk1/2 and P38MAPK are pivotal for full transcriptional activity of STAT3 13, 14. We propose that Anxa2 may play an important role in regulating proliferation of MCF‐7 cells and MCF‐7/ADR cells by influencing expression of c‐myc and cyclin D1 via activation of Erk1/2 signalling pathways.
Mitogen‐activated protein kinases (MAPKs) compose a family of protein kinases involved in numerous key cellular functions including cell proliferation, apoptosis and motility. The MAPKs family, including extracellular signal‐regulated kinase (ERK) and p38 MAPK can be engaged following activation of the growth factor receptor kinases. Phosphocholine has been found to be essential for activation of C‐Raf and ERK1/2, as well as for DNA synthesis 21, 31. The activated MAPK pathway has been detected in many human tumour cells including of carcinomas of the breast, colon, kidney and lung, suggesting that MAPK may play a major role in tumour progression and metastasis 32, 33, 34, 35. In our study described here, FBS‐induced activation of ERK was found to be modulated by expression of Anxa2 in MCF‐7 cells and its multidrug‐resistant derivative (Fig. 6). The exact role played by Anxa2 in regulation of p38 MAPK signalling pathways, however, still remains unknown, but, it appears to be associated with cell response to FBS‐induced stress. Most MMPs are secreted as inactive precursor proteins, which are activated when cleaved by extracellular proteinases 32. It has been recently reported that Anxa2 can activate ERK1/2, p38MAPK signalling pathways to up‐regulate expression of MMPs during tumour invasion and metastasis 21.
Our previous research has shown that Anxa2 plays an essential role in MDR‐induced tumour invasion. MCF‐7/ADR cells had a more than 4‐fold increase in invasiveness compared to MCF‐7 cells, and expression of AnnexinA2 is increased in metastatic cancer, while down‐regulation of Anxa2 reduced invasion of MCF‐7/ADR cells 8. These findings suggest that Anxa2 may play a key role in migration and invasion of MCF‐7 cells, and it might also contribute to invasion of drug‐resistant breast tumour cells. Anxa2 was overexpressed in MCF‐7/ADR cells compared to wild‐type MCF‐7 cells. Down‐regulation of Anxa2 did not reverse adriamycin resistance in MCF‐7/ADR cells, which demonstrated that Anxa2 was not required for drug resistance (Fig. 1f). Despite these findings, cell proliferation, migration and invasion activity were significantly reduced after knockdown of Anxa2 in MCF‐7/ADR cells. In the present study, western blot analyses suggest that chemotherapeutics may induce elevation of Anxa2. We suggest that Anxa2 is up‐regulated along with acquisition of the MDR phenotype. In addition, up‐regulation of Anxa2 promoted proliferation and migration of MCF‐7 cells both in vitro and in vivo. In summary, our findings suggest that expression of Anxa2 may play an important role in regulating proliferation, migration and invasion of MCF‐7 cells and MCF‐7/ADR cells by influencing expression of c‐myc and cyclin D1 via activation Erk1/2 signalling pathways.
Acknowledgements
Studies in our laboratory were supported by Funds of the National Natural Science Foundation of China (No. 81071731), NSFC(81001188) and Tianjin Higher Education Science & Technology Fund Planning Project (20100120). We thank members of our laboratory for helpful discussions.
The first two authors contributed equally to this work.
References
- 1. Beck WT (1990) Mechanisms of multidrug resistance in human tumor cells. The roles of P‐glycoprotein, DNA topoisomerase II, and other factors. Cancer Treat. Rev. 17, 11–20. [DOI] [PubMed] [Google Scholar]
- 2. Beck WT (1987) Pharmacological, molecular, and cytogenetic analysis of “atypical” multidrug‐resistant human leukemic cells. Cancer Res. 47, 5455–5460. [PubMed] [Google Scholar]
- 3. Yao HX, Zhang ZQ, Xiao ZQ, Chen YH, Li C, Zhang PF et al (2009) Identification of metastasis associated proteins in human lung squamous carcinoma using two‐dimensional difference gel electrophoresis and laser capture microdissection. Lung Cancer 65, 41–48. [DOI] [PubMed] [Google Scholar]
- 4. Ohno Y, Izumi M, Kawamura T, Nishimura T, Mukai K, Tachibana M (2009) Annexin II represents metastatic potential in clear‐cell renal cell carcinoma. Br. J. Cancer 101, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pei HP, Zhu H, Zeng S, Li YX, Yang HX, Shen LF et al (2007) Proteome analysis and tissue microarray for profiling protein markers associated with lymph node metastasis in colorectal cancerProteome analysis and tissue microarray for profiling protein markers associated with lymph node metastasis in colorectal cancer. J. Proteome Res. 6, 2495–2501. [DOI] [PubMed] [Google Scholar]
- 6. Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC (2006) Angiogenesis ‐associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp. Mol. Pathol. 81, 146–156. [DOI] [PubMed] [Google Scholar]
- 7. Wu W, Tang X, Hu W, Lotan R, Hong WK, Mao L (2002) Identification and validation of metastasis‐associated proteins in head and neck cancer cell lines by two‐dimensional electrophoresis and mass spectrometry. Clin. Exp. Metastasis 19, 319–326. [DOI] [PubMed] [Google Scholar]
- 8. Zhang F, Zhang L, Zhang B, Wei XY, Yang Y, Qi RZ et al (2009) Anxa2 plays a critical role in enhanced invasiveness of the multidrug resistant human breast cancer cells. J. Proteome Res. 8, 5041–5047. [DOI] [PubMed] [Google Scholar]
- 9. Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM et al (2007) Involvement of CD147 in regulation of multidrug resistance to P‐gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 98, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM et al (2007) Up‐regulation of CD147 and matrix metalloproteinase‐2, ‐9 induced by P‐glycoprotein substrates in multidrug resistant breast cancer cells. Cancer Sci. 98, 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y et al (2007) Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 101, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang JP, Tan CP, Li J, Siddique MM, Guo K, Chan SW et al (2010) VHZ is a novel centrosomal phosphatase associated with cell growth and human primary cancers. Mol Cancer. 9, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burysek L, Syrovets T, Simmet T (2002) The serine protease plasmin triggers expression of MCP‐1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 277, 33509–33517. [DOI] [PubMed] [Google Scholar]
- 14. Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO et al (2005) Role of the protein kinase C‐epsilon‐Raf‐1‐MEK‐1/2‐p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase‐2 after ischemic preconditioning. Circulation 112, 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caplan JF, Filipenko NR, Fitzpatrick SL, Waisman DM (2004) Regulation of annexin A2 by reversible glutathionylation. J. Biol. Chem. 279, 7740–7750. [DOI] [PubMed] [Google Scholar]
- 16. Chen CL, Loy A, Cen L, Chan C, Hsieh FC, Cheng G et al (2007) Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer 7, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takano S, Togawa A, Yoshitomi H, Shida T, Kimura F, Shimizu H et al (2008) Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine‐adjuvant chemotherapy. Ann. Surg. Oncol. 15, 3157–3168. [DOI] [PubMed] [Google Scholar]
- 18. Motokura T, Arnold A (1993) PRAD1/cyclin D1 proto‐oncogene: genomic organization, 5′ DNA sequence, and sequence of a tumor‐specific rearrangement breakpoint. Genes Chromosomes Canc. 7, 89–95. [DOI] [PubMed] [Google Scholar]
- 19. Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar‐Sagi D, Roussel MF et al (1993) Overexpression of mouse D‐type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 7, 1559–1571. [DOI] [PubMed] [Google Scholar]
- 20. Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G et al (2008) Annexin A2 expression and phosphorylation are up‐regulated in hepatocellular carcinoma. Int. J. Oncol. 33, 1157–1163. [PubMed] [Google Scholar]
- 21. Zhang Y, Zhou ZH, Bugge TH, Wahl LM (2007) Urokinase‐type plasminogen activator stimulation of monocyte matrix metalloproteinase‐1 production is mediated by plasmin‐dependent signaling through annexin A2 and inhibited by inactive plasmin. J. Immunol. 179, 3297–3304. [DOI] [PubMed] [Google Scholar]
- 22. Alfonso P, Canamero M, Fernandez‐Carbonie F, Nunez A, Casal JI (2008) Proteome analysis of membrane fractions in colorectal carcinomas by using 2D‐DIGE saturation labeling. J. Proteome Res. 7, 4247–4255. [DOI] [PubMed] [Google Scholar]
- 23. Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T et al (2001) Annexin II overexpression correlates with stromal tenascin‐C overexpression: a prognostic marker in colorectal carcinoma. Cancer 92, 1419–1426. [DOI] [PubMed] [Google Scholar]
- 24. Emoto K, Sawada H, Yamada Y, Fujimoto H, Takahama Y, Ueno M et al (2001) Annexin II overexpression is correlated with poor prognosis in human gastric carcinoma. Anticancer Res. 21, 1339–1345. [PubMed] [Google Scholar]
- 25. Spano F, Raugei G, Palla E, Colella C, Melli M (1990) Characterization of the human lipocortin‐2‐encoding multigene family: its structure suggests the existence of a short amino acid unit undergoing duplication. Gene 95, 243–251. [DOI] [PubMed] [Google Scholar]
- 26. Cole SP, Pinkoski MJ, Bhardwaj G, Deeley RG (1992) Elevated expression of annexin II (lipocortin II, p36) in a multidrug resistant small cell lung cancer cell line. Br. J. Cancer 65, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Hong X, Hussain M, Sarkar SH, Li R, Sarkar FH (2005) Gene expression profiling revealed novel molecular targets of docetaxel and estramustine combination treatment in prostate cancer cells. Mol. Cancer Ther. 4, 389–398. [DOI] [PubMed] [Google Scholar]
- 28. Chuthapisith S, Bean BE, Cowley G, Eremin JM, Samphao S, Layfield R et al (2009) Annexins in human breast cancer: possible predictors of pathological response to neoadjuvant chemotherapy. Eur. J. Cancer 45, 1274–1281. [DOI] [PubMed] [Google Scholar]
- 29. Mickleburgh I, Burtle B, Hollas H, Campbell G, Chrzanowska‐Lightowlers Z, Vedeler A et al (2005) Annexin A2 binds to the localization signal in the 3′ untranslated region of c‐myc mRNA. FEBS J. 272, 413–421. [DOI] [PubMed] [Google Scholar]
- 30. Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM (2004) Annexin A2 is a novel RNA‐binding protein. J. Biol. Chem. 5, 8723–8731. [DOI] [PubMed] [Google Scholar]
- 31. Jimenez B, del Peso L, Montaner S, Esteve P, Lacal JC (1995) Generation of phosphorylcholine as an essential event in the activation of Raf‐1 and MAP‐kinases in growth factors‐induced mitogenic stimulation. J. Cell. Biochem. 57, 141–149. [DOI] [PubMed] [Google Scholar]
- 32. Shah BH, Catt KJ (2004) Matrix metalloproteinases in reproductive endocrinology. Trends Endocrinol. Metab. 15, 47–49. [DOI] [PubMed] [Google Scholar]
- 33. Ye S (2006) Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc. Res. 69, 636–645. [DOI] [PubMed] [Google Scholar]
- 34. Beloueche‐Babari M, Jackson LE, Al‐SAffar NM, Workman P, Leach MO, Ronen SM (2005) Magnetic resonance spectroscopy monitoring of mitogen‐activated protein kinase signaling inhibition. Mounia Beloueche‐Babari Cancer Res. 65, 3356–3363. [DOI] [PubMed] [Google Scholar]
- 35. Johnson GL, Lapadat R (2002) Mitogen‐activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912. [DOI] [PubMed] [Google Scholar]


