Abstract
Objectives
Cancer stem cells (CSCs) compose a subpopulation of cells within a tumour that can self‐renew and proliferate. Growth factors such as epidermal growth factor (EGF) and basic fibroblast growth factor (b‐FGF) promote cancer stem cell proliferation in many solid tumours. This study assesses whether EGF, bFGF and IGF signalling pathways are essential for colon CSC proliferation and self‐renewal.
Material and methods
Colon CSCs were cultured in serum‐free medium (SFM) with one of the following growth factors: EGF, bFGF or IGF. Characteristics of CSC gene expression were evaluated by real time PCR. Tumourigenicity of CSCs was determined using a xenograft model in vivo. Effects of EGF receptor inhibitors, Gefitinib and PD153035, on CSC proliferation, apoptosis and signalling were evaluated using fluorescence‐activated cell sorting and western blotting.
Results
Colon cancer cell HCT116 transformed to CSCs in SFM. Compared to other growth factors, EGF was essential to support proliferation of CSCs that expressed higher levels of progenitor genes (Musashi‐1,LGR5) and lower levels of differential genes (CK20). CSCs promoted more rapid tumour growth than regular cancer cells in xenografts. EGFR inhibitors suppressed proliferation and induced apoptosis of CSCs by inhibiting autophosphorylation of EGFR and downstream signalling proteins, such as Akt kinase, extracellular signal‐regulated kinase 1/2 (ERK 1/2).
Conclusions
This study indicates that EGF signalling was essential for formation and maintenance of colon CSCs. Inhibition of the EGF signalling pathway may provide a useful strategy for treatment of colon cancer.
Introduction
Despite overall reduced cancer incidence in men (of 1.3% per year from 2000 to 2006) and women (of 0.5% per year from 1998 to 2006), colorectal cancer remains the second leading cause of cancer death among men ages 40–79 years 1. Although most newly diagnosed colorectal cancers compose a resectable localized disease, many patients die from advanced metastatic or recurrent disease within 5 years, with limited effectiveness of surgery and adjuvant chemotherapy. Colorectal cancers are thought to originate from a group of stem cells that have acquired a series of mutations leading to aberrant activation of the Wnt/β‐catenin pathway 2. In contrast to the conventional stochastic model, a new hierarchical tumour model indicates that a small fraction of so called cancer stem cells (CSCs) [also referred to as tumour‐initiating cells (TICs)], are capable of initiating and maintaining tumour growth. CSCs distinguish themselves from the other heterogeneous tumour cells by their self‐renewing capability, and perpetuate long‐term tumour growth by providing more differentiated cancer cells 3. Meanwhile, there is increasing evidence to suggest that CSCs are responsible for cancer metastasis and therapy resistance. Most patients with metastatic disease relapse after chemotherapy, probably due to CSC resistance to chemotherapeutic agents 4, 5.
Proliferation and differentiation of normal intestinal cells from stem/progenitor cells is modulated in a specific microenvironment, termed ‘the stem‐cell niche′ which provides self‐renewing undifferentiated stem cells 6. Presence of extracellular matrix (ECM), cell‐to‐cell direct contact and humoural factors, are essential for the niche 7 to support stem cells in normal tissues as well as in cancer 8. Humoural factors, including epidermal growth factor (EGF) and basic fibroblast growth factor (b‐FGF) are essential for activation of proliferation and to inhibit apoptosis of intestinal stem/progenitor cells 9, 10. EGF and b‐FGF have routinely been used as supplements in serum‐free medium to culture CSCs of many solid tumours 11, 12. However, there is little knowledge concerning the role of EGF and b‐FGF signalling in colon CSC formation and proliferation. In this study, we have examined the role of EGF and b‐FGF, and their downstream signalling pathways, on proliferation of colon CSCs.
Materials and methods
Cell culture and sphere formation assay
Adherent HCT116 colon cancer cells were dissociated into single cell suspension, and then were cultured in serum‐free medium (SFM) consisting of DMEM/F12 (1:1) (Invitrogen, Carlsbad, CA, USA), 10% BSA, 2% B27 supplement (Gibco, Grand Island, CA, USA), 20 ng/ml of EGF and b‐FGF (PeproTech, Princeton, NJ, USA) in ultralow attachment T25 slide flasks or dishes (Greiner Bio‐one, Frickenhausen, Germany). A quantity of at least 1000 cells/ml was established for forming tumourospheres/colonospheres (in vitro model of cancer stem cells). In all experiments, cells were maintained at 37 °C in a humidified 5% CO2 95% air atmosphere.
Subsequently, primary tumourospheres were collected by gentle centrifugation after 7–10 days and digested with 0.05% trypsin‐EDTA, dissociated into single cell suspension, then transferred to 96‐well polystyrene suspension culture, F‐bottom microplates (Greiner Bio‐one, Frickenhausen, Germany) at 200 cells/well density, with 200 μl SFM, containing 20 ng/ml of single growth factor (EGF, b‐FGF, or IGF) respectively for 7–10 days, until formation of spheres. Sphere numbers of each well were calculated.
Real time PCR assay
Expression of progenitor and differentiation genes in tumourospheres and the cell line was determined by real time PCR. Adherent cells and suspension tumourospheres were harvested, and total RNA was purified with TRIzol reagent (Invitrogen). cDNA was synthesized using oligo(dT) primer and amplified with Taq DNA polymerase. Real time PCR was performed on an ABI Prism 7900HT detection system (Applied Biosystems, Foster City, CA, USA) using SYBR green primescript RT‐PCR kit (TaKaRa, Shiga, Japan), according to the manufacturer's instructions. Primers for targeted genes are listed in Table 1. Negative controls were performed without cDNA in the reaction mixture. Results were normalized against glyceraldehyde‐3‐phosphate dehydrogenase gene expression. Relative quantification of target genes was performed using a standard curve or comparative cycle threshold (C T) method.
Table 1.
Primers of target genes
| Gene | Primer sequence |
|---|---|
| LGR‐5 | F: CTCCCAGGTCTGGTGTGTTG |
| R: GTGAAGACGCTGAGGTTGGA | |
| Musashi‐1 | F: GGACTCAGTTGGCAGACTACG |
| R: CTGGTCCATGAAAGTGACGAA | |
| CK20 | F: ATGGATTTCAGTCGCAGAAGC |
| R: CTCCCATAGTTCACCGTGTGT |
Animal experimentation
Tumourigenicity was determined by injecting 2 × 105 colon cancer cell line cells, and colon tumourospheres, subcutaneously into 5‐week‐old nude mice (BALB/c, nu/nu); cells dissociated from the colon cancer cell line or tumourospheres were injected. Tumour growth was measured (width, length) on alternate days after diameter of tumours reached 5 mm, and up to 18 days after injection. Tumour size (mm3) was calculated using the formula: length × width2/2. All mice were maintained in a pathogen‐free, temperature and light‐controlled facility at the animal experiment centre of Fudan Medical University. All animal experiments fulfilled the criteria for humane treatment of laboratory animals, and were approved by Shanghai Medical Center of Fudan University.
Cell proliferation, viability and apoptosis assay
HCT116 tumourospheres were digested and dissociated into single cell suspension with SFM, which contained 10 ng/ml EGF only. Cells were plated in 96‐well polystyrene suspension culture microplates at 200 cells/well in the SFM containing Gefitinib (Tocris Bioscience, Bristol, UK) or PD153035 (Merck, Whitehouse Station, NJ, USA), synthetic EGFR inhibitors at 0–20 μm for 72 h. Numbers of newly formed tumourospheres were then counted.
To determine cell viability, cells were incubated with MTT(3‐(4,5‐dimethylthiazol‐e‐yl)‐2,5‐diphenyltetrazoliumbromide) at final concentration of 5 mg/ml for 4 h. After MTT removal, 150 μl dimethyl sulphoxide (DMSO) was added to each well, and absorbance was determined at 570 nm wavelength, using a spectra microplate reader (Beckman Coulter, Brea, CA, USA). Apoptosis assays were performed using annexin V‐ FITC staining and concurrent incubation with propidium iodide (PI). Apoptotic cells are annexin‐V+/PI−. Data were collected using FACS Aria (BD Biosciences, Franklin Lakes, NJ, USA) equipment and analysed with Win MDI versions 2.9.
Western blot analysis
To analyze EGFR downstream signals, the inhibitor of the ERK pathway, PD98059 (10 μm; Merck) and inhibitor for the phosphatidylinosito‐l, 3‐kinase(PI3K)/Akt pathway, LY294002 (10 μm; Beyotime, Shanghai, China) were added to cell cultures as described above. Tumourospheres were collected and washed in PBS before lysis in RAPI buffer containing a cocktail of protease inhibitors. Whole protein extracts of tumourospheres (15–30 μg protein/sample) were separated using 7.5–10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Whatman, Kent, UK). Membranes were first blocked with 5% non‐fat dried milk for 1 h at room temperature, then incubated overnight at 4 °C with antibodies against EGFR, phospho‐EGFR, Akt, phospho‐Akt, ERK and phosphor‐ERK (Cell Signaling Technology, Danvers, MA) respectively. After extensive washing in TBS‐T, membranes were further incubated with horseradish peroxidase‐conjugated anti‐rabbit or anti‐mouse secondary antibodies (1:1000) for 1 h at room temperature. Detection was performed using enhanced chemiluminescence reagent according the manufacturer's protocol. Chemiluminescent signals were quantified by density measurement using Image J software (NIH, USA).
Statistical analysis
Data are reported as means ± SE of triplicate experiments. Statistical analysis (analysis of variance) was performed using spss 13.0 (IBM, NY, USA) and P < 0.05 was considered statistically significant.
Results
EGF stimulated tumourosphere formation
HCT116 colon cancer cells grown in SFM formed tumourospheres (Fig. 1a). Ttumourospheres can be expanded for several months in this medium as described previously in neural and breast cancer stem cells 13, 14. We then investigated ability of different growth factors (EGF, IGF and b‐FGF) to stimulate colon cancer cell tumourosphere formation in serum‐free media. Only EGF was able to stimulate more tumourosphere formation by HCT116 cells (Fig. 1b). Efficiency of sphere formation by EGF was dose‐dependent up to 10 ng/ml (Fig. 1c). These findings indicated that EGF signalling promotes tumour sphere formation.
Figure 1.
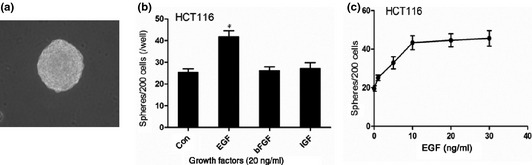
Tumorosphere formation in colon cancer. (a) Tumorosphere derived from HCT116 cell lines cultured in SFM condition (×200); (b) Compared with other growth factors, EGF was more effective stimulating tumorosphere formation in HCT116; (c) Concentration‐dependent effect of EGF in stimulating tumorsphere forming in HCT116.
Gene expression and tumourigenicity of the tumourospheres
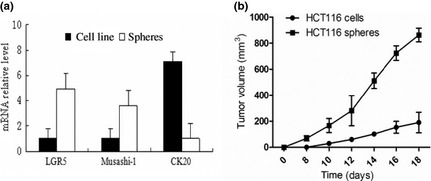
To confirm that cells in a tumourosphere maintain stem‐cell characteristics, gene expression profile of the progenitor genes Musashi‐1 15 and LGR5 16, 17, and of the differentiation gene CK20 18 were evaluated. Expressions of LGR5 and Musashi‐1 were higher in tumourospheres than in cell lines, while CK20 expression was lower (Fig. 2a), indicating that the tumourospheres were indeed are aggregations of cancer stem cells.
Figure 2.
The characteristics of colon tumorosphere. (a) The expression of progenitor genes, Musashi‐1, and LGR5, and the differentiation gene, CK20 as determined by real‐time PCR; (b) 2 × 105 cells from either HCT116 cell line or tumorospheres were injected i.p. into 5‐week‐old male nude mice. Growth curves of xenograft tumors were measured.
To determine whether tumourospheres were tumourigenic, we injected 2 × 105 of either colon cancer cell line or tumourosphere cells subcutaneously into mice to evaluate their ability to form subcutaneous xenotransplanted tumours (Fig. 2b). Spheroid cells were significantly more tumourigenic and formed larger and faster growing tumours than did cell line cells. This indicated that spheroid cells, representing cancer stem cells, were more tumourigenic compared to cell line cells.
EGFR inhibitor reduced colon CSC proliferation
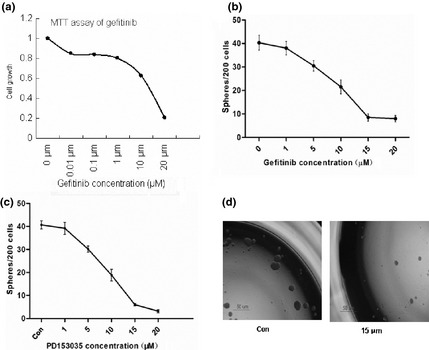
To further evaluate the role of EGF signalling pathways on CSC formation and proliferation, we applied EGFR inhibitors (Gefitinib and PD153035) to CSC cultures. Effects of EGFR inhibitors on cell viability and tumourosphere formation were measured, and were found to reduced CSC viability (Fig. 3a) and tumourospheres formation (Fig.3b,c) in a dose‐dependent manner. IC50 of Gefitinib was in the region of 12.5 μm. At 15 μm, Gefitinib and PD153035 effectively inhibited tumourosphere formation (Fig. 3d).
Figure 3.
Inhibition of tumorosphere formation by EGFR inhibitors. (a) The cell viability was determined by MTT assay after 72 h incubation EGFR inhibitors, gefitinib; (b,c) Concentration‐dependent inhibition of tumorosphere formation by the EGFR inhibitors, gefitinib and PD153035; (d) HCT116 forms spheres in the presence or absence of gefitinib (×40).
Gefitinib‐induced apoptosis
EGFR inhibitors not only inhibited tumourosphere formation, but also greatly reduced tumourosphere number, to levels even below those of the control group (Fig. 3b,c and Fig. 1b,c). To further examine the mechanism by which EGFR inhibitors inhibit colon CSCs, we examined CSC survival by evaluating their apoptosis. We treated spheroid cultures with Gefitinib, in a variety of concentrations from 1 to 15 μm, and determined the level of apoptosis after 72 h. Gefitinib caused significantly higher apoptosis of colon CSCs, specially when at 15 μm concentration (Fig. 4).
Figure 4.
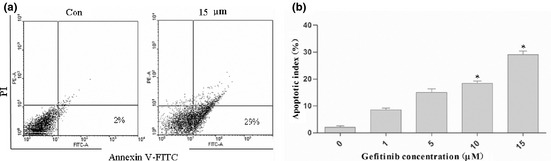
Effects of EGFR inhibitor on colon CSC s apoptosis. Colon CSCs were treated with gefitinib (0, 1, 5, 10, 15 μm) for 72 h. (a) Representative dot plots showing the apoptotic effect of gefitinib at a concentration of 15 μm. Flow cytometric detection of apoptosis via AnnexinV‐FITC/PI. The apoptotic cells were defined as Annexin‐V+ and PI−; (b) Data analysis of apoptotic index. There was a significant difference [P < 0.05 between control (without gefitinib) and gefitinib treatment]. Data are representative of values from at least three independent experiments.
Effects of Gefitinib on EGFR signalling pathways of tumourospheres
To evaluate EGFR signalling pathways in colon CSCs, we evaluated the ratios of EGFR/p‐EGFR, ERK/p‐ERK, Akt/p‐Akt by western blotting. Gefitinib inhibits EGF‐induced phosphorylation of EGFR and its downstream signalling molecules 19, such as ERK1/2 and Akt, in a dose‐dependent manner (Fig. 5a). A selective inhibitor of the PI3K/Akt pathway (LY294002) and an inhibitor of the ERK pathway (PD98509) also significantly inhibited tumourosphere formation (Fig. 5b). These results further support the notion that colon tumourosphere formation is dependent on EGF signalling.
Figure 5.
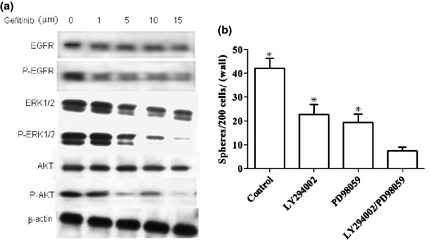
The effects of ERFR , ERK and PI 3K inhibitors on the EGF signaling pathway. (a) The effects of gefitinib on EGFR, ERK1/2, and Akt phosphorylation. Colon CSCs were incubated with different concentrations of gefitinib for 72 h. The total and phosphorylated EGFR, ERK1/2, and Akt levels were determined by Western blot analysis; (b) Colon CSCs were cultured in the absence or presence of ERK inhibitor, PD98059 (10 μm) and/or the PI3K inhibitor, LY294002 (10 μm). The number of formed spheres was determined. Mean ± SE of three independant experiments were graphed. There was a significant difference between the independent or combination LY294002 with PD98059 treatment versus the control (in the absence of an inhibitor) P < 0.05, but the combination treatment was the most efficient in inhibiting sphere formation
Discussion
The discovery that there are cancer stem cells in many solid tumours, including colon cancer 20, demonstrates the heterogeneity of cancer cells and has resulted in new concepts for therapeutic approaches to cancer 21, 22. CSCs show pluripotential differentiation, self‐renewal and tumourigenic properties when transplanted into immunodeficient mice 23. Therefore, colon CSCs are likely to be important for development, relapses and metastasis of colon cancer. Moreover, they may provide useful information for diagnostic and therapeutic approaches. Recent studies show that CSCs derived from colon cancer and cultured in SFM supplemented with EGF and b‐FGF are capable of tumour spheroid growth, which was originally observed during isolation and maintenance of neural stem cells. These tumourospheres, enriched with tumour stem cells, more closely resemble primary tumours in their phenotype and genotype than cells of lines cultured in serum media 24. In the current study, we generated human colonospheres that could be maintained in SFM for several months. The spheroid formation assay is typical for enrichment of cancer cells with stem cell‐like properties. In our study, we assessed ‘stemness′ of tumourospheres cultured by spheroid formation assay; progenitor gene (Musashi‐1, LGR5), and differentiation gene (CK20) expression were evaluated. One key method to investigate ‘stemness′ of CSCs is the capacity for tumourigenicity in SICD mice. Our results demonstrated the stem‐cell characteristics and tumourigenicity of colon CSCs, to prove their tumour‐initiating and growth potential.
Recently, studies have shown that growth factors produced by a microenvironment can revert differentiated cells to a more stem cell‐like state 25. Many studies have suggested that the EGF signalling pathway regulates intestinal epithelial cell and stem/progenitor cell growth and differentiation 9, 26. The EGF‐EGFR signalling pathway could provide critical function for self‐renewal of prostate cancer stem‐like cells 27. In many other solid tumours, EGFR plays critical roles in maintenance, proliferation and self‐renewal of cancer stem cells 28, 29. However, there is little knowledge concerning the role of growth factors in mediating proliferation and self‐renewal of colon CSCs. In our study, we examined the effect of growth factors in formation and proliferation of colon CSCs. We have shown that EGF signalling was necessary to promote formation and self‐renewal of colon CSCs, with EGF signalling being crucial to maintaining both normal and malignant epithelial cells. In normal intestinal epithelia, EGFR activation drives cell cycle progression, supports migration and differentiation, and inhibits apoptosis. In cancer cells, EGFR activation is believed to promote cancer cell growth, differentiation and survival 19. Following EGFR activation, three major intracellular signalling pathways are activated, including the Ras‐ERK cascade 30, 31, PI3K/Akt kinase pathway 31 and STAT3‐dependent signalling events 19, 32. In our current study, EGFR inhibitor, Gefitinib, inhibited both ERK and PI3K/Akt pathways and reduced colon CSC survival (Figs 4 and 5a). Independent inhibition of ERK and PI3K/Akt (Fig. 5b) further illustrated importance of these signalling pathways for maintenance of colon CSC 19, 33. Understanding the mechanisms of these signalling pathways may open new approaches for diagnosis and treatment of colon cancer.
In summary, we have demonstrated that EGF signalling, and less likely IGF and b‐FGF, is important to maintain colon CSC self‐renewal and proliferation. These results do not exclude that other signalling pathways may also be essential to maintain CSCs. Further studies of EGFR activation in relation to other stem cell‐related signalling molecules (e.g. Notch, Shh, Oct3/4, and Wnt) will provide additional useful information. It is also possible that CSCs reprogram and dedifferentiate in vitro from a more differentiated cell type in response to signal transduction cascade(s) 34. Further studies, especially in vivo, are needed to better understand colon CSCs biology, and also to evaluate implications for diagnostic and therapeutic approaches to colon cancer.
Acknowledgements
This work was supported by Grants from the Shanghai Commission of Science and Technology (09ZR1418700). We thank Ying Chen for the technical support in our experiments, Yuxing Zhang and Xiujuan Zhang for their advice in the study.
References
- 1. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J. Clin. 60, 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Dick JE (2008) Stem cell concepts renew cancer research. Blood 112, 4793–4807. [DOI] [PubMed] [Google Scholar]
- 3. Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. [DOI] [PubMed] [Google Scholar]
- 4. Zhang M, Rosen JM (2006) Stem cells in the etiology and treatment of cancer. Curr. Opin. Genet. Dev. 16, 60–64. [DOI] [PubMed] [Google Scholar]
- 5. Oh PS, Patel VB, Sanders MA, Kanwar SS, Yu Y, Nautiyal J et al (2011) Schlafen‐3 decreases cancer stem cell marker expression and autocrine/juxtacrine signaling in FOLFOX‐resistant colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G347–G355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore KA, Lemischka IR (2006) Stem cells and their niches. Science 311, 1880–1885. [DOI] [PubMed] [Google Scholar]
- 7. Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359. [DOI] [PubMed] [Google Scholar]
- 8. Lobo NA, Shimono Y, Qian D, Clarke MF (2007) The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki A, Sekiya S, Gunshima E, Fujii S, Taniguchi H (2010) EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long‐term monolayer cell culture. Lab. Invest. 90, 1425–1436. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS (2004) Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 59 (2 Suppl.), 21–26. [DOI] [PubMed] [Google Scholar]
- 11. Kondo T, Setoguchi T, Taga T (2004) Persistence of a small subpopulation of cancer stem‐like cells in the C6 glioma cell line. Proc. Natl. Acad. Sci. USA 101, 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828. [PubMed] [Google Scholar]
- 13. Singh SK, Clarke ID, Hide T, Dirks PB (2004) Cancer stem cells in nervous system tumors. Oncogene 23, 7267–7273. [DOI] [PubMed] [Google Scholar]
- 14. Dontu G, Al‐Hajj M, Abdallah WM, Clarke MF, Wicha MS (2003) Stem cells in normal breast development and breast cancer. Cell Prolif. 36(Suppl. 1), 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H et al (2003) Candidate markers for stem and early progenitor cells, Musashi‐1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 535, 131–135. [DOI] [PubMed] [Google Scholar]
- 16. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 17. Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al (2009) Crypt stem cells as the cells‐of‐origin of intestinal cancer. Nature 457, 608–611. [DOI] [PubMed] [Google Scholar]
- 18. Chan CW, Wong NA, Liu Y, Bicknell D, Turley H, Hollins L et al (2009) Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene. Proc. Natl. Acad. Sci. USA 106, 1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kari C, Rocha de Quadros M, Rodeck U (2003) Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res. 63, 1–5. [PubMed] [Google Scholar]
- 20. Ricci‐Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C et al (2007) Identification and expansion of human colon‐cancer‐initiating cells. Nature 445, 111–115. [DOI] [PubMed] [Google Scholar]
- 21. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951. [DOI] [PubMed] [Google Scholar]
- 22. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al (2004) Identification of human brain tumor initiating cells. Nature 432, 396–401. [DOI] [PubMed] [Google Scholar]
- 23. O'Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature 445, 106–110. [DOI] [PubMed] [Google Scholar]
- 24. Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM et al (2006) A tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum‐cultured cell lines. Cancer Cell 9, 391–403. [DOI] [PubMed] [Google Scholar]
- 25. Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH et al (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476. [DOI] [PubMed] [Google Scholar]
- 26. Jiang H, Edgar BA (2009) EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mimeault M, Johansson SL, Batra SK (2012) Pathobiological implications of the expression of EGFR, pAkt, NF‐kappaB and MIC‐1 in prostate cancer stem cells and their progenies. PLoS ONE 7, e31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abhold EL, Kiang A, Rahimy E, Kuo SZ, Wang‐Rodriguez J, Lopez JP et al (2012) EGFR kinase promotes acquisition of stem cell‐like properties: a potential therapeutic target in head and neck squamous cell carcinoma stem cells. PLoS ONE 7, e32459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S et al (2008) Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J. Biol. Chem. 283, 10958–10966. [DOI] [PubMed] [Google Scholar]
- 30. Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL (1999) Epidermal growth factor protects epithelial cells against fas‐induced apoptosis. J. Biol. Chem. 274, 17612–17618. [DOI] [PubMed] [Google Scholar]
- 31. Ono M, Hirata A, Kometani T, Miyagawa M (2004) Sensitivity to gefitinib (Iressa, ZD1839) in non‐small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal‐regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Mol. Cancer Ther. 3, 465–472. [PubMed] [Google Scholar]
- 32. Dowlati A, Nethery D, Kern JA (2004) Combined inhibition of epidermal growth factor receptor and JAK/STAT pathways results in greater growth inhibition in vitro than single agent therapy. Mol. Cancer Ther. 3, 459–463. [PubMed] [Google Scholar]
- 33. Nunes M, Shi C, Greenberger LM (2004) Phosphorylation of extracellular signal‐regulated kinase 1 and 2, protein kinase B, and signal transducer and activator of transcription 3 are differently inhibited by an epidermal growth factor receptor inhibitor, EKB‐569, in tumor cells and normal human keratinocytes. Mol. Cancer Ther. 3, 21–27. [PubMed] [Google Scholar]
- 34. Inagaki A, Soeda A, Oka N, Kitajima H, Nakagawa J, Motohashi T et al (2007) Long‐term maintenance of brain tumor stem cell properties under at non‐adherent and adherent culture conditions. Biochem. Biophys. Res. Commun. 361, 586–592. [DOI] [PubMed] [Google Scholar]


