Campylobacter jejuni is a leading cause of diarrheal disease worldwide, and currently no preventative interventions are available. C. jejuni is an invasive mucosal pathogen that has a variety of polysaccharide structures on its surface, including a capsule. In phase 1 studies, a C. jejuni capsule conjugate vaccine was safe but poorly immunogenic when delivered alone or with aluminum hydroxide. Here, we report enhanced immunogenicity of the conjugate vaccine delivered with liposome adjuvants containing monophosphoryl lipid A without or with QS-21, known as ALF and ALFQ, respectively, in preclinical studies. Both liposome adjuvants significantly enhanced immunity in mice and nonhuman primates and improved protective efficacy of the vaccine compared to alum in a nonhuman primate C. jejuni diarrhea model, providing promising evidence that these potent adjuvant formulations may enhance immunogenicity in upcoming human studies with this C. jejuni conjugate and other malaria and HIV vaccine platforms.
KEYWORDS: campylobacter, Campylobacter jejuni, adjuvants, conjugate, liposomes, vaccines
ABSTRACT
Campylobacter jejuni is among the most common causes of diarrheal disease worldwide and efforts to develop protective measures against the pathogen are ongoing. One of the few defined virulence factors targeted for vaccine development is the capsule polysaccharide (CPS). We have developed a capsule conjugate vaccine against C. jejuni strain 81-176 (CPS-CRM) that is immunogenic in mice and nonhuman primates (NHPs) but only moderately immunogenic in humans when delivered alone or with aluminum hydroxide. To enhance immunogenicity, two novel liposome-based adjuvant systems, the Army Liposome Formulation (ALF), containing synthetic monophosphoryl lipid A, and ALF plus QS-21 (ALFQ), were evaluated with CPS-CRM in this study. In mice, ALF and ALFQ induced similar amounts of CPS-specific IgG that was significantly higher than levels induced by CPS-CRM alone. Qualitative differences in antibody responses were observed where CPS-CRM alone induced Th2-biased IgG1, whereas ALF and ALFQ enhanced Th1-mediated anti-CPS IgG2b and IgG2c and generated functional bactericidal antibody titers. CPS-CRM + ALFQ was superior to vaccine alone or CPS-CRM + ALF in augmenting antigen-specific Th1, Th2, and Th17 cytokine responses and a significantly higher proportion of CD4+ IFN-γ+ IL-2+ TNF-α+ and CD4+ IL-4+ IL-10+ T cells. ALFQ also significantly enhanced anti-CPS responses in NHPs when delivered with CPS-CRM compared to alum- or ALF-adjuvanted groups and showed the highest protective efficacy against diarrhea following orogastric challenge with C. jejuni. This study provides evidence that the ALF adjuvants may provide enhanced immunogenicity of this and other novel C. jejuni capsule conjugate vaccines in humans.
IMPORTANCE Campylobacter jejuni is a leading cause of diarrheal disease worldwide, and currently no preventative interventions are available. C. jejuni is an invasive mucosal pathogen that has a variety of polysaccharide structures on its surface, including a capsule. In phase 1 studies, a C. jejuni capsule conjugate vaccine was safe but poorly immunogenic when delivered alone or with aluminum hydroxide. Here, we report enhanced immunogenicity of the conjugate vaccine delivered with liposome adjuvants containing monophosphoryl lipid A without or with QS-21, known as ALF and ALFQ, respectively, in preclinical studies. Both liposome adjuvants significantly enhanced immunity in mice and nonhuman primates and improved protective efficacy of the vaccine compared to alum in a nonhuman primate C. jejuni diarrhea model, providing promising evidence that these potent adjuvant formulations may enhance immunogenicity in upcoming human studies with this C. jejuni conjugate and other malaria and HIV vaccine platforms.
INTRODUCTION
Campylobacter infections are major causes of bacterial diarrhea worldwide, with the majority identified as being caused by Campylobacter jejuni. C. jejuni is a leading cause of foodborne illness in North America and Europe and was identified in the Global Enteric Multicenter Study (GEMS) and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) multisite birth cohort studies as a significant attributable cause of severe-to-moderate diarrhea that is associated with growth stunting in middle-to-low-income countries (1–4). C. jejuni infection typically results in acute inflammatory gastroenteritis that is self-limiting, but in more severe cases, the disease can progress to dysentery. In addition to acute disease, C. jejuni infections are associated with long-term sequelae, including reactive arthritis, inflammatory bowel syndrome, and most frequently with Guillain-Barré syndrome (GBS) (5–9). GBS is caused by development of autoantibodies to a subset of C. jejuni strains that express lipooligosaccharides (LOS) that mimic human gangliosides in structure, and it is currently estimated that C. jejuni infection precedes GBS in 20 to 50% of cases in the developed world and in other regions may be even higher (8).
The global burden imposed by the morbidity of Campylobacter disease and its related sequelae drives efforts to develop protective interventions. Measures to influence disease prevention, including source eradication, personal protective measures, and chemoprophylaxis, have been moderately successful thus far. Moreover, the rise of antibiotic-resistant C. jejuni point to the need for other types of interventions (10–12). There are no licensed vaccines available for Campylobacter. One critical hurdle in vaccine development is that, unlike other enteric pathogens, there are few defined virulence factors that have been targeted as subunit vaccine approaches. C. jejuni expresses a polysaccharide capsule (CPS) that is the major serodeterminant of the Penner heat-stable (HS) serotyping scheme (13). There are 47 known HS serotypes of C. jejuni resulting from 35 chemically diverse CPS structures (14, 15). Importantly, CPS has been shown to be an important virulence factor modulating invasion and disease and contributing to serum resistance (16, 17). We have developed a CPS conjugate approach utilizing the diphtheria toxin mutant CRM197 as a carrier protein (18). CPS isolated from strain 81-176 (type HS23/36) was conjugated to CRM197 (CPS-CRM) as a prototype vaccine candidate. The prototype CPS-CRM vaccine was immunogenic in mice without an adjuvant, generated high levels of anti-CPS IgG and was protective when mice were intranasally challenged with C. jejuni strain 81-176. CPS-CRM vaccine efficacy was also tested in a New World nonhuman primate (NHP), Aotus nancymaae, model. NHPs animals receiving a CPS-CRM dose comparable to 2.5 μg of polysaccharide plus aluminum hydroxide (alum) were protected from diarrhea after challenge with 81-176 (18).
To follow on the success of the preclinical studies, a CPS-CRM conjugate vaccine GMP-manufactured from a mutant strain of 81-176 lacking LOS, known as CJCV1, was tested in a phase 1 study with or without alum at 4-week intervals (ClinicalTrials.gov identifier NCT02067676) (19). Although the vaccine was safe, it was weakly immunogenic, likely because it was delivered in only two doses unlike all the preclinical studies which utilized three doses. In addition, adjuvants other than alum have not been extensively explored with our C. jejuni conjugate vaccine in preclinical models, where an immunopotentiating adjuvant might enhance immunogenicity of the CPS-CRM conjugate in humans. These adjuvants often incorporate components that stimulate innate immune system receptors like Toll-like receptors (TLRs) and the inflammasome. The Walter Reed Army Institute of Research Laboratory of Adjuvant and Antigen Research has developed an Army Liposome Formulation (ALF) family of adjuvants that increase immunogenicity of a number of different vaccine platforms against a variety of pathogens, including HIV, Plasmodium falciparum, and Neisseria meningitidis (20–24). ALF is a liposome-based formulation containing a potent activator of TLR4, a synthetic form of monophospholipid A (MPLA) known as 3D-PHAD (Avanti Polar Lipids). The addition of components like alum and a detoxified saponin derivative QS-21 has enhanced the effectiveness of MPLA-containing liposomes further. Although both alum and QS-21 can activate the NOD-like receptor P3 (NLRP3) inflammasome complex in antigen-presenting cells (25, 26), they differ in the type of immune response induced; alum primarily skews immunity toward a Th2 response, and QS-21 induces Th1 responses. Here, CPS-CRM was tested with a series of ALF adjuvants: ALF, ALF plus alum (ALFA), ALF plus QS-21 (ALFQ), and ALFQ plus alum (ALFQA). We show that ALF and, to a greater extent, ALFQ enhance the CPS-specific antibody response generated against our CPS-CRM vaccine. Functional bactericidal CPS-specific antibodies were generated at the highest level in ALFQ-adjuvanted mice and NHPs vaccinated with CPS-CRM plus ALFQ (CPS-CRM + ALFQ) showed the greatest protective efficacy against diarrhea in a C. jejuni challenge model.
RESULTS
Coadministration of ALF adjuvants enhances the immunogenicity of a C. jejuni CPS-CRM vaccine.
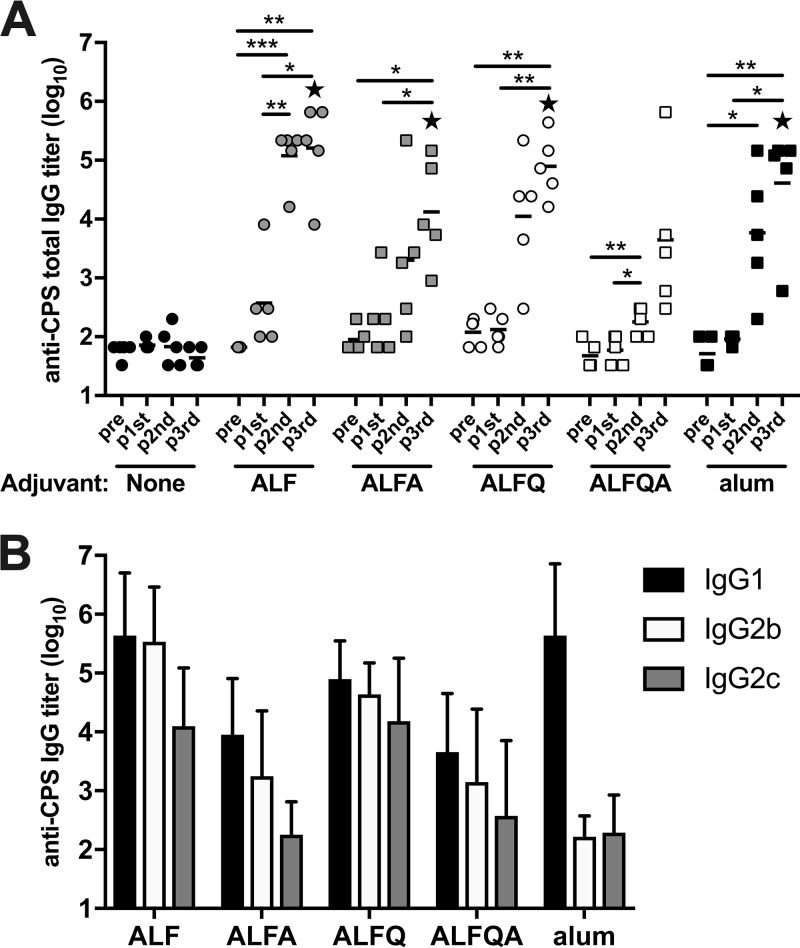
Previous studies have demonstrated that the CPS-CRM vaccine is immunogenic in mice at three doses without the addition of an adjuvant and in NHPs when delivered with alum (18). However, the vaccine was poorly immunogenic in humans at two doses, even when coadministered with alum. To test the ability of liposome-based immunopotentiating adjuvants to augment immunogenicity of CPS-CRM, we tested four ALF formulation adjuvants and alum with a very low dose of CPS-CRM by immunizing mice three times intramuscularly (i.m.) at 4-week intervals. Serum anti-CPS IgG titers were measured by ELISA prevaccination and 2 weeks after each immunization. Without an adjuvant, 0.1 µg of CPS-CRM (based on CPS weight) was not immunogenic in mice after three doses (Fig. 1A). The addition of an adjuvant induced a modest increase in anti-CPS IgG titers after one dose, and titers were boosted by the second and third doses of vaccine plus adjuvant in groups with ALF, ALFA, ALFQ, and alum (Fig. 1A). ALFQA did not enhance CPS-specific responses to the same extent as alum and other ALF formulations. After three doses, all adjuvanted groups had significantly higher CPS-specific total IgG titers than the vaccine-alone group, but no difference was observed in titers between adjuvanted groups. Because differences in qualitative antibody responses have been observed depending on adjuvant properties, we measured IgG subclasses. As expected, alum induced primarily a Th2-mediated antibody response characterized by anti-CPS IgG1 and little IgG2b or IgG2c (Fig. 1B). Conversely, ALF and ALFQ adjuvants induced Th1-biased CPS-specific IgG2b and 2c titers at levels higher than alum-adjuvanted mice. ALF- and ALFQ-adjuvanted mice showed equivalent levels of anti-CPS IgG1 compared to alum. The presence of alum in the ALFA or ALFQA groups dampened the production of CPS-specific IgG2b and 2c in these groups, and the levels were significantly lower than the levels observed in the ALF and ALFQ groups. Based on these results, we selected ALF and ALFQ adjuvants for further testing because they induced a robust CPS-specific antibody response characterized by high titers of anti-CPS IgG1, IgG2b, and IgG2c.
FIG 1.
ALF adjuvant formulations enhance immunogenicity of a C. jejuni CPS-CRM vaccine in mice. Mice were immunized i.m. three times at 4-week intervals with 0.1 µg of CPS-CRM alone or with the indicated ALF adjuvant or alum. (A) Kinetics of the CPS-specific antibody response. Anti-CPS IgG titers were measured in serum collected prevaccination and 2 weeks after each immunization. Log10 titers of individual mice (n = 5 per group) are shown, where the horizontal line indicates median of the group at the respective time point. Repeated-measures one-way ANOVA with multiplicity-adjusted P values for statistical significance among groups was performed (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Statistical significance between groups was determined by ordinary one-way ANOVA with multiplicity-adjusted P values. Stars indicate significantly different values from CPS-CRM alone (P ≤ 0.05). (B) Anti-CPS IgG1, IgG2b or IgG2c titers were measured in serum 2 weeks after the third dose. The bar graph represents means plus the standard deviations of five mice per group immunized with CPS-CRM plus the indicated adjuvant.
Dose escalation of CPS-CRM vaccine with ALF and ALFQ.
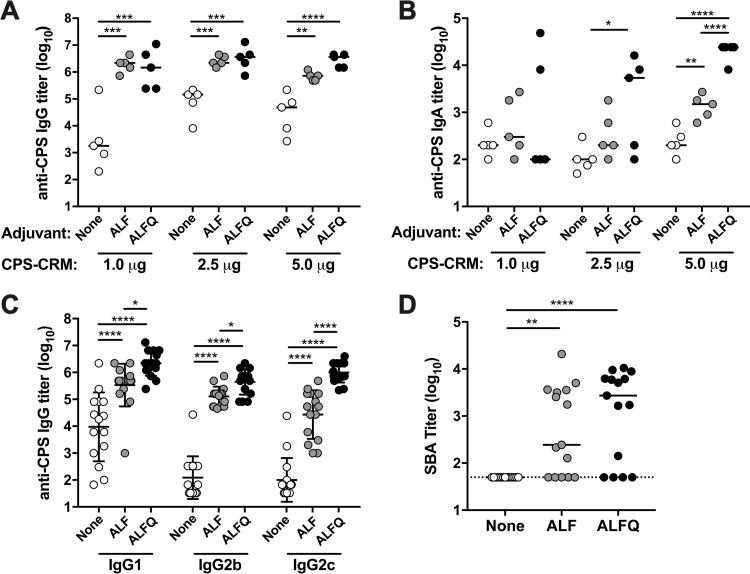
ALF and ALFQ were further compared in a subsequent study with a dose escalation of CPS-CRM. 1.0, 2.5, and 5.0 µg of CPS-CRM (based on CPS weight) was delivered alone or admixed with ALF or ALFQ adjuvants. CPS titers were measured 2 weeks after the third dose. At elevated doses, CPS-CRM vaccine was immunogenic without the use of an adjuvant, and we observed a dose-dependent increase in anti-CPS IgG titers (Fig. 2A). We observed a 2.6-fold increase in IgG titers between the 1.0- and 2.5-µg doses, and IgG titers plateaued at 2.5 µg with no further increase in anti-CPS IgG at the 5.0-µg dose. Administration of CPS-CRM with ALF or ALFQ induced significantly higher titers of anti-CPS IgG at every dose of CPS-CRM tested compared to vaccine alone (Fig. 2A). These results suggest that ALF and ALFQ may allow dose sparing of a C. jejuni conjugate vaccine formulation in the clinic. Differences between the vaccine-alone and the ALF or ALFQ treatment groups were not as striking at the 2.5- and 5.0-µg doses, and yet both adjuvants enhanced IgG titers at every dose of vaccine tested. We also measured CPS-specific IgA responses in serum after three doses of CPS-CRM with or without adjuvants (Fig. 2B). Overall, the IgA titers were much lower than those observed for IgG. CPS-CRM alone did not induce substantial levels of anti-CPS IgA at any of the doses tested. Significantly higher IgA titers were observed at the highest 5.0-µg dose of CPS-CRM when coadministered with ALF, whereas ALFQ induced significantly higher levels of anti-CPS IgA at both the 2.5- and 5.0-µg doses compared to vaccine alone. The highest IgA titers were observed with 5.0 µg of CPS-CRM + ALFQ, where titers were significantly higher than those observed with vaccine alone and CPS-CRM + ALF (75- and 14-fold, respectively). Notably, this is the first time that we have observed substantial levels of CPS-specific IgA after vaccination with this CPS-CRM vaccine.
FIG 2.
ALF and ALFQ adjuvants induce high levels of anti-CPS IgG and functional serum bactericidal antibodies at higher doses of CPS-CRM in mice. Mice were immunized i.m. three times at 4-week intervals with 1.0, 2.5, or 5.0 µg of CPS-CRM alone or with ALF or ALFQ. (A and B) Anti-CPS IgG (A) and IgA (B) antibody responses were measured 2 weeks after the third vaccination. Log10 titers of individual mice (n = 5 per group) are shown, where the horizontal line indicates median of the group. (C) Anti-CPS IgG1, IgG2b, and IgG2c titers were measured 2 weeks after the third vaccination. Log10 titers of individual mice combining dose levels (n = 15 per group) are shown, where the horizontal line indicates the median of the group. (D) Functional antibody responses were measured after the third immunization by F-SBA. Log10 F-SBA titers of individual mice with combined dose levels are shown, where the horizontal line indicates the median of the group. The dotted line indicates the limit of detection of F-SBA. Statistical significance between groups was determined by ordinary one-way ANOVA with multiplicity-adjusted P values (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Because we observed differences in CPS-specific IgG subclass responses at a very low dose of 0.1 µg of CPS-CRM + ALF or ALFQ, we measured these responses at the higher vaccine doses (Fig. 2C). CPS-CRM at higher doses delivered without an adjuvant primarily induced CPS-specific IgG1. IgG1, IgG2b, and IgG2c levels were significantly higher in ALF-adjuvanted mice than in mice given vaccine alone. The titers of CPS-specific IgG1, IgG2b, and IgG2c were the highest in the ALFQ groups, with levels that were significantly higher than both vaccine alone and ALF. Subclass analysis suggests that ALF and, to a greater extent, ALFQ can bias the CPS-specific response toward Th1-mediated IgG2b and IgG2c antibodies.
Coadministration of CPS-CRM with ALF or ALFQ induces bactericidal antibody responses.
Because CPS conjugate vaccines against other Gram-negative bacteria, such as Neisseria meningitidis, have been shown to generate functional serum bactericidal antibody (SBA) responses (27–31) and because bactericidal activity has been described previously after C. jejuni infection (32–34), we measured whether our CPS-CRM vaccine generated SBA responses. We developed an improved flow cytometric-based SBA (F-SBA) to measure responses in mice and NHPs (described in detail in the supplementary material and in Fig. S1). No C. jejuni F-SBA activity was detected in mouse serum prior to immunization (data not shown). After three immunizations with the CPS-CRM vaccine at 1.0, 2.5, or 5.0 µg, no SBA activity was detected in the serum of mice that received the vaccine without an adjuvant (Fig. 2D). Conversely, functional F-SBA titers were observed in animals vaccinated with CPS-CRM + ALF or ALFQ adjuvants, which is consistent with an increased production of Th1-mediated CPS-specific IgG2b and IgG2c antibody responses in the ALF and ALFQ groups. Indeed, we observed significant correlation of F-SBA and anti-CPS IgG2b or IgG2c titers (see Fig. S2 in the supplemental material). A weaker correlation was observed between anti-CPS IgG1 and F-SBA.
A flow cytometric-based SBA using PI to measure CJ viability after exposure of 81-176 cells to sera from mice or NHP immunized with a CPS-CRM vaccine. (A) PI staining on untreated or methanol-treated 81-176 cells serve as negative and positive controls for PI staining, respectively. (B) PI staining of BRC-only controls where 81-176 cells were incubated with increasing amounts of BRC without heat-inactivated (HI) serum. (C) PI staining of 81-176 cells exposed to immune CPS-CRM + ALFQ HI mouse serum in the presence or absence of 6% BRC. Dot plots show representative PI staining on 81-176 cells from one experiment and are representative of at least two experiments. Numbers within the plot represent the percentage of PI+ 81-176 cells and numbers above the dot plot represent the dilution of HI serum. (D) F-SBA endpoint titers calculated by four-parameter logistic regression analysis. The % PI+ 81-176 cells (% PI+ 6% BRC + HI-sera – % PI+ 0% BRC HI-serum alone) is plotted versus the log10 serum dilution and IC50 is calculated using 4PL analysis as the F-SBA titer. The plot shows representative 4PL in sera from mice immunized with CPS-CRM, CPS-CRM + ALF, or CPS-CRM + ALFQ and analyzed on the same day. LOD of the assay of %PI+ 6% BRC alone + 3 standard deviations (SD) is shown as a dashed black line. (E) F-SBA 4PL analysis of NHP serum from an animal immunized with CPS-CRM + ALFQ and F-SBA titers measured against four different CJ strains: 81-176, CG8421, 81-176 kpsM mutant, and CG8486 (HS4 CPS type). The LOD of % PI+ 4% BRC alone + 3 SD is shown for each strain in dotted line corresponding to strain color in the legend. Download FIG S1, TIF file, 1.1 MB (1.1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Correlation between F-SBA and CPS-specific IgG1 (A), IgG2b (B), and IgG2c (C) antibody titers in mice. Log10 transformed titers were analyzed and Pearson correlation coefficient and P values are displayed within the graph. Download FIG S2, EPS file, 0.2 MB (163.8KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
ALFQ enhances development of multifunctional CD4+ T cell responses.
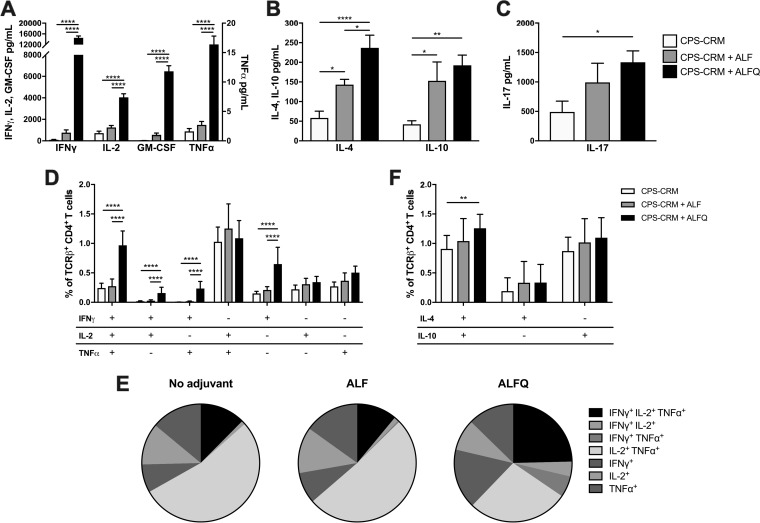
To investigate T cell responses in mice immunized with CPS-CRM ± ALF or ALFQ, we isolated spleens from animals 2 weeks after the third immunization and restimulated splenocytes with CPS-CRM to measure CRM-specific T cell responses. After 3 days, the Th1, Th2, and IL-17 cytokine levels were measured in the culture supernatant. Mice immunized with CPS-CRM + ALFQ showed superior production of Th1 cytokines gamma interferon (IFN-γ), interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α) compared to vaccine alone or CPS-CRM + ALF (Fig. 3A). No difference in Th1 cytokine production was observed between the vaccine-alone and CPS-CRM + ALF groups, indicating that the inclusion of QS-21 in the ALFQ adjuvant was responsible for the considerable enhancement of Th1 cytokine production. Both ALF and ALFQ augmented production of the Th2 cytokines IL-4 and IL-10 compared to vaccine alone (Fig. 3B). Higher levels of IL-4 were observed in ALFQ-adjuvanted animals compared to both vaccine-alone and CPS-CRM + ALF treatment groups. Similar levels of IL-10 were observed in ALF- and ALFQ-adjuvanted groups. Both ALF and ALFQ induced higher levels of IL-17 production compared to vaccine alone, but only the levels induced by ALFQ were significantly higher (Fig. 3C).
FIG 3.
ALFQ enhances T cell responses to the CPS-CRM vaccine in mice. Splenocytes were harvested 2 weeks after the third vaccination to measure T cell-mediated cytokine production. Splenocytes were cultured for 72 h with medium alone or restimulated with CPS-CRM, and Th1 (A), Th2 (B), and IL-17 (C) cytokines were measured in culture supernatants. Cytokine levels were normalized by subtracting medium-alone from CPS-CRM-stimulated samples. (D) Flow cytometric phenotypic analysis of IFN-γ, TNF-α, and IL-2-producing CD4+ TCRβ+ T cells in CPS-CRM-restimulated splenocytes. (E) Pie charts show the distribution of Th1-cytokine-producing cells among samples for vaccine-alone/no adjuvant or CPS-CRM delivered with ALF or ALFQ determined by phenotypical analysis. (F) Flow cytometric phenotypic analysis of IL-4- and IL-10-producing CD4+ TCRβ+ T cells in CPS-CRM-restimulated splenocytes. In all graphs, data for 1.0-, 2.5-, and 5.0-µg CPS-CRM dose levels were combined. Bar graphs represent the means plus the standard deviations of n = 15 for vaccine alone or for CPS-CRM + ALF or ALFQ. Statistical significance between groups was determined by ordinary one-way ANOVA with multiplicity-adjusted P values (*, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001).
Concurrent with cytokine analysis in the supernatant, we stimulated splenocytes to characterize CD4+ Th1 and Th2 cell subsets. Consistent with the very high levels of IFN-γ detected in CPS-CRM-restimulated supernatants of CPS-CRM + ALFQ-immunized mice, we observed a significantly higher frequency of IFN-γ-producing CD4+ T cell subsets by flow cytometry (Fig. 3D). Significantly higher frequencies of CD4+ IFN-γ+ single cytokine-producing cells, double cytokine-producing IFN-γ+ IL-2+ and IFNγ+ TNF-α+ cells, and IFN-γ+ IL-2+ TNF-α+ triple-producing CD4+ T cells were detected compared to both vaccine-alone and CPS-CRM + ALF groups. As a whole, very little difference in CD4+ Th1 cell phenotypes was observed between nonadjuvanted mice and mice adjuvanted with ALF (Fig. 3E), and similar frequencies of Th1 cytokine-producing cells were generated (Fig. S3). The most striking difference in CD4+ T cell phenotypes induced in ALFQ-adjuvanted mice was IFN-γ+ IL-2+ TNF-α+ triple-producing CD4+ T cells (Fig. 3E; black pie slice), and a greater frequency of total CD4+ Th1 cytokine-producing cells was observed (Fig. S3). We also analyzed CD4+ Th2 cells and found that ALFQ induced higher frequencies of IL-4+ IL-10+ double-producing cells compared to vaccine alone, but not higher frequencies of single cytokine-producing CD4+ Th2 cells compared to vaccine alone or ALF-adjuvanted groups (Fig. 3F). Taken together, the cytokines in the supernatant and the flow phenotypic analysis data support that inclusion of QS-21 in the ALFQ liposome induces the most robust CRM-specific CD4+ T cell responses.
Analysis of the total frequency of Th1 cytokine-producing CD4+ T cells in vaccinated mice. Splenocytes were restimulated with CPS-CRM and stained for flow cytometric phenotypic analysis of IFN-γ-, TNF-α-, and IL-2-producing CD4+ TCRβ+ T cells. The total frequencies of CD4+ TCRβ+ T cells producing IFN- γ, TNF-α, and IL-2 for each individual mouse (n = 15 per group) are displayed, where the horizontal line indicates the mean + the SD of the group. 1.0-, 2.5-, and 5.0-µg CPS-CRM dose levels were combined. Ordinary one-way ANOVA with multiplicity-adjusted P values for statistical significance were determined (****, P ≤ 0.0001). Download FIG S3, EPS file, 0.1 MB (119KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
ALFQ enhances immunogenicity and efficacy of CPS-CRM in A. nancymaae.
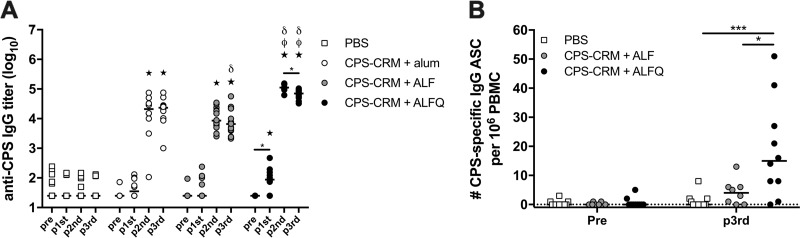
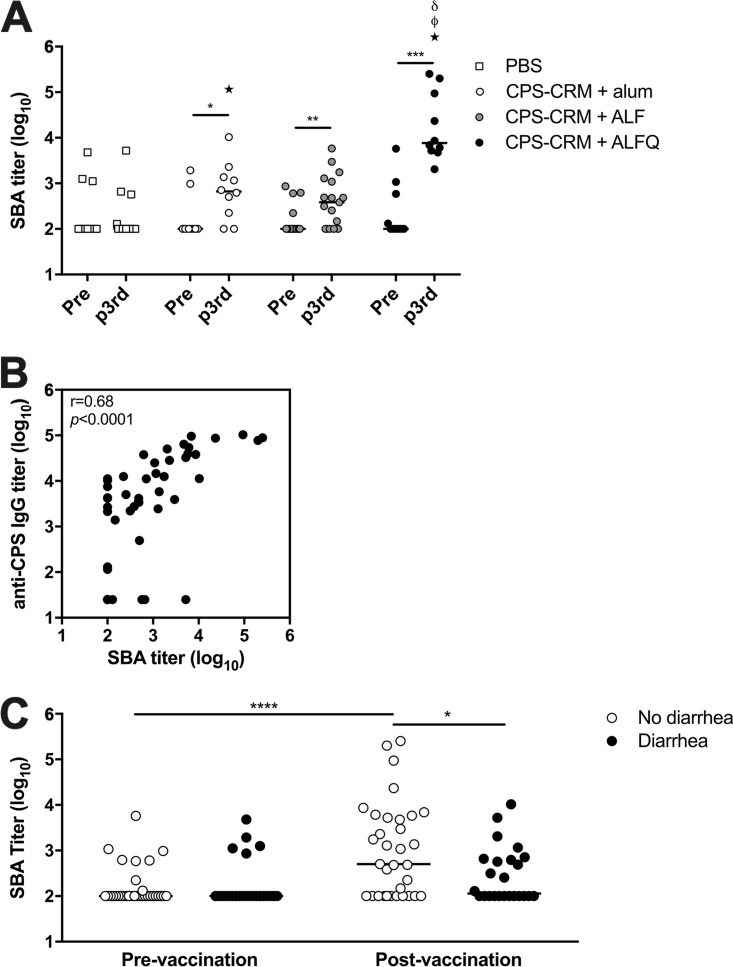
ALF and ALFQ adjuvants showed substantial enhancement to CPS-CRM vaccine immunogenicity in our mouse model; however, a reliable wild-type mouse model for C. jejuni-mediated diarrhea has only recently been published and has not yet been tested for vaccine efficacy (35). We have previously developed an A. nancymaae NHP C. jejuni challenge model with HS23/36-expressing strain 81-176 (36) and demonstrated that our prototype CPS-CRM conjugate vaccine is protective when delivered with alum subcutaneously (s.c.) in three doses at 6-week intervals (18). This same strain 81-176 has been utilized in human controlled human infection models (CHIMs); however, since the discovery that LOS mimicry causes GBS and since strain 81-176 expresses LOS capable of inducing this mimicry, 81-176 can no longer be utilized in CHIMs (37, 38). We developed an alternative CHIM with an HS23/36-expressing strain CG8421 (39) and also established an A. nancymaae model to unite both human and primate studies. Here, we evaluated the immunogenicity and protective efficacy of our CPS-CRM vaccine adjuvanted with alum, ALF, or ALFQ against CG8421 challenge. For logistic purposes, animals were divided among two separate cohorts. For comparison against the historic vaccine experiment (18), we immunized NHP with 3.5 µg of CPS-CRM + alum s.c. We delivered 3.5 µg of CPS-CRM ± ALF or ALFQ or phosphate-buffered saline (PBS) sham i.m. All groups received three doses at 4-week intervals, which is consistent with the dosing schedule in our mouse model. Anti-CPS IgG titers developed in animals immunized with CPS-CRM + alum after two doses, and no boost was observed after the third dose (Fig. 4A). Titers were significantly higher than PBS sham-immunized animals after the second and third doses. In NHPs treated with CPS-CRM + ALF, similar levels of anti-CPS IgG were observed compared to alum-adjuvanted animals after the second dose, and titers decreased slightly between the second and third dose of CPS-CRM + ALF. Titers were significantly lower in ALF-adjuvanted animals compared to alum-adjuvanted animals after the third dose, although the difference was not striking (mean log10 titer of 4.2 in alum versus 3.8 in the ALF group). The most prominent enhancement of CPS-specific IgG was observed in animals immunized with CPS-CRM + ALFQ (Fig. 4A). After a single immunization, anti-CPS IgG titers were significantly higher than baseline and PBS-immunized animals. After two doses, titers were significantly higher than both alum- and ALF-adjuvanted groups (mean log10 ALFQ titer of 5.1). Although we observed a small but significant drop in titers between doses 2 and 3 in ALFQ-adjuvanted animals (log10 titer of 5.1 versus 4.8), the titers remained higher than both alum- and ALF-treated animals at the same time point.
FIG 4.
ALFQ significantly enhances the CPS-specific antibody responses in A. nancymaae immunized with CPS-CRM. NHPs were immunized three times at 4-week intervals with PBS or 3.5 µg of CPS-CRM coadministered with alum, ALF, or ALFQ. Animals were divided among two cohorts. (A) Serum anti-CPS IgG titers were measured pre- and postvaccination in individual animals from both cohorts. Log10 titers of individual animals are shown, where the horizontal line indicates median of the group (PBS, n = 20; alum, n = 10; ALF, n = 17; ALFQ, n = 10). Statistical differences among each group were determined by repeated-measures one-way ANOVA with multiplicity-adjusted P values (*, P ≤ 0.05). Statistical significance between groups was determined by ordinary one-way ANOVA with multiplicity-adjusted P values. Stars indicate significant differences from PBS, δ symbols indicate significantly different from alum, and ϕ symbols indicate significant differences from ALF (P ≤ 0.05). (B) Number of CPS-specific IgG ASCs detected in peripheral blood prevaccination and 7 days after the third vaccination in cohort 2 NHPs immunized with PBS or CPS-CRM + ALF or ALFQ. The numbers of ASCs per 106 PBMC of individual animals are shown, where the horizontal line indicates the median of the group. Ordinary one-way ANOVA with multiplicity-adjusted P values for statistical significance between groups were determined (*, P ≤ 0.05; ***, P ≤ 0.001).
In a cohort that included a PBS sham-, ALF-, and ALFQ-vaccinated NHPs, we had the opportunity to measure CPS-specific IgG antibody-secreting cells (ASCs) in peripheral blood by enzyme-linked immunospot (ELISPOT) assay (Fig. 4B). At 7 days after the third immunization, CPS-specific IgG ASCs were detectable in ALF-adjuvanted NHPs at levels higher than in PBS-immunized animals, although the difference was not statistically significant (values for CPS-specific IgG ASCs per 106 peripheral blood mononuclear cells (PBMC): PBS [median, 0; range, 0 to 8] and ALF [median, 4; range, 0 to 13]). Consistent with the highest levels of anti-CPS IgG observed in the serum after vaccination, NHPs immunized with CPS-CRM + ALFQ had significantly higher levels of CPS-specific IgG ASCs compared to animals immunized with PBS and CPS-CRM + ALF (ASCs: ALFQ [median, 15; range, 0 to 51]).
All sham-immunized and CPS-CRM-immunized animals were challenged with C. jejuni strain CG8421 at 4 weeks after the last immunization and monitored for diarrhea development (Table 1). The attack rate in PBS-immunized animals was 70%, as expected. The protective efficacy (PE) of NHPs immunized with CPS-CRM + alum was 29%, which is lower than previously reported for our prototype HS23/36 conjugate delivered at approximately 2.5 µg (based on CPS, not the total conjugate weight) with alum at 6-week intervals (18). Enhanced protective efficacies were observed with the liposome adjuvants, where ALF-adjuvanted animals showed 66% PE, and ALFQ-adjuvanted animals showed 86% PE. No differences in diarrhea duration, levels of colonization or duration of colonization were observed among the all groups.
TABLE 1.
Protective efficacy of the CPS-CRM vaccine in A. nancymaae NHPs using various adjuvants
| Group | No. of animals | Diarrhea attack rate, n (%) | Protective efficacy against diarrhea (%)a | Pb |
|---|---|---|---|---|
| CPS-CRM + alum | 10 | 5 (50) | 29 | 0.43 |
| CPS-CRM + ALF | 17 | 4 (24) | 66 | 0.008 |
| CPS-CRM + ALFQ | 10 | 1 (10) | 8 | 0.005 |
| PBS | 20 | 14 (70) |
Protective efficacy was calculated as follows: [(attack rate of PBS-treated animals − attack rate of vaccinated animals)/attack rate of PBS-treated animals] × 100.
Determined using a Fisher exact test with no adjustment for multiple comparisons.
ALFQ induces high levels of functional CPS-specific bactericidal antibodies in NHPs that are associated with protection from C. jejuni-mediated diarrhea.
Because we observed enhanced PE in NHPs that were immunized with CPS-CRM + ALF or CPS-CRM + ALFQ, we measured F-SBA responses before and after the third immunization to look for association of bactericidal activity with protection from diarrhea (Fig. 5A). A few animals in each group had background F-SBA titers against C. jejuni strain 81-176 detectable before vaccination potentially indicating a previous environmental exposure to C. jejuni. No change in bactericidal activity was observed between pre- and postvaccination in PBS sham-immunized NHPs. CPS-CRM + alum-vaccinated animals developed F-SBA titers against C. jejuni strain 81-176 after the third dose, where 50% (5/10) of the NHPs were classified as responders by a 4-fold rise in F-SBA titers from prevaccination, and 41% (7/17) of ALF-adjuvanted animals developed F-SBA responses. ALFQ-adjuvanted animals showed the most robust increase in F-SBA responses after three vaccinations with a 100% (10/10) response rate and an average of 499-fold rise in bactericidal activity over baseline (fold increase, 6 to 2,524). Although alum- and ALF-adjuvanted animals had higher F-SBA titers compared to PBS controls after vaccination, ALFQ-adjuvanted NHPs had significantly higher titers than did the PBS-, alum-, or ALF-treated groups (147-, 35-, and 64-fold higher, respectively).
FIG 5.
Functional antibody responses are induced in A. nancymaae immunized with CPS-CRM. (A) Functional antibody responses were measured by F-SBA against strain 81-176 in NHPs prevaccination and after the third vaccination. Log10 F-SBA titers of individual animals are shown, where the horizontal line indicates the median of the group. Paired t tests were performed to determine the significance between pre- and post-third vaccinations (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Statistical significance between groups was determined at each time point by ordinary one-way ANOVA with multiplicity-adjusted P values. Stars indicate significant differences from PBS, δ symbols indicate significant differences from alum, and ϕ symbols indicate significant differences from ALF (P ≤ 0.05). (B) Pearson correlation analysis of F-SBA responses after the third vaccination versus anti-CPS IgG responses. The correlation coefficient and P value are shown within the plot. (C) Association of functional F-SBA titer with disease outcome. Pre- and postvaccination F-SBA titers were analyzed in animals with or without diarrhea after challenge with CJ strain CG8421 (paired t test for significance; ****, P ≤ 0.0001; unpaired t test for significance; *, P ≤ 0.05).
We verified the HS23/36 CPS specificity of the F-SBA response in a number of NHP responders by measuring bactericidal activity against an 81-176 mutant C. jejuni strain that does not express CPS (kpsM mutant) and in strain CG8486, which expresses an unrelated CPS of the HS4 type (16, 18, 40). Bactericidal activity is readily detected against the HS23/36 CPS+ 81-176 strain; however, there is no F-SBA response against the CPS− kpsM mutant or against the unrelated CG8486 strain (Fig. S1E). In addition, similar F-SBA titers were generated against the challenge strain CG8421, which also expresses a HS23/36 CPS type (Fig. S1E). Importantly, F-SBA titers measured after the third vaccination show a significant correlation with anti-CPS IgG titers measured at the same time point (Fig. 5B). To investigate the effect of bactericidal activity on disease outcome, we compared the F-SBA titers before and after the third vaccination to whether the animals did or did not develop diarrhea and found that there was a statistically significant trend toward higher F-SBA titers in animals who did not develop diarrhea after challenge (Fig. 5C). These data suggest that CPS-specific bactericidal activity may play a role in protection against C. jejuni-mediated diarrhea in the A. nancymaae model.
DISCUSSION
Given the previous lack of immunogenicity with a C. jejuni conjugate delivered with or without alum in humans, we sought here to identify potential immunomodulatory adjuvants to enhance both humoral and cellular immune responses to CPS conjugate vaccines in animal models. We evaluated the Army Liposome Formulation adjuvants, ALF and ALFQ, admixed with soluble CPS-CRM or the conjugate vaccine adsorbed to alum before combination with the liposome adjuvants forming ALFA or ALFQA. Initial analysis compared these adjuvants to CPS-CRM + alum. Although alum has been the adjuvant that has dominated the vaccine field for decades, there are now a number of potent adjuvants that are being evaluated and even licensed for human use. Most notably, the proprietary adjuvant system AS01 has been licensed as Shingrix (zoster vaccine recombinant; GlaxoSmithKline). AS01 is a liposome-based adjuvant formulation containing MPLA and QS-21 that is similar to the liposome adjuvants utilized in this study, specifically ALFQ. The two adjuvants that best enhanced immunogenicity to the C. jejuni CPS conjugate in mice and NHPs were ALF and ALFQ. Both ALF and ALFQ contain the TLR4 agonist MPLA, and ALFQ also incorporates QS-21. Although alum induced high anti-CPS IgG titers in mice at a low dose of 0.1 µg of CPS-CRM, the response was dominated by IgG1, whereas ALF and ALFQ induced IgG2b and IgG2c CPS responses at all doses of CPS-CRM evaluated. These data are consistent with reports that MPLA favors induction of IFN-γ-producing CD4+ T cells and antibody class switching to Th1-mediated subtypes (41–44). In preliminary studies, the addition of alum to ALF and ALFQ to form ALFA and ALFQA in mice, respectively, did not provide an advantage over ALF and ALFQ and were not pursued for further analysis.
Dose escalation of the CPS-CRM vaccine with ALF and ALFQ in mice showed that high IgG titers were achieved at 1.0 µg of CPS-CRM and were not significantly increased by escalating the levels to 2.5 or 5.0 µg. These data suggest that potent adjuvants like ALF or ALFQ may allow dose sparing of polysaccharide conjugate vaccines, which is a noteworthy consideration for a multivalent C. jejuni conjugate vaccine. Our current estimate of the valency required for an effective C. jejuni CPS vaccine would be 8 (45; F. Poly et al., unpublished data). This is likely achievable based upon the successful licensure of the S. pneumoniae CPS conjugate vaccine Prevnar13, where the total amount of capsule delivered is approximately 30.8 µg (4.4 µg of serotype 6B saccharides and 2.2 µg of each of the remaining 12 serotypes). Future studies will test whether inclusion of ALF or ALFQ with a multivalent C. jejuni conjugate vaccine might allow dose sparing of each CPS conjugate. One notable difference in the antibody response at the high 5-µg dose of CPS-CRM + ALFQ was the generation of high levels of anti-CPS IgA antibodies (mean log10 titer of 4.29). These IgA levels are higher than we had previously observed in mice vaccinated with CPS-CRM (18), although the IgA levels were lower than the IgG levels. It is not clear whether CPS-specific mucosal IgA can play a role in protection against C. jejuni-mediated disease since we did not measure fecal IgA. However, serum anti-CPS IgA was undetectable in CPS-CRM + ALFQ-vaccinated NHPs (data not shown), where we observed 86% PE against diarrhea, suggesting that IgA may not be important for protection in this NHP model. Future studies will further investigate the role of anti-CPS IgA in the mouse and NHP models by measuring mucosal IgA responses.
Previous work with the prototype CPS-CRM vaccine demonstrated that the vaccine alone at approximately 2.5 µg (based on CPS weight, 25 µg of total conjugate weight) at 4-week intervals was protective against strain 81-176 in a mouse intranasal challenge model and 2.5 µg of CPS-CRM + alum delivered s.c. at 6-week intervals was 100% efficacious against diarrhea in A. nancymaae NHPs (18). In this study, we observed reduced protective efficacy against diarrhea (29% PE) in the group given 3.5 µg of CPS-CRM + alum and challenged with strain CG8421, but what caused reduced PE compared to the historic experiments with strain 81-176 is not clear. In this study, vaccination intervals were reduced from 6 to 4 weeks to mirror intervals in the mouse studies and to align with 4-week intervals used in the CJCV1 phase 1 clinical study. Interestingly, reduced vaccination intervals had a positive effect on anti-CPS IgG titers after the third vaccination in CPS-CRM + alum-treated animals with a mean log10 titer of 4.2 at 4-week intervals compared to 2.6 (loge titer of 6) in the historic experiment (18). PE was measured against a different C. jejuni strain CG8421 in this study, although the CPS type expressed by CG8421 is also of the HS23/36 type, and we have observed cross-reactivity of anti-CPS antibodies between 81-176 and CG8421 in vaccinated animals by F-SBA, Western blotting, and flow cytometry binding experiments (Fig. S1E and data not shown). Nonetheless, ALF and, to a greater extent, ALFQ enhanced the immunogenicity of the conjugate vaccine in both animal models. In NHPs, CPS-CRM + ALFQ generated the highest levels of anti-CPS IgG, CPS-specific ASCs, and the highest F-SBA titers.
We currently do not know what immune mechanism(s) provide protection from C. jejuni-mediated disease in the NHP model or following human infection. Humoral responses certainly play a role in protection as evidenced by persistent C. jejuni infections in agammaglobulinemic and immunocompromised individuals (46, 47). Bactericidal activity mediated by complement activation by C. jejuni-specific antibodies in serum has been reported (32–34). SBA appears as early as 36 to 48 h after the onset of diarrhea and can be detected in convalescent-phase sera 40 days later (34). Capsule conjugate vaccines have been shown to induce bactericidal activity against other Gram-negative pathogens (48–50). We hypothesize that similar to Neisseria meningitidis, Haemophilus influenzae type B, and Shigella conjugate vaccines, the C. jejuni CPS conjugate vaccine induces functional antibody titers against the CPS that can be measured in a bactericidal assay. Here, we report that SBA responses develop against the homologous 81-176 strain in mice and NHPs immunized with the CPS-CRM conjugate vaccine and that higher SBA titers in NHPs are associated with protection from diarrhea. Unlike other studies that utilize culture-based SBA methods to measure bactericidal activity, we have developed a flow cytometry-based SBA assay that allows rapid, high-throughput analysis. Due to the specialized culture requirements for C. jejuni growth, we experienced problems developing a culture-based SBA assay where multiple repeats were necessary due to control failures. The F-SBA method is time-conserving and serum-sparing. These methods can be easily adapted to measure bactericidal responses against different C. jejuni strains, other bacteria, and in multiple animal models and is currently being developed for human sera. We report here that the CPS-CRM vaccine delivered without an adjuvant does not induce functional bactericidal responses in mice. Only with the addition of ALF and ALFQ does SBA activity develop, likely due to the generation of CPS-specific IgG2b and IgG2c that are present in ALF- and ALFQ-adjuvanted mice, but not in animals receiving vaccine alone. We observed a significant correlation between SBA and anti-CPS IgG2b and IgG2c titers (P ≤ 0.0001; Pearson r = 0.60 and 0.62, respectively). In NHPs, CPS-CRM delivered with alum, ALF, and ALFQ induced bactericidal activity against 81-176. The SBA activity was CPS specific since F-SBA titers correlated with the anti-CPS IgG response and higher SBA titers were associated with protection from diarrhea after three immunizations. Because A. nancymaae is an understudied NHP model, IgG subclasses have not yet been identified, and there are no reagents available to measure these responses. No SBA responses were measurable against a nonencapsulated 81-176 mutant, indicating that anti-CPS antibodies alone are able to bind CPS and activate the complement cascade to kill wild-type C. jejuni. Although complement-dependent activity was previously described following infection in humans (33, 34), the target(s) of the functional antibody response remains unidentified. Antigens suggested as targets include outer membrane proteins and flagellin, although there is no direct evidence that antibodies specific for any one C. jejuni protein are bactericidal. C. jejuni’s outermost surface is covered by CPS, and we report here for the first time that vaccination with a conjugate vaccine induces CPS-specific antibodies with bactericidal activity against C. jejuni. Future studies with multivalent C. jejuni capsule conjugate vaccine will measure SBA responses against multiple CPS types and look for potential association with protection.
One of the most striking effects of the ALF liposome adjuvants was their effect on T cell responses measured in mice. While it was evident from the production of anti-CPS IgG2b and IgG2c titers measured in the serum that both ALF and ALFQ induced Th1 cell development, phenotypic analysis revealed a striking difference in the quantity and quality of the T cell response between these two adjuvants. The inclusion of QS-21 in the ALFQ adjuvant significantly enhanced the development of multifunctional CD4+ T cell responses to the carrier protein CRM in mice to a greater extent than ALF. ALFQ induced higher levels of Th1 cytokines compared to vaccine alone and vaccine plus ALF, with the most notable increase observed in IFN-γ+ IL-2+ TNF-α+ triple-producing Th1 cells. Interestingly, ALFQ also induced higher levels of IL-4+ IL-10+ Th2 cells and the production of IL-17 measured in cell supernatants. Further phenotypic analysis is needed to determine whether ALFQ specifically induces Th17 cell development or if IL-17 production is being produced by a different cell subset. Nonetheless, this study sheds light on ALFQ as a potent adjuvant to enhance T cell responses. We were unable to measure T cell responses in A. nancymaae due to limited sample availability and because few T cell assays and reagents have been developed for this NHP model. Nonetheless, enhanced T cell responses in A. nancymaae are inferred in this model in CPS-CRM + ALFQ-immunized animals, as evidenced by higher CPS-specific IgG levels and significantly higher numbers of ASCs measured in peripheral blood. Future work in clinical studies will focus on defining T cell responses to CPS-CRM plus ALFQ to include Th1, Th2, and Th17 development, as well as antigen-specific T follicular helper responses.
Taken together, these studies demonstrate that ALF and ALFQ liposome-based adjuvants are compatible with a bacterial polysaccharide conjugate vaccine platform. ALF and, to a greater extent, ALFQ significantly enhanced anti-polysaccharide antibody responses, which lends promise to their usefulness to induce immunity to polysaccharide antigens in traditionally difficult populations such as children or the elderly. In addition, ALFQ administration with CRM-containing conjugate vaccines may overcome any immune cell anergy or interference in humans from earlier administration of childhood diphtheria-containing vaccines. CRM197 was chosen for our prototype C. jejuni polysaccharide conjugate vaccine due to its well-characterized and safe use as a licensed carrier protein. There are now many reports using alternate carrier proteins in an effort to include other important antigenic targets and expand the effectiveness of conjugate vaccines (51–54). The administration of ALFQ with our recently developed multipathogen conjugate vaccine platform combining C. jejuni and Shigella polysaccharides with recombinant enterotoxigenic Escherichia coli tip-adhesin proteins (55) has great potential to enhance vaccine-mediated immunity to not only C. jejuni but also two other important enteric pathogens.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed under an Institutional Animal Care and Use Committee (IACUC) approved protocol at the Naval Medical Research Center (mouse) or NAMRU-6, Peru (A. nancymaae), in compliance with all applicable Federal regulations governing the protection of animals in research.
Bacterial strains and growth conditions.
C. jejuni strains 81-176 and CG8421 have been described previously, and both express an HS23/36 CPS type (37). For the bactericidal assays, C. jejuni strain 81-176 was grown on Muller-Hinton (MH) agar plates (MH broth [21 g/liter] and Bacto agar [15 g/liter]; BD, Sparks, MD) at 37°C in a microaerobic environment (nitrogen, 85%; carbon dioxide, 10%; oxygen, 5%) for 20 h. Cells were harvested in dextrose-gelatin-Veronal buffer (DGV; Lonza, Walkersville, MD) and set to an optical density at 600 nm of 0.1, equivalent to a concentration of 3 × 108 CFU/ml. For the A. nancymaae challenge, C. jejuni strain CG8421 was grown on MH plates at 42°C under microaerobic conditions and expanded over a 2-day period. Bacteria from MH plates were pooled in ice-cold PBS, and the concentration was adjusted photometrically with PBS to reach the target dose of 5 × 1011 CFU in 5 ml. The actual doses determined by serial dilution onto MH plates were 4.3 × 1011 and 4.5 × 1011 CFU in cohorts 1 and 2, respectively.
Capsule conjugate vaccine.
Capsules were extracted from strain 81-176 and conjugated to CRM197 as previously described (18). The CPS content was determined by anthrone assay (56), and the protein content was determined by a bicinchoninic acid assay. Two lots of vaccines (batch 4 and CJCV1) were produced by Dalton Pharma Services (Toronto, Ontario, Canada) under contract.
Vaccine and adjuvant formulations.
The following CPS-CRM vaccines were utilized: lot CJCV1 for all mouse studies and lot batch 4 for A. nancymaae studies. Lyophilized CPS-CRM was reconstituted in sterile water to an isosmotic concentration. ALF and ALF plus QS-21 (ALFQ) were prepared by the U.S. Military HIV Research Program and have been described previously (21, 57, 58). Mouse doses of ALF and ALFQ contained 20 µg/mouse 3D-PHAD and 10 µg/mouse QS-21. A. nancymaae doses of ALF and ALFQ contained 50 µg/NHP 3D-PHAD and 25 µg/NHP QS-21. For formulation with ALF or ALFQ, reconstituted CPS-CRM was added to dried liposomes or liquid formulation, respectively. For ALFA and ALFQA formulations, 0.1 µg of reconstituted CPS-CRM was adsorbed to 30 µg of alum (Brenntag Biosector; Brenntag, Frederikssund, Denmark) for 1 h at room temperature before being added to dried ALF or liquid ALFQ liposomes. All ALF, ALFQ, ALFA, and ALFQA formulations were vortexed at a slow speed for 10 min at room temperature and then incubated at 4°C for an additional hour with occasional shaking. Mouse vaccines were delivered in a total volume of 50 µl per dose. Each CJCV1 conjugate dose contained a total of 0.1, 1.0, 2.5, or 5.0 µg based on CPS weight. A. nancymaae vaccines were delivered in a total volume of 500 µl per dose for CPS-CRM + alum and 250 µl per dose for the PBS sham, CPS-CRM + ALF, or CPS-CRM + ALFQ treatment groups. Each CPS-CRM batch 4 conjugate dose contained a total of 3.5 µg based on CPS weight. A total of 300 µg/NHP of alum was absorbed to CPS-CRM (batch 4) in the A. nancymaae study.
Animal immunizations.
Groups of five 6- to 8-week-old female C57BL6/J mice (The Jackson Laboratory, Bar Harbor, ME) were immunized i.m. in alternating rear thighs at 0, 4, and 8 weeks. Mice were tail bled 2 weeks after each immunization and bled by cardiac puncture without recovery 2 weeks after the third immunization. A. nancymaae NHPs (captive-born) were purchased from the Instituto Veterianario de Investigaciones Tropicales y de Altura (Iquitos, Peru). Male and female animals (29 male, 28 female; average age, 15 months; weight, 670 to 980 g) were randomized into vaccine or control groups before immunization. The animals were stool culture negative for Campylobacter and seronegative (i.e., IgG titer < 1:400) against glycine-extracted surface antigens of C. jejuni strain CG8421, as measured by enzyme-linked immunosorbent assay (ELISA) (59). Prior to immunizations or blood draw, NHPs were anesthetized with ketamine hydrochloride (10 mg/kg). Vaccine plus ALF or ALFQ or PBS was administered i.m. in 250 µl in alternating thighs, and vaccine plus alum was administered s.c. at 0, 4, and 8 weeks. NHP studies were separated into two cohorts due to facility size restrictions: cohort 1 was composed of CPS-CRM + alum (n = 10), CPS-CRM + ALF (n = 10), or PBS (n = 9) treatments, and cohort 2 was composed of CPS-CRM + ALF (n = 7), CPS-CRM + ALFQ (n = 10), or PBS (n = 11) treatments.
Oral challenge of A. nancymaae with CG8421.
NHPs were challenged with 5 × 1011 CFU of strain CG8421 delivered in 5.0 ml saline via an oral-gastric tube with procedures as previously described for challenge with C. jejuni strain 81-176 (18, 36). Stools were monitored three times daily for 10 days for signs of diarrhea. Animals with two consecutive days of loose to watery stools met the endpoint criteria of diarrhea. C. jejuni colonization was monitored daily for 10 days by serially diluting stool in PBS and cultured on BBL Campylobacter CSM agar (BD) with CCDA selective supplement (Thermo Fisher, Waltham, MA) under microaerobic conditions at 42°C for 48 h.
Anti-CPS ELISA.
Anti-CPS total IgG or IgG subclass analysis was performed using oxidized CPS and Carbo-BIND plates as previously described (60). The following horseradish peroxidase-conjugated secondary antibodies were used for detection: goat anti-mouse IgG or goat anti-mouse IgA and A. nancymaae anti-CPS IgG responses were measured using goat anti-human IgG (SeraCare, Gaithersburg, MD). Mouse IgG subclass secondary antibodies and isotype controls were purchased from SouthernBiotech (Birmingham, AL).
Mouse splenocyte restimulation and T cell cytokine expression analysis.
Single-cell suspensions from individual mouse spleens were generated, and 5 × 105 cells per well were cultured in duplicate in complete Dulbecco modified Eagle medium (cDMEM) with either cDMEM alone, 5 µg/ml CPS-CRM (based on protein content), or on immobilized 0.5 µg of anti-CD3 (145-2C11) plus 0.1 µg of anti-CD28 (37.51) at 37°C in 5% CO2. Supernatants were harvested after 72 h and analyzed using a Bio-Plex mouse cytokine Th1/Th2 kit spiked with IL-17A, and plates were read using a Bio-Plex 100 (Bio-Rad, Hercules, CA). Cytokine expression was normalized by subtracting the pg/ml in control medium-alone wells from CPS-CRM-restimulated wells.
T cell phenotypic analysis was performed on splenocytes stimulated for 24 h under the conditions described above. After 18 h, GolgiPlug and GolgiStop (BD Biosciences) were added for the last 5 h of culture. Samples were fixed in formaldehyde, Fc blocked with anti-CD16/32 (93), and then surface stained with the antibodies TCRβ (H57-597), CD4 (GK1.5), and CD8α (53-6.7). Cells were permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained intracellularly with IL-2 (JES6-5H4), IL-4 (11B11), IL-10 (JES5-16E3), IFN-γ (XMG1.2), and TNF-α (MP6-XT22). Data were acquired using a LSRFortessa (BD Biosciences) and analyzed using FlowJo 10 software (Tree Star, Ashland, OR). CD4+ TCRβ+ T cells were gated on cytokine positive cells, and Boolean gating analysis was applied to determine multifunctional T cell populations.
A. nancymaae PBMC isolation and ELISPOT analysis.
To isolate PBMCs, whole blood by density gradient centrifugation was performed. Freshly isolated PBMCs were directly used in an ELISPOT assay. Multiscreen HTS plates (Millipore, catalog no. MSIPS4W10) were coated with CPS conjugated to bovine serum albumin at a concentration of 15 μg/ml diluted in 1× PBS overnight at 4°C. Plates were blocked for 1 h with complete RPMI. Freshly isolated PBMCs were added at 0.5 × 106 cells/well, followed by incubation overnight at 37°C and 5% CO2. Plates were washed three times with 1× PBS–0.05% Tween and incubated for 3 h at room temperature with anti-human IgG (SeraCare, catalog no. 474-1006). Spots were developed using TMB substrate (Mabtech, Cincinnati, OH). The number of CPS-specific antigen-secreting cells (ASCs) are expressed as the number of CPS-specific IgG ASCs per 106 PBMC.
Serum bactericidal assay.
Heat-inactivated (HI) sera were serial diluted in DGV in a 96-well plate, and baby rabbit complement (BRC [Cedarlane, Burlington, NC]; 4 or 6% by volume for NHP or mice, respectively) was added to a total volume of 90 μl. Then, 10 μl of 3 × 108 CFU/ml (3 × 106 CFU) 81-176 was added to serum + BRC (final volume, 100 μl), followed by incubation at 37°C for 1 h. Next, 100 μl of a propidium iodide (PI; Molecular Probes, Eugene, OR) dye solution was added directly to the wells, followed by incubation at room temperature for 15 min in the dark. Samples were acquired immediately after PI staining on a FACSCanto II cytometer (BD), and the percentages of PI+ 81-176 cells in controls and experimental SBA samples were analyzed using FlowJo. The data were normalized to the control for the amount of nonspecific killing by HI sera alone by subtracting the percentage of PI+ cells in the HI sera without BRC (0%) from the percentage of PI+ cells in HI sera plus BRC at each serum dilution. The normalized percentage of PI+ cells was plotted versus the log10 serum dilution and analyzed by four-parameter logistic curve fitting model for four-parameter logistic (4PL) analysis using Prism (v7; GraphPad, La Jolla, CA), and the F-SBA titer was defined as the calculated 50% inhibitory concentration (IC50).
Statistics.
Data points were analyzed using GraphPad Prism (v7). ELISA and F-SBA titers were log10 transformed. Paired t tests were used to compare preimmune to postimmune titers. A repeated-measure one-way analysis of variance (ANOVA) with Tukey’s multiplicity-adjusted P values was used to compare titers at different time points among a vaccine group. An ordinary one-way ANOVA with Tukey’s multiplicity-adjusted P values was used to test for statistical significance for data sets with multiple groups. P values if ≤0.05 were considered statistically significant. The Pearson’s correlation coefficient was used to describe the correlation between 81-176 F-SBA and anti-CPS IgG ELISA titers. For all NHP experiments, the proportion of animals with diarrhea in each test group was compared to that in the control PBS group with a Fisher exact test. Frequency analyses were not adjusted for multiple comparisons.
Detailed methods describing flow cytometric-based serum bactericidal assay. Download Text S1, DOCX file, 0.03 MB (30.7KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
ACKNOWLEDGMENTS
This study was funded by Navy Work Unit 6000.RAD1.DA3.A0308.
A.R., R.M.L., F.P., A.J.M., and P.G. designed all experiments and prepared the manuscript. Z.B., G.R.M., and C.R.A. provided expertise and all ALF adjuvant materials. N.M.S., C.L.G., H.E., G.N., N.E., M.N., R.C., and J.R. performed animal experiments and collected and analyzed all data.
The views expressed here are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of the Army, the Department of Defense, or the U.S. government. G.R.M., F.P., and P.G. are/were employees of the U.S. government, and this work was performed as part of their official duties. Title 17 USC 105 provides that “copyright protection under this title is not available for any work of the United States government.” Title 17 USC 101 defines a U.S. government work as a work prepared by an employee of the U.S. government as part of that person’s official duties.
P.G. is a coinventor on a capsule conjugate vaccine patent. C.R.A. is a coinventor on pending patents for ALFQ and ALFA, and Z.B. is a coinventor on ALFQ.
REFERENCES
- 1.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman JC, McCormick BJ, Shrestha S, Samie A, Mahfuz M, Qureshi S, Hotwani A, Babji S, Trigoso DR, Lima AA, Bodhidatta L, Bessong P, Ahmed T, Shakoor S, Kang G, Kosek M, Guerrant RL, Lang D, Gottlieb M, Houpt ER, Platts-Mills JA, Etiology Risk Factors, Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health Development Project Network Investigators. 2016. Epidemiology and impact of campylobacter infection in children in 8 low-resource settings: results from the MAL-ED Study. Clin Infect Dis 63:1171–1179. doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. 2002. Human campylobacteriosis in developing countries. Emerg Infect Dis 8:237–244. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, Investigators M-E. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. 2007. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum 37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuki N. 2010. Human gangliosides and bacterial lipo-oligosaccharides in the development of autoimmune neuropathies. Methods Mol Biol 600:51–65. doi: 10.1007/978-1-60761-454-8_4. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen NP, Kuijf ML, Ang CW, Schiellerup P, Krogfelt KA, Jacobs BC, van Belkum A, Endtz HP, Bergman MP. 2009. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect 11:988–994. doi: 10.1016/j.micinf.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Nachamkin I, Szymanski CM, Blaser MJ. 2008. Campylobacter, 3rd ed. ASM Press, Washington, DC. [Google Scholar]
- 9.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM, Walkerton Health Study Investigators. 2006. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 131:445–450. quiz 660. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Engberg J, Neimann J, Nielsen EM, Aerestrup FM, Fussing V. 2004. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerg Infect Dis 10:1056–1063. doi: 10.3201/eid1006.030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering LK. 2004. Antimicrobial resistance among enteric pathogens. Semin Pediatr Infect Dis 15:71–77. doi: 10.1053/j.spid.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz J, Marco F, Oliveira I, Vila J, Gascon J. 2007. Trends in antimicrobial resistance in Campylobacter spp. causing traveler’s diarrhea. APMIS 115:218–224. doi: 10.1111/j.1600-0463.2007.apm_567.x. [DOI] [PubMed] [Google Scholar]
- 13.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. 2000. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol 35:529–541. [DOI] [PubMed] [Google Scholar]
- 14.Penner JL, Hennessy JN. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol 12:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poly F, Serichantalergs O, Kuroiwa J, Pootong P, Mason C, Guerry P, Parker CT. 2015. Updated Campylobacter jejuni capsule PCR multiplex typing system and its application to clinical isolates from South and Southeast Asia. PLoS One 10:e0144349. doi: 10.1371/journal.pone.0144349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol 40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 17.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, Ferderber JS, Porter CK, Trent MS, Guerry P. 2013. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 81:665–672. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. 2009. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun 77:1128–1136. doi: 10.1128/IAI.01056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poly F, Noll AJ, Riddle MS, Porter CK. 2018. Update on Campylobacter vaccine development. Hum Vaccin Immunother 25:1–12. doi: 10.1080/21645515.2018.1528410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck Z, Matyas GR, Jalah R, Rao M, Polonis VR, Alving CR. 2015. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine 33:5578–5587. doi: 10.1016/j.vaccine.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Genito CJ, Beck Z, Phares TW, Kalle F, Limbach KJ, Stefaniak ME, Patterson NB, Bergmann-Leitner ES, Waters NC, Matyas GR, Alving CR, Dutta S. 2017. Liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013. Vaccine 35:3865–3874. doi: 10.1016/j.vaccine.2017.05.070. [DOI] [PubMed] [Google Scholar]
- 22.Seth L, Bingham Ferlez KM, Kaba SA, Musser DM, Emadi S, Matyas GR, Beck Z, Alving CR, Burkhard P, Lanar DE. 2017. Development of a self-assembling protein nanoparticle vaccine targeting Plasmodium falciparum circumsporozoite protein delivered in three army liposome formulation adjuvants. Vaccine 35:5448–5454. doi: 10.1016/j.vaccine.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Zollinger WD, Babcock JG, Moran EE, Brandt BL, Matyas GR, Wassef NM, Alving CR. 2012. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine 30:712–721. doi: 10.1016/j.vaccine.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 24.Torres OB, Matyas GR, Rao M, Peachman KK, Jalah R, Beck Z, Michael NL, Rice KC, Jacobson AE, Alving CR. 2017. Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. NPJ Vaccines 2:13. doi: 10.1038/s41541-017-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Willingham SB, Ting JP, Re F. 2008. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol 181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, Lien E. 2016. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J Biol Chem 291:1123–1136. doi: 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 1976. Requirements for meningococcal polysaccharide vaccine. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 28.Wong KH, Barrera O, Sutton A, May J, Hochstein DH, Robbins JD, Robbins JB, Parkman PD, Seligmann EB Jr.. 1977. Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand 5:197–215. doi: 10.1016/S0092-1157(77)80005-X. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JD, Edelman R, King JC Jr, Papa T, Ryall R, Rennels MB. 2002. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis 186:1848–1851. doi: 10.1086/345763. [DOI] [PubMed] [Google Scholar]
- 30.Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. 2010. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 6:881–887. doi: 10.4161/hv.6.11.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 1999. Standardization and validation of serological assays for evaluation of immune responses to Neisseria meningitidis serogroup A/C vaccines. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 32.Jones DM, Eldridge J, Dale B. 1980. Serological response to Campylobacter jejuni/coli infection. J Clin Pathol 33:767–769. doi: 10.1136/jcp.33.8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaser MJ, Smith PF, Kohler PF. 1985. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis 151:227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- 34.Pennie RA, Pearson RD, Barrett LJ, Lior H, Guerrant RL. 1986. Susceptibility of Campylobacter jejuni to strain-specific bactericidal activity in sera of infected patients. Infect Immun 52:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giallourou N, Medlock GL, Bolick DT, Medeiros PH, Ledwaba SE, Kolling GL, Tung K, Guerry P, Swann JR, Guerrant RL. 2018. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog 14:e1007083. doi: 10.1371/journal.ppat.1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. 2006. New World monkey Aotus nancymaae as a model for Campylobacter jejuni infection and immunity. Infect Immun 74:790–793. doi: 10.1128/IAI.74.1.790-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, Porter CK, Tribble D, Darsley M, Guerry P. 2008. Characterization of two Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun 76:5655–5667. doi: 10.1128/IAI.00780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, Burg EF, III, Moran AP, Applebee L, Bourgeois AL. 2010. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect. Immun 78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tribble DR, Baqar S, Carmolli MP, Porter C, Pierce KK, Sadigh K, Guerry P, Larsson CJ, Rockabrand D, Ventone CH, Poly F, Lyon CE, Dakdouk S, Fingar A, Gilliland T, Daunais P, Jones E, Rymarchyk S, Huston C, Darsley M, Kirkpatrick BD. 2009. Campylobacter jejuni strain CG8421: a refined model for the study of campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis 49:1512–1519. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 40.Chen YH, Poly F, Pakulski Z, Guerry P, Monteiro MA. 2008. The chemical structure and genetic locus of Campylobacter jejuni CG8486 (serotype HS:4) capsular polysaccharide: the identification of 6-deoxy-d-ido-heptopyranose. Carbohydr Res 343:1034–1040. doi: 10.1016/j.carres.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Casella CR, Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattom A, Li X, Cho YH, Burns A, Hawwari A, Shepherd SE, Coughlin R, Winston S, Naso R. 1995. Effect of conjugation methodology, carrier protein, and adjuvants on the immune response to Staphylococcus aureus capsular polysaccharides. Vaccine 13:1288–1293. doi: 10.1016/0264-410X(95)00052-3. [DOI] [PubMed] [Google Scholar]
- 43.Moore A, McCarthy L, Mills KH. 1999. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17:2517–2527. doi: 10.1016/S0264-410X(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 44.Neuzil KM, Johnson JE, Tang YW, Prieels JP, Slaoui M, Gar N, Graham BS. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525–532. doi: 10.1016/S0264-410X(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 45.Pike BL, Guerry P, Poly F. 2013. Global distribution of Campylobacter jejuni Penner serotypes: a systematic review. PLoS One 8:e67375. doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman AF, Holland SM. 2007. Persistent bacterial infections and primary immune disorders. Curr Opin Microbiol 10:70–75. doi: 10.1016/j.mib.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Johnson RJ, Nolan C, Wang SP, Shelton WR, Blaser MJ. 1984. Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann Intern Med 100:832–834. doi: 10.7326/0003-4819-100-6-832. [DOI] [PubMed] [Google Scholar]
- 48.Kayhty H, Makela O, Eskola J, Saarinen L, Seppala I. 1988. Isotype distribution and bactericidal activity of antibodies after immunization with Haemophilus influenzae type b vaccines at 18-24 months of age. J Infect Dis 158:973–982. doi: 10.1093/infdis/158.5.973. [DOI] [PubMed] [Google Scholar]
- 49.Miller E, Salisbury D, Ramsay M. 2001. Planning, registration, and implementation of an immunization campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20(Suppl 1):S58–S67. doi: 10.1016/S0264-410X(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 50.Riddle MS, Kaminski RW, Di Paolo C, Porter CK, Gutierrez RL, Clarkson KA, Weerts HE, Duplessis C, Castellano A, Alaimo C, Paolino K, Gormley R, Gambillara Fonck V. 2016. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study. Clin Vaccine Immunol 23:908–917. doi: 10.1128/CVI.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels J-P, Schuerman L. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a double blind efficacy study. Lancet 367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 52.Yang H-H, Mascuch SJ, Madoff LC, Paoletti LC. 2008. Recombinant group B streptococcus alpha-like protein 3 is an effective immunogen and carrier protein. Clin Vaccine Immunol 15:1035–1041. doi: 10.1128/CVI.00030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baraldo K, Mori E, Bartoloni A, Petracca R, Giannozzi A, Norelli F, Rappuoli R, Grandi G, Del Giudice G. 2004. N19 polyepitope as a carrier for enhanced immunogenicity and protective efficacy of meningococcal conjugate vaccines. Infect Immun 72:4884–4887. doi: 10.1128/IAI.72.8.4884-4887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szu SL, Schneerson R, Vickers JH, Bryla D, Robbins JB. 1989. Comparative immunogenicities of Vi polysaccharide protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high or lower molecular weight Vi. Infect Immun 57:3823–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laird RM, Ma Z, Dorabawila N, Pequegnat B, Omari E, Liu Y, Maue AC, Poole ST, Maciel M, Satish K, Gariepy CL, Schumack NM, McVeigh AL, Poly F, Ewing CP, Prouty MG, Monteiro MA, Savarino SJ, Guerry P. 2018. Evaluation of a conjugate vaccine platform against enterotoxigenic Escherichia coli (ETEC), Campylobacter jejuni, and Shigella. Vaccine 36:6695–6702. doi: 10.1016/j.vaccine.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leyva A, Quintana A, Sanchez M, Rodriguez EN, Cremata J, Sanchez JC. 2008. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 36:134–141. doi: 10.1016/j.biologicals.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Matyas GR, Muderhwa JM, Alving CR. 2003. Oil-in-water liposomal emulsions for vaccine delivery. Methods Enzymol 373:34–50. doi: 10.1016/S0076-6879(03)73003-1. [DOI] [PubMed] [Google Scholar]
- 58.Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng K, Iyer MR, Li F, Beck Z, Janda KD, Alving CR. 2013. Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine 31:2804–2810. doi: 10.1016/j.vaccine.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baqar S, Rice B, Lee L, Bourgeois AL, El Din AN, Tribble DR, Heresi GP, Mourad AS, Murphy JR. 2001. Campylobacter jejuni enteritis. Clin Infect Dis 33:901–905. doi: 10.1086/322594. [DOI] [PubMed] [Google Scholar]
- 60.Pequegnat B, Laird RM, Ewing CP, Hill CL, Omari E, Poly F, Monteiro MA, Guerry P. 2017. Phase-variable changes in the position of O-methyl phosphoramidate modifications on the polysaccharide capsule of Campylobacter jejuni modulate serum resistance. J Bacteriol 199:e00027-17. doi: 10.1128/JB.00027-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A flow cytometric-based SBA using PI to measure CJ viability after exposure of 81-176 cells to sera from mice or NHP immunized with a CPS-CRM vaccine. (A) PI staining on untreated or methanol-treated 81-176 cells serve as negative and positive controls for PI staining, respectively. (B) PI staining of BRC-only controls where 81-176 cells were incubated with increasing amounts of BRC without heat-inactivated (HI) serum. (C) PI staining of 81-176 cells exposed to immune CPS-CRM + ALFQ HI mouse serum in the presence or absence of 6% BRC. Dot plots show representative PI staining on 81-176 cells from one experiment and are representative of at least two experiments. Numbers within the plot represent the percentage of PI+ 81-176 cells and numbers above the dot plot represent the dilution of HI serum. (D) F-SBA endpoint titers calculated by four-parameter logistic regression analysis. The % PI+ 81-176 cells (% PI+ 6% BRC + HI-sera – % PI+ 0% BRC HI-serum alone) is plotted versus the log10 serum dilution and IC50 is calculated using 4PL analysis as the F-SBA titer. The plot shows representative 4PL in sera from mice immunized with CPS-CRM, CPS-CRM + ALF, or CPS-CRM + ALFQ and analyzed on the same day. LOD of the assay of %PI+ 6% BRC alone + 3 standard deviations (SD) is shown as a dashed black line. (E) F-SBA 4PL analysis of NHP serum from an animal immunized with CPS-CRM + ALFQ and F-SBA titers measured against four different CJ strains: 81-176, CG8421, 81-176 kpsM mutant, and CG8486 (HS4 CPS type). The LOD of % PI+ 4% BRC alone + 3 SD is shown for each strain in dotted line corresponding to strain color in the legend. Download FIG S1, TIF file, 1.1 MB (1.1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Correlation between F-SBA and CPS-specific IgG1 (A), IgG2b (B), and IgG2c (C) antibody titers in mice. Log10 transformed titers were analyzed and Pearson correlation coefficient and P values are displayed within the graph. Download FIG S2, EPS file, 0.2 MB (163.8KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Analysis of the total frequency of Th1 cytokine-producing CD4+ T cells in vaccinated mice. Splenocytes were restimulated with CPS-CRM and stained for flow cytometric phenotypic analysis of IFN-γ-, TNF-α-, and IL-2-producing CD4+ TCRβ+ T cells. The total frequencies of CD4+ TCRβ+ T cells producing IFN- γ, TNF-α, and IL-2 for each individual mouse (n = 15 per group) are displayed, where the horizontal line indicates the mean + the SD of the group. 1.0-, 2.5-, and 5.0-µg CPS-CRM dose levels were combined. Ordinary one-way ANOVA with multiplicity-adjusted P values for statistical significance were determined (****, P ≤ 0.0001). Download FIG S3, EPS file, 0.1 MB (119KB, eps) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Detailed methods describing flow cytometric-based serum bactericidal assay. Download Text S1, DOCX file, 0.03 MB (30.7KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.