Summary
Aims
In previous studies, transplantation of bone marrow mononuclear cells (BMMCs) in epileptic animals has been found to be neuroprotective. However, the mechanism by which the BMMCs act remains unclear. We hypothesize that BMMCs may provide neuroprotection to the epileptic brain through trophic support. To test our hypothesis, we studied the temporal expression of neurotrophins after BMMC transplantation in the epileptic rat hippocampus.
Methods
Chronically epileptic rats were intravenously transplanted with 1 × 107 BMMCs isolated from GFP transgenic mice. Expression levels of BDNF, GDNF, NGF, VEGF, and TGF‐β1, and their receptors, were evaluated by ELISA and/or qRT‐PCR analysis.
Results
Our data revealed increased protein expression of BDNF, GDNF, NGF, and VEGF and reduced levels of TGF‐β1 in the hippocampus of transplanted epileptic animals. Additionally, an increase in the mRNA expression of BDNF, GDNF, and VEGF, a reduction in TGF‐β1, and a decrease in mRNA levels of the TrkA and TGFR‐β1 receptors were also observed.
Conclusion
The gain provided by transplanted BMMCs in the epileptic brain may be related to the ability of these cells in modulating the network of neurotrophins and angiogenic signals.
Keywords: Epilepsy, Neurotrophic factors, Pilocarpine, Stem cells
Introduction
Temporal lobe epilepsy (TLE) is the most frequent form of epilepsy in adults 1. It is characterized by the progressive occurrence of spontaneous recurrent seizures (SRS) and affects mainly the hippocampus 2. Unfortunately, 30% of the patients become refractory to the antiepileptic drugs 3. Surgical treatment is a therapeutic option for these patients, but it is restricted due to possible brain function sequelae.
Cellular therapy‐based studies have grown exponentially in the field of epilepsy research and have shown benefits in experimental models 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. We have previously reported that bone marrow mononuclear cells (BMMCs) that are intravenously transplanted reduce seizure frequency and improve cognitive deficits in chronic epileptic rats 8, 13. However, the grafted cells uptake in the brain is low and poorly explains the benefits of the cellular treatment 7, 8, 13. Trophic factors are key elements during embryogenesis 16 and together with their receptors are selectively expressed in different parts of the central nervous system (CNS) during development. It has been demonstrated that stem cells have the ability to generate a variety of growth factors both in vitro and in vivo 17, 18, 19. Therefore, it is possible that the transplanted BMMCs could secrete and/or promote the cerebral expression of trophic factors that subsequently stimulate endogenous mechanisms of neuroprotection.
Bao et al. 20 showed that increased levels of neurotrophic factors were associated with functional improvement following transplantation of human bone marrow‐derived mesenchymal stem cells in ischemic rats. Furthermore, a large array of growth factors was shown to improve cognitive deficits and to be neuroprotective in animal models of epilepsy 21. To understand better the mechanisms of action of cellular treatment in epilepsy, we investigated whether BMMC transplantation modulates brain expression of brain‐derived neurotrophic factor (BDNF), glial cell‐derived neurotrophic factor (GDNF), neural growth factor (NGF), vascular endothelial growth factor (VEGF), or transforming growth factor β1 (TGF‐β1), and whether they modulate the receptors of these proteins, in rats subjected to pilocarpine‐induced chronic epilepsy.
Materials and Methods
Animals
Adult male Wistar rats (40–50 days of age, 150–200 g) were used for the epilepsy and controls groups. Enhanced green fluorescent protein (eGFP) transgenic adult male C57BL/6 mice were used as BMMC donors. This study was performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Pontifícia Universidade Católica do Rio Grande do Sul (11/00265).
Epilepsy Model
Epilepsy was induced by pilocarpine injection to produce SRS 22. Rats were treated with methylscopolamine (1 mg/kg i.p.; Sigma‐Aldrich, St. Louis, MO, USA) 15–30 min prior to pilocarpine (320 mg/kg, i.p.; Sigma‐Aldrich) to minimize peripheral side effects. Systemic administration of pilocarpine induced status epilepticus (SE). The seizures score was obtained from Racine scale 23, and only animals that were scored as grade 5 were included in the study. Seizures were controlled with diazepam (10 mg/kg, i.p.) 90 min after administration of pilocarpine. SRS were video‐monitored from day 15 to 21 (8 h/day) after pilocarpine injection. Animals that did not exhibit SRS were excluded from the study 13.
Isolation and Processing of BMMCs
BMMCs were obtained from C57BL/6 mice expressing eGFP. The animals were euthanized, and fresh bone marrow was extracted from the long bones. The material was centrifuged at 400 × g for 10 min. The cell pellet was resuspended with RPMI 1640 medium and fractionated on a density gradient generated by centrifugation at 400 × g over a Ficoll‐Paque solution (Histopaque 11191; Sigma‐Aldrich). The mononuclear fraction was collected and washed with DPBS. Cell concentrations were determined with Neubauer counting chamber and the number of viable cells by trypan blue exclusion. For the detection of surface antigens, BMMCs were incubated with conjugated antibodies against CD34, CD11b, CD117, CD45, and Sca1 (BD Biosciences, San Jose, CA, USA). Labeled cells were collected and analyzed using a FACSCalibur cytometer 13.
Transplantation of BMMCs
Pilocarpine‐injected animals were randomly divided into two groups: Pilo (pilocarpine‐treated rats receiving a saline injection) and Pilo + BMMC (Pilo rats transplanted with BMMCs). A control group composed of healthy rats received BMMCs (Control + BMMC). For each experimental group, the animals were further divided as follows: 12 animals for ELISA (four animals for each time point) and 12 animals for RT‐PCR (four animals for each time point). BMMCs were prepared for transplantation in saline at a concentration of 1 × 107 cells in 100 μL total volume. Cells or saline were administered via tail vein injection 22 days after SE. Animals were euthanized at 3 (3D), 7 (7D), and 14 (14D) days posttransplantation for ELISA and RT‐PCR analysis of hippocampal tissue (Figure 1). An additional group of healthy rats that received a saline injection instead of BMMC (Control group, four animals for each analysis) were euthanized only at 14 days after saline administration.
Figure 1.
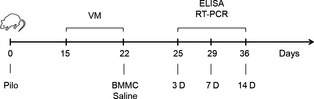
Experimental procedures. Pilo: pilocarpine; VM: video monitoring; BMMC: bone marrow mononuclear cell; periods posttransplantation: 3D, 7D, and 14D (days or 25, 29, and 36 days after pilocarpine injection).
Enzyme‐linked Immunosorbent Assay (ELISA)
Dissected hippocampal tissues were homogenized in lysis buffer (137 mM NaCl, 20 mM Tris–HCl (pH 8.0), 1% NP40, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 50 mM NaF, 1 mM PMSF, and a proteinase inhibitor) and centrifuged at 8000 × g for 10 min at 4°C. To measure the total level of trophic factors, the samples were acidified and neutralized. Evaluation of BDNF, GDNF, NGF, and TGF‐β1 expression was determined using Immunoassay Systems Emax® (Promega, Madison, WI, USA) and VEGF using the ELISA Development Kit (PeproTech, Rocky Hill, NJ, USA); all were carried out according to the manufacturer's instructions. The optical density of each well was determined within 30 min using a microplate reader set to 450–620 nm. The protein levels are presented as pg/mg of total protein.
Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT–PCR)
Extraction of RNA and synthesis of cDNA tissue RNA were performed using an Rneasy® Protect Mini Kit (Qiagen, Chatsworth, CA, USA). The quantitation of total RNA was assessed using a Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). Six micrograms of total RNA were used to synthesize cDNA with a MultiScribe Reverse Transcriptase Kit (Applied Biosystems, Foster, CA, USA). Forty nanograms of cDNA were used per reaction, and quantitative real‐time RT–PCR was performed using Master Mix SYBR Green (Quatro G, Porto Alegre, RS, Brazil) on a 7500 Real‐Time PCR System thermal cycler (Applied Biosystems). The endogenous gene was glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), and the expression was normalized for the Control group. The RNAs were quantified, and the same initial RNA concentration was used in all RTs. Relative quantitation was performed, and all assays were run at least in duplicate. Primers used in this study were described in Table 1.
Table 1.
Primer sequences
| Gene | Forward sequences | Reverse sequences |
|---|---|---|
| BDNF | AGCTGAGCGTGTGTGACAGT | ACCCATGGGATTACACTTGG |
| TrkB | TGCCGTGGTGGTGATTGCCTCTGTG | GTTCTCTCCTACCAAGCAGTTCCGG |
| GDNF | ATGAAGTTATGGGATGTCGTGGCTG | ACCGTTTAGCGGAATGCTTTCTTAG |
| GFR‐α1 | TAGCCACTCTGTACTTCGCG | GCTTGCAGCGGCAGTTGTACA |
| NGF | GCCAAGGACGCAGCTTTCTA | GCCTGTACGCCGATCAAAA |
| TrkA | TGGCTGCCTTCGCCTCAACCAG | ATGGTGGACACAGGTATCACTG |
| VEGF | CACATAGGAGAGATGAGCTTC | CCGCCTTGGCTTGTCACAT |
| VEGFR‐2 | CATTGTGTCCTGCATCCGGGATAACCT | TGTACACGATGCCATGCTCGTCACTGA |
| TGF‐β1 | AGAACCCCCATTGCTGTCCC | GAAAGCCCTGTATTCCGTCTCC |
| TGF‐βR1 | AGAAAGCATCGGCAAAGGTC | CCCAGGATATTTTCATGGCG |
| GAPDH | TGCCACTCAGAAGACTGTGGATG | GCCTGCTTCACCACCTTCTTGAT |
Statistical Analysis
Quantitative data were expressed as mean ± SEM, and multiple comparisons between the different groups were made using one‐way ANOVA followed by Tukey's posttests. Differences of P < 0.05 were considered significant. Analyses were performed using Prism Graph 5.0 software (GraphPad Software, San Diego, CA, USA).
Results
BMMC Transplantation Modulates Hippocampal BDNF Levels in Epileptic Rats
BDNF protein levels were significantly elevated in epileptic animals 7 days after saline administration when compared to the Control group (P < 0.05). However, we did not find any difference in protein levels when Pilo animals were compared at different time points, showing no variation in BDNF protein in the temporal window (Figure 2A).
Figure 2.
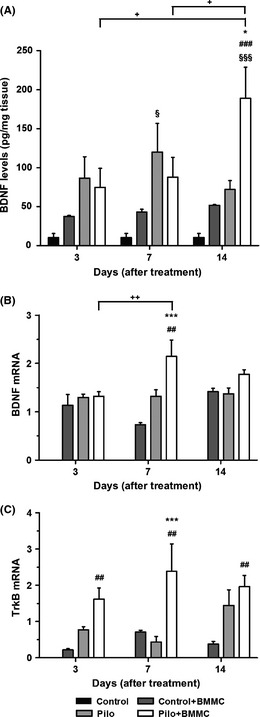
BMMC transplantation modulates the hippocampal levels of BDNF in chronically epileptic animals. (A) BDNF protein levels (n = 4 per group). (B) BDNF mRNA expression (n = 4 per group). (C) TrkB mRNA expression (n = 4 per group). All analyses were evaluated at 3, 7, and 14 days after BMMC or saline transplantation. *P < 0.05 and ***P < 0.001 vs. Pilo; # P < 0.05, ## P < 0.01, and ### P < 0.001 vs. Control + BMMC; § P < 0.05 and §§§ P < 0.001 vs. Control; + P < 0.05 and ++ P < 0.01 vs. Pilo or Pilo + BMMC in Tukey's post hoc test after one‐way ANOVA.
Conversely, in Pilo + BMMC animals, BDNF protein levels were significantly elevated 14 days posttransplantation when compared to the Pilo group (P < 0.05), cellular control group (P < 0.001), and control animals (P < 0.001) (Figure 2A). Additionally, in Pilo + BMMC rats, the BDNF protein expression showed higher levels 14 days after BMMC transplantation when compared to Pilo + BMMC 3D and 7D groups (P < 0.05). No statistical differences between control healthy and cellular control groups were found.
Regarding the modulation of mRNA levels, our results show that BMMC significantly elevated BDNF mRNA levels at 7 days (P < 0.01) when compared to Pilo and Control + BMMC groups (Pilo + BMMC 7D, P < 0.001) (Figure 2B). In Pilo + BMMC 7D animals, BDNF mRNA was also increased when compared to the Pilo + BMMC 3D group (P < 0.01). In addition, BMMC increases TrkB mRNA levels in epileptic rats 7 days after transplantation when compared to Pilo animals (P < 0.001). When Pilo + BMMC groups were compared with Control + BMMC for each time point, the TrkB mRNA expression was increased in Pilo + BMMC animals versus their respective Control + BMMC groups (3D, P < 0.05; 7D and 14D, P < 0.01) (Figure 2C).
BMMC Transplantation Modulates Hippocampal Levels of NGF in Epileptic Rats
No difference in NGF protein levels was found between Pilo and control animals. However, they were significantly higher in Pilo + BMMC rats 7 days after transplantation when compared to the Pilo, Control + BMMC, and Control groups (P < 0.01, P < 0.05, and P < 0.001, respectively). We also found significantly higher levels of NGF protein in Pilo + BMMC groups 3 (P < 0.05), 7 (P < 0.001), and 14 days (P < 0.001) after BMMC transplantation when compared to the Control group. At day 14, Control + BMMC rats showed higher levels of NGF protein when compared to the Control group (P < 0.01) (Figure 3A). As for NGF mRNA levels, we did not observe any statistical significant differences in the analyzed time points (Figure 3B). Conversely, TrkA mRNA levels were reduced in Pilo + BMMC animals 14 days posttransplantation when compared to the Pilo 14D group (P < 0.05). At day 7, Pilo animals showed decreased TrkA mRNA levels when compared to Control + BMMC 7D (P < 0.05). The temporal profile of TrkA mRNA expression in Pilo rats demonstrated increased levels at 14 days when compared to Pilo 3D and Pilo 7D (P < 0.05 and P < 0.001) (Figure 3C).
Figure 3.
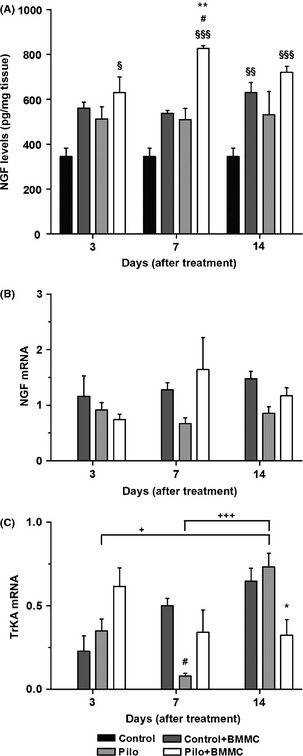
BMMC transplantation modulates the hippocampal levels of NGF in chronically epileptic animals. (A) NGF protein expression (n = 4 per group). (B) NGF mRNA expression (n = 4 per group). (C) TrkA mRNA expression (n = 4 per group). All analyses were evaluated at 3, 7, and 14 days after BMMC or saline transplantation. *P < 0.05 and **P < 0.01 vs. Pilo; # P < 0.05 vs. Control + BMMC; § P < 0.05, §§ P < 0.01, and §§§ P < 0.001 vs. Control; + P < 0.05 and +++ P < 0.001 vs. Pilo in Tukey's post hoc test after one‐way ANOVA.
BMMC Transplantation Modulates Hippocampal Levels of GDNF in Epileptic Rats
No difference in GDNF protein levels was found between Pilo and control animals. However, GDNF protein levels were increased in Pilo + BMMC rats at 7 and 14 days when compared to Pilo 7D and Pilo 14D (P < 0.01), to the Control + BMMC 7D and 14D (P < 0.01 and P < 0.001, respectively), and to control animals (P < 0.001) (Figure 4A). Additionally, GDNF protein expression increased 7 and 14 days posttransplantation in the Pilo + BMMC when compared to Pilo + BMMC 3D group (P < 0.05). GDNF mRNA levels were significantly increased in Pilo + BMMC animals 3 and 7 days after transplantation when compared to their respective Pilo (3D, P < 0.05 and 7D, P < 0.01) and Control + BMMC groups (3D and 7D, P < 0.01) (Figure 4B). We did not observe statistical significant differences in the GFR‐α1 receptor mRNA levels at the time points analyzed (Figure 4C).
Figure 4.
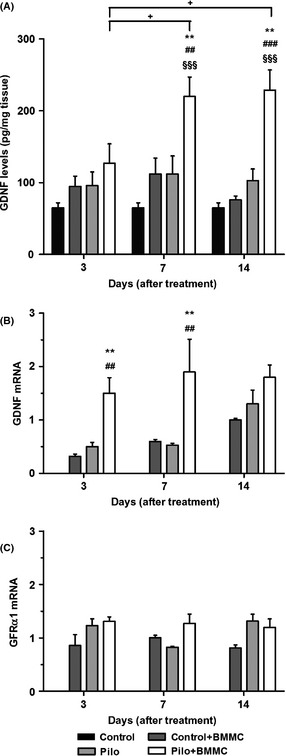
BMMC transplantation modulates the hippocampal levels of GDNF in chronically epileptic animals. (A) GDNF protein expression (n = 4 per group). (B) GDNF mRNA expression (n = 4 per group). (C) GFR‐α1 mRNA expression (n = 4 per group). All analyses were evaluated at 3, 7, and 14 days after BMMC or saline transplantation. *P < 0.05 and **P < 0.01 vs. Pilo; ## P < 0.01 and ### P < 0.001 vs. Control + BMMC; §§§ P < 0.001 vs. Control; + P < 0.05 vs. Pilo + BMMC in Tukey's post hoc test after one‐way ANOVA.
BMMC Transplantation Modulates Hippocampal Levels of VEGF in Epileptic Rats
We observed a significant increase in the expression of VEGF protein in Pilo + BMMC rats at 7 and 14 days after transplantation when compared to the Pilo groups (7D and 14D, P < 0.001). VEGF protein levels significantly increased in Pilo + BMMC animals at 3, 7, and 14 days posttransplantation when compared to their respective Control + BMMC groups (3D, P < 0.05; 7D and 14D, P < 0.001) and to the Control group (3D, P < 0.05; 7D and 14D, P < 0.001). In addition, Pilo + BMMC 14D animals showed gradually higher levels of VEGF expression 14 days after BMMC transplantation when compared to Pilo + BMMC 3D (P < 0.001) and Pilo + BMMC 7D (P < 0.05) (Figure 5A). When we analyzed temporal levels of VEGF in Pilo animals, we did not find any significant difference to control healthy animals. VEGF mRNA levels of Pilo + BMMC animals were significantly increased 3 and 7 days posttransplantation when compared to the Pilo groups (P < 0.001) (Figure 5B). Pilo + BMMC groups evaluated at 3, 7, and 14 days posttransplantation showed a significant increase in VEGF mRNA levels compared to the respective cellular control group (3D, P < 0.01; 7D and 14D, P < 0.001). Also, VEGF mRNA levels were increased in Pilo 14D animals when compared to Control + BMMC 14D group (P < 0.05). The expression of VEGF mRNA was temporally increased, with higher levels in Pilo + BMMC 7D than those in Pilo + BMMC 3D (P < 0.05), and even higher in Pilo 14D when compared with Pilo 3D and 7D (P < 0.05). VEGFR‐2 mRNA levels were increased after the BMMC transplantation at 3 days when compared to the Pilo 3D and to cellular control groups (P < 0.001). The temporal expression of VEGFR‐2 mRNA levels was reduced 7 days after BMMC transplantation compared to the Pilo + BMMC 3D group (P < 0.01). Furthermore, VEGFR‐2 mRNA levels increased over time in Pilo animals, with higher observed levels in Pilo 14D rats than those in Pilo 3D (P < 0.05) (Figure 5C).
Figure 5.
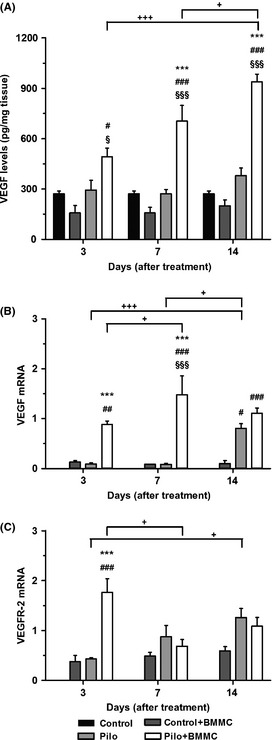
BMMC transplantation modulates the hippocampal levels of VEGF in chronically epileptic animals. (A) VEGF protein expression (n = 4 per group). (B) VEGF mRNA expression (n = 4 per group). (C) VEGFR mRNA expression (n = 4 per group). All analyses were evaluated at 3, 7, and 14 days after BMMC or saline transplantation. ***P < 0.001 vs. Pilo; # P < 0.05, ## P < 0.01 and ### P < 0.001 vs. Control + BMMC; § P < 0.05 and §§§ P < 0.001 vs. Control; + P < 0.05 and +++ P < 0.001 vs. Pilo or Pilo + BMMC in Tukey's post hoc test after one‐way ANOVA.
BMMC Transplantation Modulates Hippocampal Levels of TGF‐β1 in Epileptic Rats
TGF‐β1 protein levels were increased in the Pilo groups at 3 and 14 days compared to their respective control group (P < 0.05). However, no differences were found between Pilo groups 3D, 7D, and 14D. TGF‐β protein expression in the hippocampus of Pilo + BMMC 14D rats was significantly reduced compared to the Pilo 14D group (P < 0.05). We also observed a significant increase in the expression of TGF protein in Pilo + BMMC 3D and Pilo 3D when compared to the Control + BMMC (P < 0.001) and to the Control group (P < 0.05 and P < 0.01, respectively). In addition, the temporal expression exhibited a significant decrease between the groups evaluated at 3 and 14 days after BMMC transplantation (P < 0.01) (Figure 6A). TGF‐β1 mRNA levels were significantly different in all Pilo + BMMC (3D, P < 0.001; 7D, P < 0.01 and 14D, P < 0.001) or Pilo groups (3D and 7D P < 0.001; 14D P < 0.01) when compared to the cellular control group. TGF‐β1 mRNA levels significantly decreased with time when Pilo + BMMC 7D and Pilo + BMMC 14D groups were compared to Pilo + BMMC 3D (P < 0.001). In Pilo groups, TGF‐β1 mRNA levels gradually decreased with time (3D vs. 7D, P < 0.01; 7D vs. 14D, P < 0.05; and 3D vs. 14D, P < 0.001) (Figure 6B). TGFR‐β1 receptor mRNA levels were decreased in epileptic animals 7 days after BMMC transplantation compared to Pilo 7D (P < 0.05). In addition, TGFR‐β1 receptor mRNA levels were increased in Pilo + BMMC 7D (P < 0.05) and Pilo 7D groups (P < 0.001) when compared to their cellular control group. TGF‐βR1 receptor mRNA levels were significantly higher in Pilo 7D animals when compared to Pilo 3D rats (P < 0.05), but decreased in the Pilo 14D group when compared to the Pilo 7D one (P < 0.001) (Figure 6C).
Figure 6.
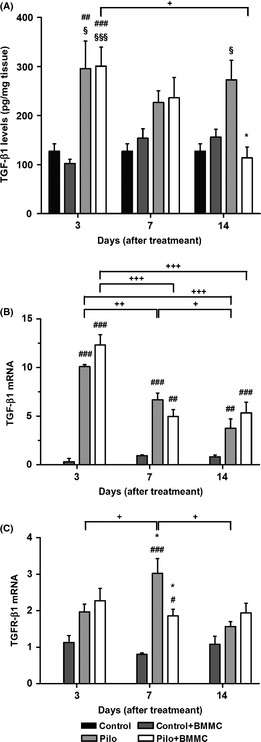
BMMC transplantation modulates the hippocampal levels of TGF‐β1 in chronically epileptic animals. (A) TGF‐β1 protein expression (n = 4 per group). (B) TGF‐β1 mRNA expression (n = per group). (C) TGF‐βR1 mRNA expression (n = 4 per group). All analyses were evaluated at 3, 7, and 14 days after BMMC or saline transplantation. *P < 0.05 vs. Pilo; # P < 0.05, ## P < 0.01, and ### P < 0.001 vs. Control + BMMC; § P < 0.05 and §§ P < 0.01 vs. Control; + P < 0.05, ++ P < 0.01, and +++ P < 0.001 vs. Pilo or Pilo + BMMC in Tukey's post hoc test after one‐way ANOVA.
Discussion
Many studies show increased expression of trophic factors in epileptic animals, but most of these studies examine animals in the acute period of the disease 24. Our study was conducted on chronically epileptic animals, because this period would be the most appropriate for translational cell therapy‐based interventions. Recent data from our research group have shown that treatment with BMMCs after temporal lobe epilepsy improves functional outcomes in rats and has great potential for therapeutic applications 7, 8, 13. The present study was not designed to investigate the functional effects of BMMC transplantation, which were already demonstrated in our previous publications 7, 8, 13, but to elucidate whether their neuroprotective effects were associated with the modulation of neurotrophic growth factors.
A large array of growth factors has control upon cell survival, proliferation, and differentiation in the central nervous system 25. In our study, we focused on BDNF, NGF, GDNF, VEGF, and TGF‐β1 because several reports in the literature suggest that these factors are involved in epileptogenic processes 26, 27, 28, 29, 30. Here, we showed that BDNF protein was increased 14 days posttransplantation, and BDNF mRNA and TrkB mRNA receptor expressions were increased 7 days posttransplantation in relation to saline‐treated epileptic animals. In fact, BDNF has been found to be a therapeutic target for epilepsy treatment 21, 31. In previous studies, BDNF was shown to modulate neurogenesis, decrease neuronal death and consequently the SRS occurrence 26, and prevent the development of SE 32. Conversely, knockout mice lacking BDNF and the TrkB receptor show reduced epileptogenesis 33. Regarding the neurotrophin NGF, we found that protein levels increased after 7 days of BMMC transplantation in epileptic rats. However, TrkA mRNA levels demonstrated a reduction at 14 days posttransplantation. Studies show that NGF facilitates neuronal repair against injuries 34. NGF is known to play an important role in the remodeling of brain networks after seizures 27. The importance of NGF as a neuroprotective factor has also been shown in distinct models of brain lesion 35, 36. Thus, the high levels of NGF and BDNF following BMMC transplantation in epileptic rats could be responsible for the tissue repair and consequently the reduction in the neurological deficits.
We also demonstrated that BMMC transplantation continuously increased the expression of GDNF protein at 7 and 14 days after BMMC treatment. GDNF mRNA levels were increased 3 and 7 days in the hippocampus of epileptic rats posttransplantation. GDNF is considered a survival factor for different types of neurons and has anticonvulsant properties 28. Li et al. 37 demonstrated that intraventricular administration of GDNF prevented inflammation in the rodent hippocampus. Moreover, Nanobashvili et al. 38 found that the development of kindling‐induced seizures was inhibited in knockout mice for the GFRα2 receptor. These dramatic GDNF increases may be involved in decreasing inflammation and in controlling the neuronal excitability seen in chronically epileptic rats, resulting in reduced seizure frequency. Regarding VEGF, we found that protein was elevated 7 and 14 days in the BMMC‐treated epileptic rats, VEGF mRNA had an increase 3 and 7 days posttreatment, while VEGFR‐2 mRNA receptor levels increased only 3 days after BMMC transplantation in epileptic rats. The temporal expression of VEGF protein showed a gradual increase, while VEGF mRNA and VEGFR‐2 levels had the higher expression at 7 and 3 days posttransplantation, respectively. Neuronal and glial cells regulate the VEGF expression after seizures in the rodent hippocampus, suggesting a neuroprotective role of VEGF in epilepsy 39. Besides, VEGF has also been found to significantly protect learning and memory after SE 29. Our findings that epileptic tissues promote a significant increase in VEGF production in response to BMMCs suggest that this mechanism could contribute to the cognitive preservation we observed in previous studies using BMMC transplantation in epilepsy.
Conversely, TGF‐β1 levels presented a different expression pattern from the other growth factors. It was noted that TGF‐β1 protein decreased 14 days after BMMC transplantation. Besides, TGF‐β1 mRNA levels did not show significant reduction between the untreated epileptic animals and BMMC‐treated group, but these levels had a gradual decrease in the temporal analysis in both groups. The TGFR‐β1 mRNA receptor expression significantly decreased 7 days posttransplantation compared to untreated epileptic group. A role for TGF‐β1 in epileptogenesis has been demonstrated in animals after seizures, and it is highly regulated in neurons and astrocytes 40, 41. Activation of the TGF‐β1 signaling pathway is capable of enhancing epileptiform activity in the brain, although its blockade could prevent epileptogenesis 30, 42. Studies show that blood–brain barrier (BBB) dysfunction may play a direct role in the pathogenesis of epilepsy and that TGF‐β1 increases BBB permeability, which occurs with seizures 42.
We showed that BMMC transplantation takes at least 3 days to modulate the majority of the trophic factors evaluated in the present study, as the protein levels in the treated groups were significantly increased at 7 days posttransplantation. For some trophic factors studied here, protein expression changes were not always followed by the same modulation of mRNA levels. Some dissociation between mRNA and protein expression can occur, suggesting that mechanisms other than upregulated mRNA levels explain increased proteins levels. In addition, the expression of trophic factor receptor mRNA (GFR‐α1) did not seem to change over the days after the BMMC transplantation. Alternate mechanisms include increased translation of existing mRNA, increased protein stability, or reduced degradation in neurons 43. In addition, trophic factors could interact with different types of receptors, with a greater affinity for some, and exert their mechanisms of action through distinct signaling pathways 44. As demonstrated elsewhere, the expression of some neurotrophins and its receptors could be modulated as needed, while other factors are continuously produced and secreted upon specific signals 45. Our data also corroborated the study of Kolomeyer et al. 46, where the mRNA expression does not directly correlate with the protein expression. Interestingly, TrkA receptor mRNA levels were reduced after BMMC transplantation in epileptic rats. Additionally, our results might indicate a negative feedback regulatory strategy following the overexpression of trophic factors. One study suggests that TrkA Ser/Thr phosphorylation is typically part of a negative feedback loop that responds to saturating doses of NGF by dampening and controlling a flood of incoming signal 47.
Complementary, we demonstrated that BMMC transplantation did not influence the protein expression of the trophic factors evaluated in control animals compared to saline‐treated control animals. With the exception of NGF protein expression, that was increased at 14 days posttransplantation in the cellular control group when compared to control animals. Nevertheless, this modulation is not likely to affect the behavioral response of these animals. In previous studies, we have demonstrated that control healthy rats administered with BMMC have the same behavioral outcome as untreated control animals 7, 13. Furthermore, we already demonstrated that BMMC transplantation decreased the frequency and duration of seizures independently of the cellular donor (syngeneic or xenogeneic) in chronically epileptic rats 7. Here, we showed that BMMC transplantation induces the expression of a number of trophic factors in the brain of epileptic rats. The role of trophic factors in epilepsy is still controversial, with data pointing to either increased epileptogenesis or protective effects associated with their modulation 21. In the present study, we show that the increased expression of trophic factors after BMMC transplantation might be associated with the beneficial effects previously seen in BMMC‐treated chronic epileptic rats 7, 13. The BMMC mechanism of action based on trophic factors modulation may explain the neuroprotective effects observed in our previous publications as few transplanted cells reached the brain of epileptic rats 7. Costa‐Ferro et al. 7 showed that BMMC transplantation prevents neuronal death and induces neuronal proliferation in chronically epileptic rats. It is well known that trophic factors are involved in neuronal preservation and neurogenesis 26, 35, 48, 49. Thus, a paracrine effect could be contributing to neuronal preservation in those animals.
To our knowledge, this is the first study to demonstrate that BMMCs induce an increase in the hippocampal production of trophic factors in chronically epileptic rats. This evidence is an important advance to comprehend the mechanisms of action of BMMC transplantation for the treatment of chronically epileptic rats 7, 8, 13. The present results, together with our previously findings, show that BMMC transplantation may provide neuroprotective effects via trophic factors for the recovery of epileptic patients, supporting future clinical trials and translation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by research grants from the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS; PQG‐11/1546‐1 to J.C. DaCosta., ARD‐ 11/1678‐0 to F.Simão) and Pandurata Ltda. G. Zanirati and P. Azevedo are the recipient of scholarship from Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES). J.C. DaCosta is a researcher of the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq). We acknowledge Dr. Denise Cantarelli Machado for her support in bone marrow mononuclear cell processing.
References
- 1. Engel J Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001;42:796–803. [DOI] [PubMed] [Google Scholar]
- 2. Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res 2007;163:755–773. [DOI] [PubMed] [Google Scholar]
- 3. Loscher W. Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol Sci 2002;23:113–118. [DOI] [PubMed] [Google Scholar]
- 4. Abdanipour A, Tiraihi T, Mirnajafi‐Zadeh J. Improvement of the pilocarpine epilepsy model in rat using bone marrow stromal cell therapy. Neurol Res 2011;33:625–632. [DOI] [PubMed] [Google Scholar]
- 5. Carpentino JE, Hartman NW, Grabel LB, Naegele JR. Region‐specific differentiation of embryonic stem cell‐derived neural progenitor transplants into the adult mouse hippocampus following seizures. J Neurosci Res 2008;86:512–524. [DOI] [PubMed] [Google Scholar]
- 6. Chu K, Kim M, Jung KH, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine‐induced status epilepticus in adult rats. Brain Res 2004;1023:213–221. [DOI] [PubMed] [Google Scholar]
- 7. Costa‐Ferro ZS, Souza BS, Leal MM, et al. Transplantation of bone marrow mononuclear cells decreases seizure incidence, mitigates neuronal loss and modulates pro‐inflammatory cytokine production in epileptic rats. Neurobiol Dis 2012;46:302–313. [DOI] [PubMed] [Google Scholar]
- 8. Costa‐Ferro ZS, Vitola AS, Pedroso MF, et al. Prevention of seizures and reorganization of hippocampal functions by transplantation of bone marrow cells in the acute phase of experimental epilepsy. Seizure 2010;19:84–92. [DOI] [PubMed] [Google Scholar]
- 9. Guttinger M, Fedele D, Koch P, et al. Suppression of kindled seizures by paracrine adenosine release from stem cell‐derived brain implants. Epilepsia 2005;46:1162–1169. [DOI] [PubMed] [Google Scholar]
- 10. Jing M, Shingo T, Yasuhara T, et al. The combined therapy of intrahippocampal transplantation of adult neural stem cells and intraventricular erythropoietin‐infusion ameliorates spontaneous recurrent seizures by suppression of abnormal mossy fiber sprouting. Brain Res 2009;1295:203–217. [DOI] [PubMed] [Google Scholar]
- 11. Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3‐selective epileptogenesis. Epilepsy Res 2009;84:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maisano X, Litvina E, Tagliatela S, Aaron GB, Grabel LB, Naegele JR. Differentiation and functional incorporation of embryonic stem cell‐derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci 2012;32:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venturin GT, Greggio S, Marinowic DR, et al. Bone marrow mononuclear cells reduce seizure frequency and improve cognitive outcome in chronic epileptic rats. Life Sci 2011;89:229–234. [DOI] [PubMed] [Google Scholar]
- 14. Waldau B, Hattiangady B, Kuruba R, Shetty AK. Medial ganglionic eminence‐derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 2010;28:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leal MM, Costa‐Ferro ZS, Souza BS, et al. Early transplantation of bone marrow mononuclear cells promotes neuroprotection and modulation of inflammation after status epilepticus in mice by paracrine mechanisms. Neurochem Res 2014;39:259–268. [DOI] [PubMed] [Google Scholar]
- 16. Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci 1994;17:182–190. [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Katakowski M, Li Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res 2002;69:687–691. [DOI] [PubMed] [Google Scholar]
- 18. Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev 2012;21:2222–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma 2004;21:33–39. [DOI] [PubMed] [Google Scholar]
- 20. Bao X, Wei J, Feng M, et al. Transplantation of human bone marrow‐derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res 2011;1367:103–113. [DOI] [PubMed] [Google Scholar]
- 21. Simonato M, Tongiorgi E, Kokaia M. Angels and demons: neurotrophic factors and epilepsy. Trends Pharmacol Sci 2006;27:631–638. [DOI] [PubMed] [Google Scholar]
- 22. Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res 1983;9:315–335. [DOI] [PubMed] [Google Scholar]
- 23. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 24. Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol 2001;63:125–149. [DOI] [PubMed] [Google Scholar]
- 25. Mattson MP, Maudsley S, Martin B. BDNF and 5‐HT: a dynamic duo in age‐related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 2004;27:589–594. [DOI] [PubMed] [Google Scholar]
- 26. Bovolenta R, Zucchini S, Paradiso B, et al. Hippocampal FGF‐2 and BDNF overexpression attenuates epileptogenesis‐associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation 2010;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holtzman DM, Lowenstein DH. Selective inhibition of axon outgrowth by antibodies to NGF in a model of temporal lobe epilepsy. J Neurosci 1995;15:7062–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanter‐Schlifke I, Georgievska B, Kirik D, Kokaia M. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther 2007;15:1106–1113. [DOI] [PubMed] [Google Scholar]
- 29. Nicoletti JN, Lenzer J, Salerni EA, et al. Vascular endothelial growth factor attenuates status epilepticus‐induced behavioral impairments in rats. Epilepsy Behav 2010;19:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cacheaux LP, Ivens S, David Y, et al. Transcriptome profiling reveals TGF‐beta signaling involvement in epileptogenesis. J Neurosci 2009;29:8927–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koyama R, Ikegaya Y. To BDNF or not to BDNF: that is the epileptic hippocampus. Neuroscientist 2005;11:282–287. [DOI] [PubMed] [Google Scholar]
- 32. Paradiso B, Marconi P, Zucchini S, et al. Localized delivery of fibroblast growth factor‐2 and brain‐derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proc Natl Acad Sci USA 2009;106:7191–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci 2001;24:47–53. [DOI] [PubMed] [Google Scholar]
- 34. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001;24:1217–1281. [DOI] [PubMed] [Google Scholar]
- 35. Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science 1987;235:214–216. [DOI] [PubMed] [Google Scholar]
- 36. Hefti F, Weiner WJ. Nerve growth factor and Alzheimer's disease. Ann Neurol 1986;20:275–281. [DOI] [PubMed] [Google Scholar]
- 37. Li S, Xu B, Martin D, Racine RJ, Fahnestock M. Glial cell line‐derived neurotrophic factor modulates kindling and activation‐induced sprouting in hippocampus of adult rats. Exp Neurol 2002;178:49–58. [DOI] [PubMed] [Google Scholar]
- 38. Nanobashvili A, Airaksinen MS, Kokaia M, et al. Development and persistence of kindling epilepsy are impaired in mice lacking glial cell line‐derived neurotrophic factor family receptor alpha 2. Proc Natl Acad Sci USA 2000;97:12312–12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cammalleri M, Martini D, Ristori C, Timperio AM, Bagnoli P. Vascular endothelial growth factor up‐regulation in the mouse hippocampus and its role in the control of epileptiform activity. Eur J Neurosci 2011;33:482–498. [DOI] [PubMed] [Google Scholar]
- 40. Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci 2000;12:2333–2344. [DOI] [PubMed] [Google Scholar]
- 41. Plata‐Salaman CR, Ilyin SE, Turrin NP, et al. Kindling modulates the IL‐1beta system, TNF‐alpha, TGF‐beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res 2000;75:248–258. [DOI] [PubMed] [Google Scholar]
- 42. Ivens S, Kaufer D, Flores LP, et al. TGF‐beta receptor‐mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 2007;130(Pt 2):535–547. [DOI] [PubMed] [Google Scholar]
- 43. Krasnova IN, Chiflikyan M, Justinova Z, et al. CREB phosphorylation regulates striatal transcriptional responses in the self‐administration model of methamphetamine addiction in the rat. Neurobiol Dis 2013;58:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013;153:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheedlo HJ, Turner JE. Immunocytochemical characterisation of proteins secreted by retinal pigment epithelium in retinas of normal and Royal College of Surgeons dystrophic rats. J Anat 1998;193(Pt 2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kolomeyer AM, Sugino IK, Zarbin MA. Characterization of conditioned media collected from aged versus young human eye cups. Invest Ophthalmol Vis Sci 2011;52:5963–5972. [DOI] [PubMed] [Google Scholar]
- 47. Van Kanegan MJ, Strack S. The protein phosphatase 2A regulatory subunits B'beta and B'delta mediate sustained TrkA neurotrophin receptor autophosphorylation and neuronal differentiation. Mol Cell Biol 2009;29:662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 2002;99:11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line‐derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 2006;37:2361–2367. [DOI] [PubMed] [Google Scholar]


