Summary
Aim
Hemorrhagic transformation (HT) is a major complication of delayed tissue plasminogen activator (t‐PA) treatment in ischemic stroke. We aimed to explore whether peroxynitrite decomposition catalyst (PDC) could prevent such complication.
Methods
Male Sprague‐Dawley (SD) rats were subjected to middle cerebral artery occlusion (MCAO) with t‐PA (10 mg/kg) or t‐PA plus FeTMPyP (3 mg/kg, a representative PDC) at MCAO for 2 or 5 h and reperfusion for 22 or 19 h, respectively. HT was assessed with hemoglobin assay. Neurological deficit was evaluated with Modified Neurological Severity Score (mNSS). Peroxynitrite formation was examined by detecting 3‐nitrotyrosine (3‐NT) formation. The expression and activity of MMP‐9/MMP‐2 were assessed by Western blotting and gelatin zymography.
Results
t‐PA treatment at 2 h of MCAO did not induce HT but attenuated neurological deficit, whereas treatment at 5 h significantly induced HT and worsened the neurological outcome. Such complications were prevented by FeTMPyP cotreatment. Early t‐PA treatment inhibited 3‐NT and MMP‐9/MMP‐2 expression, whereas delayed treatment induced 3‐NT and MMP‐9/MMP‐2 expression and activity. FeTMPyP cotreatment downregulated 3‐NT and inhibited MMP‐9/MMP‐2 in both time points.
Conclusion
Peroxynitrite decomposition catalyst could prevent hemorrhagic transformation and improve neurological outcome ischemic rat brains with delayed t‐PA treatment via inhibiting peroxynitrite‐mediated MMP activation.
Keywords: Hemorrhage, Matrix metalloproteinases, Peroxynitrite, Tissue plasminogen activator
Introduction
Tissue plasminogen activator (t‐PA) remains the only FDA‐approved therapy for acute ischemic stroke, with a restrictive treatment time window of 4.5 h 1, 2, 3, 4. Treatment beyond this time window significantly increases risk of hemorrhagic transformation (HT) and mortality 5, 6, 7, 8, which worsens the patients' outcome 9. With the narrow therapeutic window and potential severe complications, t‐PA treatment is only applied for <5% stroke patients practically 10. Developing novel adjuvant therapeutic strategy to extend t‐PA's therapeutic time window and reduce the risk of HT is critical for improving outcome of stroke treatment.
Activating matrix metalloproteinases (MMPs), a proteolytic zinc‐containing enzyme family, could degrade neurovascular matrix, disrupt tight junction proteins and induce the BBB opening and HT in ischemic stroke 11, 12. During thrombolytic treatment, t‐PA promotes the expression of MMP‐9 13, 14 and the release of MMP‐9 from neutrophils, contributing to HT 15. Inhibition of MMP‐9 reduced incidence and severity of HT in focal ischemic rat model with t‐PA treatment 16, 17. Plasma MMP‐9 level becomes a biomarker for predicting the risk of HT in ischemic stroke patients receiving t‐PA treatment 18, 19. Meta‐analysis on 22 clinical trials with 3289 patients showed that MMP‐9 level was highly correlated with infarct volume, stroke severity, and functional outcome 20. MMP‐2 activation also contributes to the BBB leakage at the early stage of cerebral ischemia 21, 22 as well as the t‐PA‐mediated HT 23. MMP‐2 knockout mice revealed less BBB damage and HT than wild‐type mice after focal cerebral ischemia–reperfusion 24.
After t‐PA infusion, recanalization causes cerebral ischemia–reperfusion injury, producing large amounts of reactive oxygen and nitrogen species including superoxide, nitric oxide (NO), and peroxynitrite (ONOO−). Peroxynitrite is derived from the superoxide and NO, but it is much more active than those precursors 25. Peroxynitrite exerts strong cytotoxic effects via inducing protein tyrosine nitration, lipid peroxidation and DNA damage 26. Peroxynitrite could activate MMPs and disrupt tight junctions, leading to damage of neurovascular unit in ischemic brain 27, 28. Scavenging peroxynitrite with NAC or peroxynitrite decomposition catalyst FeTMPyP significantly inhibited MMP‐9 activity, reduced infarct volume, and improved neurological function during cerebral ischemia–reperfusion injury 29, 30, 31, 32, 33. Increased nitrosative oxidative stress was found in ischemic stroke patients 34. Thus, we raised the hypothesis that peroxynitrite decomposition catalyst (PDC) could prevent hemorrhage transformation through inhibiting peroxynitrite‐mediated MMP activation in ischemic brain with delayed t‐PA treatment and subsequently extend therapeutic window of t‐PA.
Materials and Methods
Middle Cerebral Artery Occlusion (MCAO) Model
Male Sprague‐Dawley (SD) rats weighing 260–290 g were obtained from Laboratory Animal Unit, The University of Hong Kong. All animal experiment protocols were approved and regulated by the Committee on the Use of Live Animal in Teaching and Research. Rats were randomly divided into the following groups: sham control, 2 h of MCAO plus 22 h of reperfusion (M2/R22), 5 h of MCAO plus 19 h of reperfusion (M5/R19), M2/R22 plus t‐PA, M5/R19 plus t‐PA, M2/R22 plus t‐PA and FeTMPyP, M5/R19 plus t‐PA and FeTMPyP. Cerebral ischemia–reperfusion MCAO model was conducted as we previously described 35. Briefly, MCAO rats were anesthetized with 4% isofluorane and maintained with 2% isofluorane via inhalation. A silicon‐coated suture (diameter 0.38 mm) was inserted from the external carotid artery (ECA) into the internal carotid artery (ICA). Sham control rats were subjected to similar surgical operation without occlusion. At designed time window of MCAO ischemia, the suture was removed to induce reperfusion. During the operation, rats were kept on warming pats until recovered from anesthesia. Success of MCAO was confirmed by TTC staining of a 1‐mm‐thick coronal slice that is 6 mm away from the frontal tip. Brains without infarction should be excluded for study. All MCAO models were successful and included in this study.
Drug Treatment
We compared the effects of FeTMPyP, a representative PDC, on HT in the brains receiving t‐PA treatment at 2 and 5 h of cerebral ischemia. At 1.5 and 4.5 h of MCAO ischemia, recombinant t‐PA (Actilyse, Boehringer Ingelheim, 10 mg/kg) was injected (10% bonus, 90% continuous infusion for 0.5 h) via femoral vein according to previously described methods 36. Accordingly, the time points of t‐PA treatment were counted as 2 and 5 h after cerebral ischemia. In parallel experiments, same volume of saline was used as control. Right after t‐PA injection, the suture was immediately removed to induce reperfusion. With t‐PA treatment at 2 and 5 h, we designed the reperfusion period for 22 and 19 h, respectively, to keep consistent timeframe to 24 h of ischemia–reperfusion. To investigate the effects of PDC on preventing t‐PA‐induced HT, FeTMPyP (3 mg/kg, Cayman, 75854) was given via femoral vein just before t‐PA treatment. We chose the dosage of 3 mg/kg FeTMPyP for this study which was based previous reports by us and others 32, 33, 37.
Hemoglobin Assay
Hemoglobin assay was used to examine the severity of hemorrhage as previously reported 38. Briefly, rats were transcardially perfused with cold PBS and hemispheres were homogenized with cold PBS. The homogenized samples were collected and centrifuged at 15 000 g for 15 min. Supernatants were collected and the level of hemoglobin was detected using QuantiChrom hemoglobin assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacture's protocol. O.D. value at 400 nm was detected with a microplate reader.
Western Blot Analysis
After homogenization, brain samples were collected and proteins were extracted with RIPA buffer (Sigma‐Aldrich, St. Louis, MO, USA). Proteins were determined by Branford assay (Bio‐Rad Laboratories, Hercules, CA, USA), loaded for electrophoresis, and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were incubated with diluted primary antibodies including anti‐MMP‐9 (Merck, IM72), anti‐MMP‐2 (Merck, IM33), anti‐3‐nitrotyrosine (3‐NT, Abcam, ab61392), and anti‐GAPDH (Millipore, abs16) overnight at 4°C and followed by incubating secondary antibody at room temperature for 2 h. After washed with TBST buffer, signal was detected using ECL advance Western blotting detection reagents (BD biosciences, San Jose, CA, USA).
Gelatin Zymography
MMP activity was measured using gelatin zymography protocol as previously described with minor modifications 28. Briefly, the same amount of total proteins was eletrophoresised in 10% SDS‐polyacrylamide gel containing 1 mg/mL gelatin. After electrophoresis, gels were collected and washed with 2.5% Triton‐100 for 1 h and then incubated with developing buffer at 37°C for 48 h. After stained with Coomassie blue, the gels were washed with ddH2O until clear bands appeared.
Neurological Deficit Measurement
Modified Neurological Severity Score (mNSS) test was adopted to evaluate the animal neurological deficit according to previous publication 39. Neurological function was graded on a series of scales from 0 to 18 (normal score, 0; maximal deficit score, 18). The mNSS includes motor, sensory, reflex, and balance tests. Tests were carried out by an investigator who was blinded to the experiments.
Statistical Analysis
Statistical analysis was performed using SPSS 18.0 statistical programs (SPSS, Chicago, IL, USA). For multiple groups designed experiments, comparisons were made by one‐way analysis of variance (ANOVA) and followed by least significant difference test for two‐group comparison within the multiple groups. Significance levels were set at P < 0.05. Data were presented as means ± SEM.
Results
PDC Treatment Abolished Hemorrhagic Transformation in Ischemia‐reperfused Brains with t‐PA Infusion at 5 h
We first investigated the different effects of early and delayed t‐PA treatments on HT in ischemic brains. T‐PA was intravenously injected into the rats at 2 and 5 h of cerebral ischemia, and then, the brain samples were collected at the time points of 22 and 19 h of reperfusion, respectively, for accessing HT. Early t‐PA infusion (10 mg/kg) at 2 h did not induce HT, whereas delayed treatment at 5 h resulted in HT in the MCAO ischemic brains (Figure 1A and B). Hemoglobin assay confirmed that HT only occurred in the ischemic brain with t‐PA treatment at 5 h. (Figure 1C and D). Notably, cotreatment of FeTMPyP (3 mg/kg, a representative PDC) abolished delayed t‐PA‐induced HT in the ischemic brains. Those results indicate that PDC could prevent HT in ischemic brains with delayed t‐PA treatment.
Figure 1.
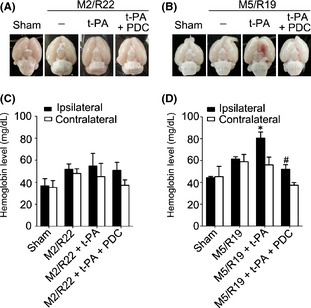
Effects of PDC (FeTMPyP, 3 mg/kg) on hemorrhagic transformation in ischemic rat brains with early and delayed t‐PA treatments. Rats were treated with t‐PA (10 mg/kg) at 2 and 5 h of MCAO ischemia following 22 and 19 h of reperfusion, respectively. M2/R22, 2 h of MCAO plus 22 h of reperfusion; M5/R19, 5 h of MCAO plus 19 h of reperfusion; (A) brain samples in M2/R22 plus t‐PA groups with or without PDC treatment; (B) brain samples in M/R22 plus t‐PA groups with or without PDC; (C) brain hemoglobin content in M2/R22 groups with t‐PA or t‐PA plus PDC treatments; (D) brain hemoglobin content in M5/R19 groups with t‐PA or t‐PA plus PDC treatments (*P < 0.05, compared to M5/R19 group; # P < 0.05, compared to M5/R19 + t‐PA group). Data are expressed as mean ± SEM (n = 6–11).
PDC Treatment Reduced Neurological Severity Scores in Ischemic Brains with t‐PA Infusion at 5 h
We then investigated the mNSS scores for neurological functions. Interestingly, t‐PA treatment at 2 h remarkably improved neurological functions whereas at 5 h worsened the neurological outcome in the MCAO ischemia‐reperfused rats, indicating that early and delayed t‐PA treatments have opposite therapeutic outcome in neurological functions (Figure 2A and B). Cotreatment of FeTMPyP not only abolished t‐PA‐induced neurological deficiency in the MCAO rats with t‐PA treatment at 5 h but also had better neurological functions than the MCAO ischemic rats with saline treatment as control (Figure 2B), indicating that PDC treatment could improve the recovery of neurological functions in cerebral ischemia‐reperfused rats with delayed t‐PA treatment.
Figure 2.
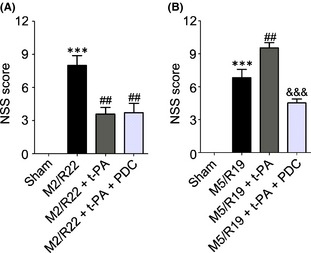
Effects of PDC (FeTMPyP, 3 mg/kg) on neurological severity scores (NSS) in MCAO ischemic rats with early and delayed t‐PA treatments. Rats were treated with t‐PA (10 mg/kg) at 2 and 5 h of MCAO ischemia following 22 and 19 h of reperfusion, respectively. M2/R22, 2 h of MCAO plus 22 h of reperfusion; M5/R19, 5 h of MCAO plus 19 h of reperfusion. A: t‐PA treatment at 2 h after MCAO ischemia with or without PDC cotreatment (***P < 0.001, VS. sham control; ## P < 0.01, compared to M2/R22). B: t‐PA treatment at 5 h after MCAO ischemia with or without PDC cotreatment (***P < 0.001, compared to sham control; ## P < 0.01, compared to M5/R19; &&& P < 0.001, compared to M5/R19 + t‐PA). Data are expressed as mean ± SEM (n = 6–11).
PDC Treatment Downregulated 3‐NT Expression and Inhibited MMP‐9/MMP‐2 Expression/activity in Ischemic Brains with Delayed t‐PA Infusion at 5 h
We finally investigated whether the underlying mechanisms of t‐PA's opposite effects and the protective effect of PDC are related to peroxynitrite and MMP activation in the ischemic brains. As showed in Figure 3, t‐PA treatment at 2 h remarkably downregulated the formation of 3‐NT, a peroxynitrite biomarker, whereas the treatment at 5 h upregulated 3‐NT level in the MCAO ischemic brains, indicating that delayed t‐PA treatment could promote peroxynitrite production. Cotreatment of FeTMPyP (3 mg/kg) significantly inhibited the expression of 3‐NT in the ischemic brains with t‐PA treatment at both 2 and 5 h.
Figure 3.
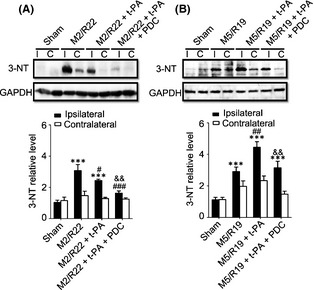
Effects of PDC (FeTMPyP, 3 mg/kg) on 3‐NT expression in MCAO ischemic rat brains with early and delayed t‐PA treatments. Rats were treated with t‐PA (10 mg/kg) at 2 and 5 h of ischemia plus 22 and 19 h of reperfusion, respectively. M2/R22, 2 h of MCAO plus 22 h of reperfusion; M5/R19, 5 h of MCAO plus 19 h of reperfusion. I, ipsilateral; C, contralateral. (A) Western blot results and statistical results in M2/R22 groups received t‐PA or t‐PA plus PDC (ipsilateral) (***P < 0.001, compared to sham control; # P < 0.05, ### P < 0.001, compared to M2/R22; && P < 0.01, compared to M2/R22 + t‐PA). (B) Western blot results and statistic results in M5/R19 groups received t‐PA or t‐PA plus PDC treatments (ipsilateral) (***P < 0.001, compared to sham control; ## P < 0.01, compared to M5/R19; & & P < 0.01, compared to M5/R19 + t‐PA). Data are expressed as mean ± SEM. (n = 3).
Meanwhile, t‐PA treatment at 2 hours significantly downregulated the expression of MMP‐9/MMP‐2 in the ischemic brains, while treatment at 5 h significantly upregulated the expression of MMP‐9/MMP‐2 and MMP‐9 activity (Figure 4). Cotreatment of FeTMPyP significantly downregulated the expression of MMP‐9/MMP‐2 and inhibited MMP‐9 activity in the ischemic brains with t‐PA treatments at both 2 and 5 h after cerebral ischemia. Those results indicate that early t‐PA treatment could reduce the production of peroxynitrite and the expression of MMPs, while delayed t‐PA treatment could exacerbate peroxynitrite production and the expression and activity of MMPs in ischemic rat brains, and PDC treatment could inhibit 3‐NT formation and the expression and activity of MMPs in the ischemic brain with delayed t‐PA treatment.
Figure 4.
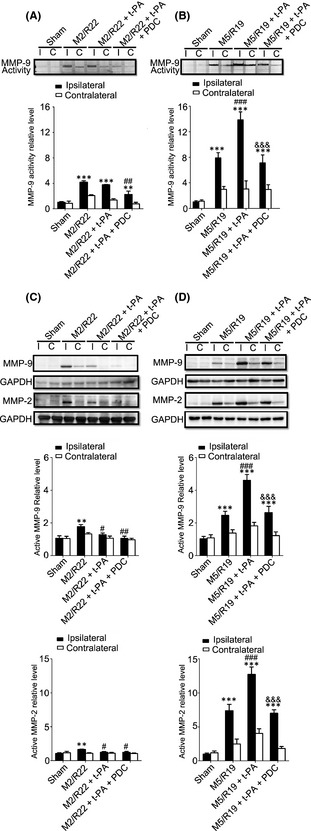
Effect of PDC (FeTMPyP, 3 mg/kg) on MMP‐9 activity and MMP‐9/MMP‐2 expression in MCAO ischemic rat brains with early and delayed t‐PA treatments. Rats were treated with t‐PA (10 mg/kg) at 2 and 5 h of MCAO ischemia following 22 and 19 h of reperfusion, respectively. M2/R22, 2 h of MCAO plus 22 h of reperfusion; M5/R19, 5 h of MCAO plus 19 h of reperfusion. I, ipsilateral; C, contralateral. (A) Representative gelatin zymography and statistical results in M2/M22 groups (ipsilateral) (**P < 0.01, ***P < 0.001, compared to sham control; ## P < 0.01, compared to M2/R22). (B) Representative gelatin zymography and statistical results in M5/M19 groups (ipsilateral) (***P < 0.001, compared to sham control; ### P < 0.001, compared to M5/R19; &&& P < 0.001, compared to M5/R19 + t‐PA). (C) Representative Western blot and statistical analysis for MMP‐9/MMP‐2 expressions in M2/R22 groups (**P < 0.01, compared to sham control; # P < 0.05, ## P < 0.01, compared to M2/R22). (D) Representative Western blot and statistical analysis for MMP‐9/MMP‐2 expressions in M5/R19 groups (***P < 0.001, compared to sham control; ### P < 0.001, compared to M5/R19; &&& P < 0.001, compared to M5/R19 + t‐PA). Data are expressed as mean ± SEM (n = 3–4).
Discussion
This is the first report that PDC could prevent hemorrhagic transformation and promote neurological functions after cerebral ischemia with delayed t‐PA treatment. PDC has the potentials to be an adjunct therapy to expend t‐PA's therapeutic time window and prevent hemorrhagic transformation in poststroke treatment.
MMPs play critical roles in inducing the BBB disruption and HT during cerebral ischemia–reperfusion injury 16, 17, 18, 19, 23. PDC could prevent MMP activation and neurovascular injury in rat brains after prolonged cerebral ischemia 32, 40, 41. Therefore, we tested the hypothesis that PDC could be effective to reduce HT through inhibiting peroxynitrite‐mediated MMP activation in ischemic brains with delayed t‐PA treatment. HT was only presented in the rats with t‐PA treatment at 5 h rather than that with t‐PA treatment at 2 h of MCAO cerebral ischemia–reperfusion. Furthermore, early t‐PA treatment at 2 h of MCAO cerebral ischemia improved neurological function scores, whereas delayed t‐PA treatment at 5 h worsened the therapeutic outcomes. These results are consistent with previous reports 42, 43, 44. Interestingly, early t‐PA treatment at 2 h significantly reduced 3‐NT formation, while delayed t‐PA treatment at 5 h oppositely induced 3‐NT formation, indicating that early recanalization could reduce the formation of peroxynitrite in the ischemic brains. Cotreatment of FeTMPyP prevented HT and improved neurological function in the rats with t‐PA treatment at 5 h of MCAO cerebral ischemia following 19 h of reperfusion. Those results indicate that peroxynitrite plays an important role in HT and neurological deficiency in ischemia‐reperfused brains with delayed t‐PA treatment. In line with those results, early and delayed t‐PA treatments had opposite effects on MMP‐9/MMP‐2 expression and MMP‐9 activity in the ischemic brains. Cotreatment of FeTMPyP revealed to inhibit the expression of MMP‐9/MMP‐2 and the activity of MMP‐9 at both early and delayed t‐PA treatments. Those results suggest that peroxynitrite‐mediated MMP activation could be the underlying mechanism of HT in ischemia‐reperfused rat brains with delayed t‐PA treatment.
It is notable that only 3 mg/kg PDC was used in the study. We designed the dosage according to our previous study that PDC treatment at 3 mg/kg significantly attenuated infarction volume and reduced apoptotic cell death in MCAO rat model of cerebral ischemia–reperfusion injury 37. Current study aimed to further explore the potentials of using PDC as an adjunct therapy with t‐PA to prevent HT and promote therapeutic outcome. Practically, we should further explore the dose‐dependent responses to the PDC treatment in protecting blood–brain barrier and attenuating HT during thrombolytic treatment for saving ischemic brain tissues as a potential therapeutic drug in future.
Taken together, we conclude that PDC could prevent hemorrhagic transformation and improve neurological outcome during cerebral ischemia–reperfusion injury with delayed t‐PA treatment via inhibiting peroxynitrite‐mediated MMP activation. Targeting peroxynitrite formation could be a potential adjuvant therapeutic strategy to reduce t‐PA‐mediated hemorrhage complication and potentially extend the current narrow treatment time window.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Hong Kong General Research Fund (GRF No. 777611M and No. 776512M), Research Grant Council, Hong Kong SAR; and Grant from National Natural Science Foundation of China (No. 31270902).
References
- 1. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke 2009;40:2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 4. Xu AD, Wang YJ, Wang DZ, Chinese Stroke Therapy Expert Panel for Intravenous Recombinant Tissue Plasminogen A . Consensus statement on the use of intravenous recombinant tissue plasminogen activator to treat acute ischemic stroke by the Chinese Stroke Therapy Expert Panel. CNS Neurosci Ther 2013;19:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue‐type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;282:2019–2026. [DOI] [PubMed] [Google Scholar]
- 6. group I, Sandercock P, Wardlaw JM, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST‐3]): a randomised controlled trial. Lancet 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep 2002;2:38–43. [DOI] [PubMed] [Google Scholar]
- 8. Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta‐analysis. Lancet 2012;379:2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011;77:341–348. [DOI] [PubMed] [Google Scholar]
- 10. Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use ICD‐9 codes substantially underestimate. Stroke 2008;39:924–928. [DOI] [PubMed] [Google Scholar]
- 11. Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab 2014;34:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase‐mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 2007;27:697–709. [DOI] [PubMed] [Google Scholar]
- 13. Park KP, Rosell A, Foerch C, et al. Plasma and brain matrix metalloproteinase‐9 after acute focal cerebral ischemia in rats. Stroke 2009;40:2836–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase‐9 upregulation after focal cerebral ischemia. Stroke 2005;36:1954–1959. [DOI] [PubMed] [Google Scholar]
- 15. Cuadrado E, Ortega L, Hernandez‐Guillamon M, et al. Tissue plasminogen activator (t‐PA) promotes neutrophil degranulation and MMP‐9 release. J Leukoc Biol 2008;84:207–214. [DOI] [PubMed] [Google Scholar]
- 16. Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)‐induced hemorrhage after thromboembolic stroke. Stroke 2000;31:3034–3040. [DOI] [PubMed] [Google Scholar]
- 17. Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis‐associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 2002;33:831–836. [DOI] [PubMed] [Google Scholar]
- 18. Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase‐9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003;34:40–46. [PubMed] [Google Scholar]
- 19. Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase‐9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107:598–603. [DOI] [PubMed] [Google Scholar]
- 20. Ramos‐Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase‐9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis 2011;20:47–54. [DOI] [PubMed] [Google Scholar]
- 21. Jin X, Liu J, Yang Y, Liu KJ, Yang Y, Liu W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3 h after ischemia onset. Neurobiol Dis 2012;48:309–316. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase‐2‐mediated occludin degradation and caveolin‐1‐mediated claudin‐5 redistribution contribute to blood‐brain barrier damage in early ischemic stroke stage. J Neurosci 2012;32:3044–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machado LS, Sazonova IY, Kozak A, et al. Minocycline and tissue‐type plasminogen activator for stroke: assessment of interaction potential. Stroke 2009;40:3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu A, Suofu Y, Guan F, Broderick JP, Wagner KR, Clark JF. Matrix metalloproteinase‐2 deletions protect against hemorrhagic transformation after 1 h of cerebral ischemia and 23 h of reperfusion. Neuroscience 2013;253:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki M, Tabuchi M, Ikeda M, Tomita T. Concurrent formation of peroxynitrite with the expression of inducible nitric oxide synthase in the brain during middle cerebral artery occlusion and reperfusion in rats. Brain Res 2002;951:113–120. [DOI] [PubMed] [Google Scholar]
- 26. Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite‐induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett 2003;140–141:113–124. [DOI] [PubMed] [Google Scholar]
- 27. Gursoy‐Ozdemir Y, Can A, Dalkara T. Reperfusion‐induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 2004;35:1449–1453. [DOI] [PubMed] [Google Scholar]
- 28. Gu Y, Zheng G, Xu M, et al. Caveolin‐1 regulates nitric oxide‐mediated matrix metalloproteinases activity and blood‐brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem 2012;120:147–156. [DOI] [PubMed] [Google Scholar]
- 29. Chen XM, Chen HS, Xu MJ, Shen JG. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia‐reperfusion injury. Acta Pharmacol Sin 2013;34:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n‐acetylcysteine on ischaemic brain injury. Br J Pharmacol 2000;130:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dhar A, Kaundal RK, Sharma SS. Neuroprotective effects of FeTMPyP: a peroxynitrite decomposition catalyst in global cerebral ischemia model in gerbils. Pharmacol Res 2006;54:311–316. [DOI] [PubMed] [Google Scholar]
- 32. Suofu Y, Clark J, Broderick J, et al. Peroxynitrite decomposition catalyst prevents matrix metalloproteinase activation and neurovascular injury after prolonged cerebral ischemia in rats. J Neurochem 2010;115:1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol 2004;142:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Nimer MS, Al‐Mahdawi AM, Sakeni RA. Assessment of nitrosative oxidative stress in patients with middle cerebral artery occlusion. Neurosciences 2007;12:31–34. [PubMed] [Google Scholar]
- 35. Shen J, Ma S, Chan P, et al. Nitric oxide down‐regulates caveolin‐1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J Neurochem 2006;96:1078–1089. [DOI] [PubMed] [Google Scholar]
- 36. Yamashita T, Kamiya T, Deguchi K, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab 2009;29:715–725. [DOI] [PubMed] [Google Scholar]
- 37. Xu M, Chen X, Gu Y, et al. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite‐mediated neurotoxicity in cerebral ischemia‐reperfusion injury. J Ethnopharmacol 2013;150:116–124. [DOI] [PubMed] [Google Scholar]
- 38. Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Normobaric hyperoxia attenuates early blood‐brain barrier disruption by inhibiting MMP‐9‐mediated occludin degradation in focal cerebral ischemia. J Neurochem 2009;108:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001;32:2682–2688. [DOI] [PubMed] [Google Scholar]
- 40. Shen J, Gu Y. Insights into mechanisms of blood‐brain barrier permeability‐roles of free radicals, matrix metalloproteinases, and caveolin‐1 In: Laher I, editor. Systems biology of free radicals and antioxidants. Berlin, Heidelberg: Springer, 2014;2049–2067. [Google Scholar]
- 41. Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood‐brain barrier disruption in acute ischemic stroke. Front Neurol 2013;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fagan SC, Nagaraja TN, Fenstermacher JD, Zheng J, Johnson M, Knight RA. Hemorrhagic transformation is related to the duration of occlusion and treatment with tissue plasminogen activator in a nonembolic stroke model. Neurol Res 2003;25:377–382. [DOI] [PubMed] [Google Scholar]
- 43. Fagan SC, Garcia JH. Hemorrhagic transformation in focal cerebral ischemia: influence of time to artery reopening and tissue plasminogen activator. Pharmacotherapy 1999;19:139–142. [DOI] [PubMed] [Google Scholar]
- 44. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt‐PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]


