Key Points
Question
Are polygenic liabilities for major depression, bipolar disorder, and schizophrenia associated with depression in the general population?
Findings
In this case-cohort study of 34 573 individuals, each 1 SD increase in the polygenic risk for major depression was associated with a 30% increase in the hazard for a depression diagnosis in hospital-based psychiatric care. Polygenic liabilities for schizophrenia and bipolar disorder were also associated with an increased hazard of depression but to a lesser extent.
Meaning
Polygenic risk scores trained in samples of prevalent major depression cases are associated with first depression in the general population.
Abstract
Importance
Although the usefulness of polygenic risk scores as a measure of genetic liability for major depression (MD) has been established, their association with depression in the general population remains relatively unexplored.
Objective
To evaluate whether polygenic risk scores for MD, bipolar disorder (BD), and schizophrenia (SZ) are associated with depression in the general population and explore whether these polygenic liabilities are associated with heterogeneity in terms of age at onset and severity at the initial depression diagnosis.
Design, Setting, and Participants
Participants were drawn from the Danish iPSYCH2012 case-cohort study, a representative sample drawn from the population of Denmark born between May 1, 1981, and December 31, 2005. The hazard of depression was estimated using Cox regressions modified to accommodate the case-cohort design. Case-only analyses were conducted using linear and multinomial regressions. The data analysis was conducted from February 2017 to June 2018.
Exposures
Polygenic risk scores for MD, BD, and SZ trained using the most recent genome-wide association study results from the Psychiatric Genomics Consortium.
Main Outcomes and Measures
The main outcome was first depressive episode (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] code F32) treated in hospital-based psychiatric care. Severity at the initial diagnosis was measured using the ICD-10 code severity specifications (mild, moderate, severe without psychosis, and severe with psychosis) and treatment setting (inpatient, outpatient, and emergency).
Results
Of 34 573 participants aged 10 to 31 years at censoring, 68% of those with depression were female compared with 48.9% of participants without depression. Each SD increase in polygenic liability for MD, BD, and SZ was associated with 30% (hazard ratio [HR], 1.30; 95% CI, 1.27-1.33), 5% (HR, 1.05; 95% CI, 1.02-1.07), and 12% (HR, 1.12; 95% CI, 1.09-1.15) increases in the hazard of depression, respectively. Among cases, a higher polygenic liability for BD was associated with earlier depression onset (β = −.07; SE = .02; P = .002).
Conclusions and Relevance
Polygenic liability for MD is associated with first depression in the general population, which supports the idea that these scores tap into an underlying liability for developing the disorder. The fact that polygenic risk for BD and polygenic risk for SZ also were associated with depression is consistent with prior evidence that these disorders share some common genetic overlap. Variations in polygenic liability may contribute slightly to heterogeneity in clinical presentation, but these associations appear minimal.
This cohort study explores the association of polygenic risk scores for major depression, bipolar disorder, and schizophrenia with the risk for depression in the Danish population.
Introduction
Genes play a moderate role in the etiology of depression, with twin-based heritability estimates ranging from 30% to 40%1 and single-nucleotide polymorphism (SNP)–based heritability estimates ranging from 9% to 29%.2,3,4 Large empirical studies of the genetic architecture of depression indicate that it is polygenic, meaning that the contribution of genetic factors is attributable to small effects of hundreds or thousands of genetic variants spread across the genome.3,5
To date, multiple studies have shown small but statistically significant associations between polygenic risk scores (PRSs) a weighted sum of the number of variants associated with the disorder in a different data set, and depression.6,7,8,9 However, these studies focused on prevalent depression, which is more likely than incident depression to be recurrent or chronic.10,11 Because the genome-wide association study (GWAS) data underpinning the PRSs are largely based on prevalent cases, this could suggest that part of the genetic architecture discovered in GWAS studies is linked to chronicity or recurrence rather than the risk of developing the disorder. Therefore, while the usefulness of PRSs as a measure of genetic liability for depression has been established, their association with depression in the general population remains, to our knowledge, relatively unexplored.
In addition to heterogeneity in chronicity and recurrence, depression is also characterized by substantial variation in characteristics, such as age at onset (AAO) and symptom severity. Research suggests that this variation may be partially due to differences in genetic liability. Family studies demonstrate that individuals with a parental history of major depression (MD) are at increased risk for onset of depression at earlier ages,12,13,14,15 and recent results from the Psychiatric Genomics Consortium (PGC) showed that the polygenic risk for depression had a stronger association with early-onset vs late-onset depression.4 Research also suggests that individuals with severe MD may have a higher genetic burden than individuals with milder symptoms. A recent GWAS in Han Chinese women found an increased genetic signal among individuals with melancholia,16 and the PGC reported higher PRSs among severe vs moderate depression cases.4
Our primary aim in this study was to evaluate the extent to which polygenic liability is associated with risk for first depressive episode in the general population. As a secondary aim, we examined whether polygenic liability is associated with severity and AAO at first depression diagnosis. Because prior evidence suggests a possible shared genetic etiology between depression and other psychiatric disorders,2,4,17 we also examined the extent to which PRSs for bipolar disorder (BD) and schizophrenia (SZ) are associated with the risk for developing depression in the general population. To accomplish these aims, we used data from the iPSYCH2012 sample, a unique data set that links genetic information with longitudinal phenotype data from Danish national registers.
Methods
Study Design
For a detailed description of the iPSYCH2012 sample, see Pedersen et al.18 Briefly, the iPSYCH2012 sample has a case-cohort design19 that consists of 2 parts: a random sample (ie, subcohort) of individuals drawn from a specified base population (ie, full cohort) and all additional cases from the full cohort that were not selected as part of the subcohort. Like a traditional cohort study, a case-cohort study can obtain accurate estimates of hazards and risks using a traditional survival analysis, provided the analyses are modified to address issues associated with point and variance estimations that are caused by oversampling cases.19,20,21,22 For a more detailed description of the case-cohort design see the eMethods in the Supplement.
In the iPSYCH2012 sample, the subcohort consists of a random sample of 30 000 individuals drawn from the full cohort of all singletons born in Denmark between May 1, 1981, and December 31, 2005, who were alive and living in Denmark on their first birthday and had known mothers (N = 1 472 762).18 The full cohort was identified using information from the Danish Civil Registration system.23 The iPSYCH2012 study includes all individuals from the full cohort who received a diagnosis of depression in a psychiatric hospital in Denmark between 1991 and 2012 at 10 years or older. Information on psychiatric diagnoses was obtained from the Danish Central Psychiatric Research Register (DCPRR).24 Diagnoses are based on the ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research (ICD-10).25
Sample Selection
We began by selecting all members of the subcohort and all cases outside the subcohort whose first depression diagnosis in the DCPRR was F32 (depressive episode). We focused exclusively on individuals with Danish-born parents to reduce heterogeneity in genetic ancestry. The sample was further restricted to individuals who were successfully genotyped as part of the iPSYCH2012 sample, passed quality control (QC) measures, and were alive, living in Denmark, and at least age 10 years by the end of follow-up (December 31, 2012). Seventy-six individuals were removed from the subcohort because their first depression diagnosis in the register was F33 (recurrent depressive disorder) and we were thus unable to identify their first depressive episode. The final sample included 34 573 individuals. Of the 14 799 members with a depression diagnosis, 308 (2.1%) were members of the subcohort and 14 491 individuals (97.9%) were cases outside the subcohort (eFigure 1 in the Supplement). This study was approved by the Danish Data Protection Agency. By Danish law, register-based studies do not require informed consent.
Genotyping
For a more detailed description of genotyping and QC in the iPSYCH2012 cohort, see Pedersen et al.18 Briefly, DNA was collected from blood spots that were drawn as part of routine clinical testing at birth and stored at the Danish Neonatal Screening Biobank.26 Blood spots were located for 80 422 of 86 189 members (93.3%) of the original iPSYCH2012 sample. Genotyping was performed at the Broad Institute of Harvard University and Massachusetts Institute of Technology (Cambridge, MA) using the Infinium PsychChip array, version 1.0 (Illumina) according to the manufacturer’s protocols.27 Quality control and imputation (using 1000 genomes as the reference panel) were conducted using the Ricopili pipeline. As the iPSYCH2012 sample is population based, individuals from the same nuclear family unit were neither purposely sampled nor purposely removed.
Measures
The AAO was operationalized as the individual’s age in years at first F32 diagnosis in the DCPRR. Information on severity was obtained from ICD-10 diagnostic codes (mild, F32.0; moderate, F32.1; severe without psychotic features, F32.2; and severe with psychotic features, F32.3). We also examined differences in treatment settings (inpatient, emergency, and outpatient) as cases treated in inpatient or emergency settings are likely more severe than cases treated in an outpatient setting.
Polygenic risk scores were calculated using a standard approach28 in which a linkage disequilibrium–pruned discovery data set is used to identify autosomal SNPs associated with the outcome at varying P value thresholds, and then a score is calculated for individuals in a target data set that corresponds to the weighted sum of each participant’s allelic burden at that threshold. Polygenic risk scores for MD and SZ were created using the Ricopili process with the most recently published GWAS results from the PGC (not including iPSYCH2012) as discovery data sets.4,29 The discovery data set for BD was composed of leave one out summary statistics provided in advance of the latest GWAS publication from the Bipolar Working Group of the PGC.30 Single-nucleotide polymorphisms from the discovery data sets were filtered at an INFO score of more than 0.9 and a minor allele frequency of more than .05, and the broad major histocompatibility complex region (chr6: 25-35 MB) was removed. Additionally SNPs were only included in the scores if they were reliably genotyped or imputed across all 23 waves of the iPSYCH2012 sample, at an INFO score of more than 0.6 and a minor allele frequency of more than .01. Ten PRSs were calculated for each disorder (30 total) at the following P value thresholds: P < .00000005, .000001, .0001, .001, .01, .05, .10, .20, .50, and >.99 (see eTable 1 in the Supplement for SNP numbers).31 Polygenic risk scores were standardized using the means and SDs from the distributions in subcohort members with Danish ancestry (eFigure in the Supplement). As the iPSYCH2012 subcohort is a simple random sample drawn from the base population, the distribution of PRSs in the subcohort approximates the distribution in the Danish-born population.
Statistical Analysis
The hazard of depression was estimated using Cox regressions with days since the participant’s tenth birthday as the timescale. Individuals entered the analysis on their tenth birthday and were censored on the date of their first F32 diagnosis in the DCPRR, death, emigration, or December 31, 2012, whichever came first. The oldest participants were age 31 years when they received their diagnosis or were censored. We used robust standard errors and Barlow weighting to account for oversampling of cases.22,32 All models were adjusted for sex and the top 4 ancestral principal components (PCs). Additionally, all models were stratified by birth year (1981-2002) to control for secular trends in diagnostic practices and because fewer bloodspots were retrievable among individuals born during earlier years.
To examine whether polygenic liability has a stronger association with early-onset depression, we conducted separate Cox regressions in each analysis with only depression with onset in a specific age range considered as an outcome. Thus, we estimated separately the association of PRSs with hazard of depression with diagnosis at ages 10 to 15, 16 to 20, 21 to 25, and 26 to 31 years, respectively. Likewise, we conducted separate Cox regressions that considered respectively mild, moderate, severe without psychotic features, severe with psychotic features, inpatient, emergency and outpatient treatment setting at first depression as outcomes in each of the analyses. We also examined the associations between PRSs, AAO, and severity among cases only. Associations with AAO in days were estimated using linear regressions. Associations with severity measures were estimated using multinomial logistic regressions. All case-only regressions were adjusted for the first 4 PCs, sex, and birth year. Statistical significance was assessed at the Bonferroni-corrected α-level P < .017. Analyses were conducted in SAS, version 9.4 (SAS Institute; eAppendix in the Supplement).
Results
Sample Characteristics
Table 1 shows sample characteristics. Of the participants without depression, 9659 (48.9%) were female, which was expected given that the subcohort is a representative sample of the Danish population. Of the participants with MD, 10 056 (68.0%) were female, which was also expected given that the typical female to male ratio of depression in the population is 2:1.33 The AAO ranged from 10 to 31 years, with a mean (SD) of 19.1 (4.1) years. Seventeen percent of the cases were classified as mild (n = 2573), 45% as moderate (n = 6635), 9% as severe (n = 1386), 3% as psychotic (n = 481), and 25% had no ICD-10 severity specification (n = 3724). Most (60%; n = 8880) were treated in an outpatient setting.
Table 1. Sample Characteristics.
| Characteristic | Case Status, No. (%) | |
|---|---|---|
| Participants Without Depression | Participants With Depression | |
| Sex | ||
| Male | 10 115 (51.2) | 4743 (32.1) |
| Female | 9659 (48.9) | 10 056 (68.0) |
| Age at depression diagnosis, y | ||
| Noncase | 19 774 (100.0) | NA |
| 10-15 | NA | 2965 (20.0) |
| 16-20 | NA | 6653 (45.0) |
| 21-25 | NA | 3981 (26.9) |
| 26-31 | NA | 1200 (8.1) |
| ICD-10 severity specifier | ||
| Noncase | 19 774 (100.0) | NA |
| Mild | NA | 2573 (17.4) |
| Moderate | NA | 6635 (44.8) |
| Severe without psychotic symptoms | NA | 1386 (9.4) |
| Severe with psychotic symptoms | NA | 481 (3.3) |
| Severity unspecified | NA | 3724 (25.2) |
| Treatment setting | ||
| Noncase | 19 774 (100.0) | NA |
| Outpatient | NA | 8880 (60.0) |
| Inpatient | NA | 2269 (15.3) |
| Emergency | NA | 3650 (24.7) |
| Total sample | 19 774 (100.0) | 14 799 (100.0) |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; NA, not applicable.
Polygenic Liability and Hazard of Depression
For all 3 PRSs (PRS-MD, PRS-BD, and PRS-SZ), the strength of the association increased as the stringency of the P value threshold decreased up to P < .05 (eTable 2 and eFigures 2-7 in the Supplement). For this reason, and to maintain consistency with prior research, we present results from the P < .05 thresholds throughout this article.
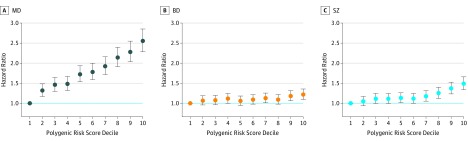
Each SD increase in PRS-MD was associated with a 30% increase in the hazard of depression (95% CI, 1.27-1.33; P < .0001). Compared with an individual with average polygenic liability, an individual who is 1 SD more than the population average had a 30% increased risk of receiving a depression diagnosis before age 31 years. The corresponding values for PRS-BD and PRS-SZ were 5% (95% CI, 1.02-1.07; P < .0001) and 12% (95% CI, 1.09-1.15; P < .0001), respectively (eTable 2 in the Supplement). Figure 1 shows the associations between PRS deciles and the hazard of depression, with the bottom decile (ie, the lowest 10% of the PRS distribution) as the reference category. Compared with individuals in the bottom decile, individuals in the top decile of PRS-MD had a hazard ratio of 2.55 (95% CI, 2.28-2.85; P < .0001). The corresponding values were 1.22 (95% CI, 1.10-1.36; P < .0001) for PRS-BD and 1.49 (95% CI, 1.34-1.66; P < .0001) for PRS-SZ (eTable 3 in the Supplement).
Figure 1. Polygenic Liability for Major Depressive Disorder (MD), Bipolar Disorder (BD), and Schizophrenia (SZ) and Hazard of Depression in the iPSYCH2012 Cohort.
A-C, The hazard of depression for each decile of polygenic liability compared with the bottom decile (ie, the bottom 10% of the polygenic liability in the Danish population). The bars represent 95% confidence intervals.
AAO
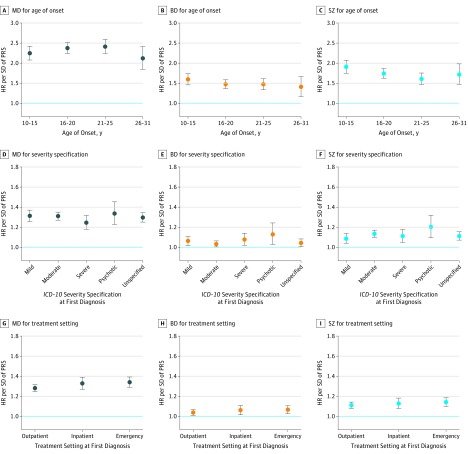
As shown in Figure 2 and Table 2, the hazard of depression per SD increase in PRS-MD was slightly higher for first diagnosis between age 16 to 20 years (1.31; 95% CI, 1.27-1.35) and age 21 to 25 years (1.32; 95% CI, 1.27-1.38) compared with age 10 to 15 years (1.27; 95% CI, 1.22-1.33) or age 26 to 31 years (1.24; 95% CI, 1.16-1.32). The hazard ratio of PRS-BD was highest for diagnosis between ages 10 to 15 years (1.08; 95% CI, 1.04-1.12) and decreased linearly with age. The hazard ratio between PRS-SZ and was highest for diagnoses between age 10 to 15 years (1.17; 95% CI, 1.12-1.22) and lowest for diagnoses between age 21 to 25 years (1.08; 95% CI, 1.04-1.13). Case-only analyses showed small associations between higher PRSs and earlier AAO across all scores, but only the association for PRS-BD survived correction for multiple testing (Table 3).
Figure 2. Polygenic Liability for Major Depressive Disorder (MD), Bipolar Disorder (BD), and Schizophrenia (SZ) and Hazard of Depression by Age and Severity.
A-C, Hazard ratios (HRs) per SD increase in polygenic liabilities for MD, BD, and SZ for a depression diagnosis within different age ranges. D-F, Hazard ratios per SD increase in polygenic liabilities for MD, BD, and SZ for a depression diagnosis with different International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) severity specifications. G-I, Hazard ratios per SD increase in polygenic liabilities for MD, BD, and SZ for a depression diagnosis within different treatment settings. The bars represent 95% confidence intervals. PRS indicates polygenic risk scores.
Table 2. Associations Between PRS-MD, PRS-BD, and PRS-SZ and Hazard of Depression Within Different Age at Onset and Severity Subgroups.
| Characteristic | MD | BD | SZ | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at depression diagnosis, y | ||||||
| 10-15 | 1.27 (1.22-1.33) | <.0001 | 1.08 (1.04-1.12) | .0002 | 1.17 (1.12-1.22) | <.0001 |
| 16-20 | 1.31 (1.27-1.35) | <.0001 | 1.04 (1.01-1.07) | .008 | 1.12 (1.09-1.16) | <.0001 |
| 21-25 | 1.32 (1.27-1.38) | <.0001 | 1.04 (1.00-1.08) | .05 | 1.08 (1.04-1.13) | 0.0001 |
| 26-31 | 1.24 (1.16-1.32) | <.0001 | 1.02 (0.95-1.10) | .55 | 1.12 (1.04-1.19) | 0.0018 |
| ICD-10 severity specifier | ||||||
| Mild | 1.31 (1.26-1.37) | <.0001 | 1.06 (1.02-1.11) | .005 | 1.09 (1.04-1.14) | 0.0002 |
| Moderate | 1.31 (1.27-1.35) | <.0001 | 1.03 (1.00-1.06) | .04 | 1.13 (1.10-1.17) | <.0001 |
| Severe | 1.24 (1.18-1.32) | <.0001 | 1.08 (1.02-1.14) | .009 | 1.11 (1.05-1.18) | 0.0004 |
| Psychotic | 1.33 (1.23-1.45) | <.0001 | 1.13 (1.03-1.24) | .01 | 1.20 (1.09-1.32) | <.0001 |
| Unspecified | 1.30 (1.25-1.35) | <.0001 | 1.04 (1.01-1.08) | .02 | 1.11 (1.07-1.15) | <.0001 |
| Treatment setting | ||||||
| Emergency | 1.34 (1.29-1.39) | <.0001 | 1.07 (1.03-1.11) | .001 | 1.14 (1.10-1.18) | <.0001 |
| Inpatient | 1.33 (1.27-1.39) | <.0001 | 1.06 (1.01-1.11) | .01 | 1.13 (1.08-1.18) | <.0001 |
| Outpatient | 1.28 (1.24-1.32) | <.0001 | 1.04 (1.01-1.07) | .008 | 1.11 (1.08-1.14) | <.0001 |
Abbreviations: BD, bipolar disorder; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; MD, major depression; PRS, polygenic risk score; SZ, schizophrenia.
Table 3. Case-Only Analyses of the Associations of PRS-MD, PRS-BD, and PRS-SZ With Age at Onset and Severity at First Depression Diagnosis.
| Characteristic | MD | BD | SZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | OR (95% CI) | P Value | β (SE) | OR (95% CI) | P Value | β (SE) | OR (95% CI) | P Value | |
| Age at depression diagnosis | −0.05 (0.02) | NA | .04 | −0.07 (0.02) | NA | .002 | −0.05 (0.02) | NA | .04 |
| ICD-10 severity specifier | |||||||||
| Mild | Reference Category | ||||||||
| Moderate | −0.00 (0.02) | 1.00 (0.95-1.05) | .99 | −0.03 (0.02) | 0.97 (0.93-1.02) | .24 | 0.04 (0.02) | 1.04 (0.99-1.09) | .08 |
| Severe | −0.05 (0.03) | 0.95 (0.89-1.01) | .13 | 0.02 (0.03) | 1.02 (0.95-1.08) | .62 | 0.02 (0.03) | 1.02 (0.95-1.09) | .58 |
| Psychotic | 0.02 (0.05) | 1.02 (0.92-1.12) | .71 | 0.06 (0.05) | 1.06 (0.97-1.17) | .21 | 0.09 (0.05) | 1.10 (1.00-1.21) | .06 |
| Unspecified | −0.01 (0.03) | 0.99 (0.94-1.04) | .77 | −0.02 (0.03) | 0.98 (0.93-1.03) | .48 | 0.02 (0.03) | 1.02 (0.97-1.07) | .51 |
| Treatment setting | |||||||||
| Outpatient | Reference Category | ||||||||
| Emergency | 0.05 (0.02) | 1.05 (1.01-1.09) | .02 | 0.03 (0.02) | 1.03 (0.99-1.07) | .18 | 0.03 (0.02) | 1.03 (0.99-1.07) | .13 |
| Inpatient | 0.04 (0.02) | 1.04 (0.99-1.09) | .13 | 0.02 (0.02) | 1.02 (0.98-1.07) | .37 | 0.02 (0.02) | 1.02 (0.97-1.07) | .46 |
Abbreviations: BD, bipolar disorder; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; MD, major depression; NA, not applicable; OR, odds ratio; PRS, polygenic risk score; SZ, schizophrenia.
Severity
The hazard ratios for PRS-MD, PRS-BD, and PRS-SZ were highest for psychotic depression (Figure 2, Table 2). The differences by severity were most pronounced for PRS-SZ (1.20; 95% CI, 1.09-1.32) and least pronounced for PRS-MD (1.33; 95% CI, 1.23-1.45). In the case-only analyses, none of the associations were statistically significant; however, the association between higher PRS-SZ and an increased odds of psychotic depression was suggestive (odds ratio, 1.10; 95% CI, 1.00-1.21; P = .06) (Table 3).
The hazard ratios for PRS-MD, PRS-BD, and PRS-SZ were higher for treatment in inpatient and emergency settings; however, these differences were small (Figure 2). Polygenic risk scores for MD were marginally associated with an increased odds of emergency treatment in the case-only analyses (odds ratio, 1.05; 95% CI, 1.01-1.09; P = .02) (Table 3).
Discussion
In this study, we found that PRSs trained using aggregated results from selected samples of prevalent, often recurrent depression cases contributed to the risk for first depression in the Danish general population. For each SD increase in polygenic liability, the hazard of depression increased by 30%. Compared with individuals in the bottom 10% of the polygenic liability distribution, the hazard of depression was 2.55 times higher among individuals in the top 10%. These results suggest that estimates of genetic liability ascertained using prevalent samples are tapping in to an underlying genetic predisposition for developing depression, not just a predisposition to maintain the disorder once it has been established. Polygenic liability for BD and SZ were associated with depression to a lesser extent than PRS-MD, which supports the well-documented finding that these disorders share some common genetic etiology.
The association of polygenic liability with AAO in this study was much smaller than prior findings from family studies would suggest. This could reflect that PRSs and family background are not entirely overlapping measures of genetic risk. Previous research suggests that some of the effect of family history of schizophrenia is mediated by polygenic risk.34 If the same holds true for depression, the larger effects identified in family studies may be attributable to the portion of the family history effect not captured by a PRS, or possibly to an increased vigilance for psychiatric disorders among multiplex families. The oldest members of the iPSYCH2012 cohort were only age 31 years at the end of follow-up, which is around the median age of onset for depression.35 As a result, the entire sample could be considered early-onset. It may be that the more pronounced differences exist in the effects of PRS-MD with the risk for depression at different points across the lifespan, but that these differences are less apparent when comparing groups of younger individuals.
We found little association between polygenic liability and greater severity at the initial depression diagnosis, which is inconsistent with recent findings from the PGC.4 In general, past studies with positive findings in this area have focused on severity measures that are associated with the illness course, such as the number of depressive episodes and the chronicity of depressive symptoms.36,37,38 It could be that polygenic liability has less of an effect on characteristics of the first depressive episode than it does on the characteristics of course.
It has been suggested previously that stratifying on the phenotype may be a viable method to increase statistical power for identifying genetic variants that are associated with depression.39 This method has been used with some success by the CONVERGE consortium, which identified a locus that was significantly associated with depression in Han Chinese women by selecting for highly severe, recurrent female cases.16 Polygenic risk was also found to be differentially associated with subtypes in autism,31 BD, and SZ.30 However, for depression, greater success in gene discovery was achieved by increasing the sample size at the expense of a carefully defined phenotype.4,40 In this vein, the results of this study indicate that the usefulness of further stratification on the phenotype for gene discovery in depression might be more limited than we may have wished.
We found some evidence of genetic heterogeneity among depression cases in terms of polygenic liabilities for BD and SZ: there was a suggestive (although not significant) association between PRS-SZ and depression with psychotic symptoms, which makes intuitive sense. The PRS for BD was significantly associated with earlier age at MD onset in the case-only analyses, and the case-cohort analyses in separate age groups suggest that PRS-BD and PRS-SZ may be particularly elevated among individuals who receive a diagnosis with MD between the ages of 10 to 15 years. These results are consistent with past studies41,42 and could suggest that a person’s degree of genetic liability for BD or SZ may place them at an increased risk for different clinical manifestations of depression. However, psychiatric diagnoses are often unstable over time, and both early AAO and greater severity/psychotic symptoms are robust risk factors for converting to BD or SZ.43,44,45 It is therefore possible that these results reflect that many individuals with BD and SZ receive a depression diagnosis during the early stages of their illness.
Strengths and Limitations
The iPSYCH2012 sample has many strengths, including a large sample size, population-based sampling, and a uniform case definition. The fact that cases were identified through clinical records rather than selected specifically for research increases the relevance for clinical practice. In addition, the ancestral homogeneity of the Danish population reduces the likelihood of confounding by population stratification.
However, it should be noted that although cases in the iPSYCH2012 sample are representative of individuals who received treatment for depression in psychiatric hospitals, they do not include people with depression who are untreated or only treated by general practitioners.46,47 To put this into perspective, currently unpublished results show that most (85%) individuals who are medically treated for depression in Denmark are treated first by their primary care doctors, although this proportion was lower in younger age groups (unpublished data, 2018). The cases in the iPSYCH2012 sample therefore represent the severe end of the depression distribution in Denmark, which is both a strength and a limitation. Severe cases are likely enriched for genetic determinants16; however, the results may not generalize to milder forms of depression, and they could be biased toward the null because of misclassification. Additionally, there may be too little variation in severity to assess the associations between polygenic liability and severity in this sample. The analyses may also be subject to selection bias if people with specialty-treated depression are more likely than people with untreated or primary-care treated depression to experience recurrent episodes.
Because we focused exclusively on first depression, we did not account for subsequent diagnostic conversions to BD or SZ. Individuals who convert may have different genetic profiles; however, there was no way to account for this without conditioning on the future, which can introduce bias.48 Further research is needed to investigate the associations between polygenic liability and the characteristics of course and outcome, including progression to other psychiatric disorders. Finally, the discovery data sets used to create the polygenic risk scores for MD, BD, and SZ had different sample sizes, which affects their statistical power.49
Conclusions
We found that polygenic liability is associated with depression in the general Danish population. Polygenic liabilities for BD and SZ were also associated with depression, which supports the idea that there is a shared genetic predisposition across these disorders. Heterogeneity in AAO might be partially attributable to underlying genetic differences among depression cases, but these associations appear minimal.
eMethods. Case-cohort study design
eTable 1. Number of SNPs in the polygenic risk scores
eTable 2. Main effects of PRS-MD, PRS-BD and PRS-SZ on hazard of depression across all p(t) thresholds
e Table 3. Associations between PRS-MD, PRS-BD and PRS-SZ deciles and hazard of depression
eFigure 1. Sample selection
eFigure 2. Associations between PRS-MD and hazard of depression across all p(t) thresholds: panel graph
eFigure 3. Associations between PRS-BD and hazard of depression across all p(t) thresholds: panel graph
eFigure 4. Associations between PRS-SZ and hazard of depression across all p(t) thresholds: panel graph
eFigure 5. Associations between PRS-MD and hazard of depression across all p(t) thresholds: overlay graph
eFigure 6. Associations between PRS-BD and hazard of depression across all p(t) thresholds: overlay graph
eFigure 7. Associations between PRS-SZ and hazard of depression across all p(t) thresholds: overlay graph
eAppendix. SAS code
References
- 1.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552-1562. doi: 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson RE, Cai N, Bigdeli TB, et al. The genetic architecture of major depressive disorder in Han Chinese women. JAMA Psychiatry. 2017;74(2):162-168. doi: 10.1001/jamapsychiatry.2016.3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537-551. doi: 10.1038/nrg3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrot WJ, Milaneschi Y, Abdellaoui A, et al. Effect of polygenic risk scores on depression in childhood trauma. Br J Psychiatry. 2014;205(2):113-119. doi: 10.1192/bjp.bp.113.143081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SC, Glymour MM, Walter S, et al. Genome-wide polygenic scoring for a 14-year long-term average depression phenotype. Brain Behav. 2014;4(2):298-311. doi: 10.1002/brb3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musliner KL, Seifuddin F, Judy JA, Pirooznia M, Goes FS, Zandi PP. Polygenic risk, stressful life events and depressive symptoms in older adults: a polygenic score analysis. Psychol Med. 2015;45(8):1709-1720. doi: 10.1017/S0033291714002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripke S, Wray NR, Lewis CM, et al. ; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium . A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497-511. doi: 10.1038/mp.2012.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41(12):1178-1182. doi: 10.1001/archpsyc.1984.01790230064010 [DOI] [PubMed] [Google Scholar]
- 11.Waltoft BL, Pedersen CB, Nyegaard M, Hobolth A. The importance of distinguishing between the odds ratio and the incidence rate ratio in GWAS. BMC Med Genet. 2015;16:71. doi: 10.1186/s12881-015-0210-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman MM, Wickramaratne P, Gameroff MJ, et al. Offspring of depressed parents: 30 years later. Am J Psychiatry. 2016;173(10):1024-1032. doi: 10.1176/appi.ajp.2016.15101327 [DOI] [PubMed] [Google Scholar]
- 13.Weissman MM, Wickramaratne P, Merikangas KR, et al. Onset of major depression in early adulthood. increased familial loading and specificity. Arch Gen Psychiatry. 1984;41(12):1136-1143. doi: 10.1001/archpsyc.1984.01790230022003 [DOI] [PubMed] [Google Scholar]
- 14.Lieb R, Isensee B, Höfler M, Wittchen HU. Parental depression and depression in offspring: evidence for familial characteristics and subtypes? J Psychiatr Res. 2002;36(4):237-246. doi: 10.1016/S0022-3956(02)00015-8 [DOI] [PubMed] [Google Scholar]
- 15.Musliner KL, Trabjerg BB, Waltoft BL, et al. Parental history of psychiatric diagnoses and unipolar depression: a Danish National Register-based cohort study. Psychol Med. 2015;45(13):2781-2791. doi: 10.1017/S0033291715000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CONVERGE consortium Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588-591. doi: 10.1038/nature14659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersson E, Larsson H, Lichtenstein P. Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol Psychiatry. 2016;21(5):717-721. doi: 10.1038/mp.2015.116 [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CB, Bybjerg-Grauholm J, Pedersen MG, et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol Psychiatry. 2018;23(1):6-14. doi: 10.1038/mp.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1-11. doi: 10.1093/biomet/73.1.1 [DOI] [Google Scholar]
- 20.Petersen L, Sørensen TI, Andersen PK. Comparison of case-cohort estimators based on data on premature death of adult adoptees. Stat Med. 2003;22(24):3795-3803. doi: 10.1002/sim.1672 [DOI] [PubMed] [Google Scholar]
- 21.Self SG, Prentice RL. Asymptotic distribution theory and efficiency results for case-cohort studies. Ann Stat. 1988;16(1):64-81. doi: 10.1214/aos/1176350691 [DOI] [Google Scholar]
- 22.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064-1072. doi: 10.2307/2533444 [DOI] [PubMed] [Google Scholar]
- 23.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 24.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7)(suppl):54-57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 26.Nørgaard-Pedersen B, Hougaard DM. Storage policies and use of the Danish Newborn Screening Biobank. J Inherit Metab Dis. 2007;30(4):530-536. doi: 10.1007/s10545-007-0631-x [DOI] [PubMed] [Google Scholar]
- 27.Gunderson KL, Steemers FJ, Ren H, et al. Whole-genome genotyping. Methods Enzymol. 2006;410:359-376. doi: 10.1016/S0076-6879(06)10017-8 [DOI] [PubMed] [Google Scholar]
- 28.Purcell SM, Wray NR, Stone JL, et al. ; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics Consortium . Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705-1715.e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grove J, Ripke S, Als TD, et al. Common risk variants identified in autism spectrum disorder [published online November 17, 2017]. bioRxiv. doi: 10.1101/224774 [DOI] [Google Scholar]
- 32.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165-1172. doi: 10.1016/S0895-4356(99)00102-X [DOI] [PubMed] [Google Scholar]
- 33.Pedersen CB, Mors O, Bertelsen A, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71(5):573-581. doi: 10.1001/jamapsychiatry.2014.16 [DOI] [PubMed] [Google Scholar]
- 34.Agerbo E, Sullivan PF, Vilhjálmsson BJ, et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA Psychiatry. 2015;72(7):635-641. doi: 10.1001/jamapsychiatry.2015.0346 [DOI] [PubMed] [Google Scholar]
- 35.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 36.Levine ME, Crimmins EM, Prescott CA, Phillips D, Arpawong TE, Lee J. A polygenic risk score associated with measures of depressive symptoms among older adults. Biodemography Soc Biol. 2014;60(2):199-211. doi: 10.1080/19485565.2014.952705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mistry S, Harrison JR, Smith DJ, Escott-Price V, Zammit S. The use of polygenic risk scores to identify phenotypes associated with genetic risk of bipolar disorder and depression: a systematic review. J Affect Disord. 2018;234:148-155. doi: 10.1016/j.jad.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 38.Ferentinos P, Rivera M, Ising M, et al. Investigating the genetic variation underlying episodicity in major depressive disorder: suggestive evidence for a bipolar contribution. J Affect Disord. 2014;155:81-89. doi: 10.1016/j.jad.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 39.Levinson DF, Mostafavi S, Milaneschi Y, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry. 2014;76(7):510-512. doi: 10.1016/j.biopsych.2014.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyde CL, Nagle MW, Tian C, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48(9):1031-1036. doi: 10.1038/ng.3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verduijn J, Milaneschi Y, Peyrot WJ, et al. Using clinical characteristics to identify which patients with major depressive disorder have a higher genetic load for three psychiatric disorders. Biol Psychiatry. 2017;81(4):316-324. doi: 10.1016/j.biopsych.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 42.Power RA, Tansey KE, Buttenschøn HN, et al. ; CONVERGE Consortium, Cardiogram Consortium, GERAD1 Consortium . Genome-wide association for major depression through age at onset stratification: major depressive disorder working group of the psychiatric genomics consortium. Biol Psychiatry. 2017;81(4):325-335. doi: 10.1016/j.biopsych.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musliner KL, Munk-Olsen T, Mors O, Østergaard SD. Progression from unipolar depression to schizophrenia. Acta Psychiatr Scand. 2017;135(1):42-50. doi: 10.1111/acps.12663 [DOI] [PubMed] [Google Scholar]
- 44.Musliner KL, Østergaard SD. Patterns and predictors of conversion to bipolar disorder in 91 587 individuals diagnosed with unipolar depression. Acta Psychiatr Scand. 2018;137(5):422-432. doi: 10.1111/acps.12869 [DOI] [PubMed] [Google Scholar]
- 45.Ratheesh A, Davey C, Hetrick S, et al. A systematic review and meta-analysis of prospective transition from major depression to bipolar disorder. Acta Psychiatr Scand. 2017;135(4):273-284. doi: 10.1111/acps.12686 [DOI] [PubMed] [Google Scholar]
- 46.Kuramoto-Crawford SJ, Han B, Jacobus-Kantor L, Mojtabai R. Receipt of depression treatment from general medical providers and specialty mental health providers. Psychiatr Serv. 2016;67(7):758-765. doi: 10.1176/appi.ps.201500113 [DOI] [PubMed] [Google Scholar]
- 47.Thornicroft G, Chatterji S, Evans-Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119-124. doi: 10.1192/bjp.bp.116.188078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen PK, Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med. 2012;31(11-12):1074-1088. doi: 10.1002/sim.4385 [DOI] [PubMed] [Google Scholar]
- 49.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9(3):e1003348. doi: 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Case-cohort study design
eTable 1. Number of SNPs in the polygenic risk scores
eTable 2. Main effects of PRS-MD, PRS-BD and PRS-SZ on hazard of depression across all p(t) thresholds
e Table 3. Associations between PRS-MD, PRS-BD and PRS-SZ deciles and hazard of depression
eFigure 1. Sample selection
eFigure 2. Associations between PRS-MD and hazard of depression across all p(t) thresholds: panel graph
eFigure 3. Associations between PRS-BD and hazard of depression across all p(t) thresholds: panel graph
eFigure 4. Associations between PRS-SZ and hazard of depression across all p(t) thresholds: panel graph
eFigure 5. Associations between PRS-MD and hazard of depression across all p(t) thresholds: overlay graph
eFigure 6. Associations between PRS-BD and hazard of depression across all p(t) thresholds: overlay graph
eFigure 7. Associations between PRS-SZ and hazard of depression across all p(t) thresholds: overlay graph
eAppendix. SAS code