Abstract
Importance
The prognosis of advanced melanoma has been greatly improved by new therapeutic agents and clinicians rely on dynamic signals to drive their therapeutic choices. Although the kinetics of metastatic disease seem to be correlated with survival, progression of the localized disease is not predictable.
Objective
To assess whether progression of metastatic disease is associated with the time to the first distant recurrence of melanoma.
Design, Setting, and Participants
This study was conducted from March 1, 2013, to September 1, 2017, among 638 adults with unresectable stage III or IV melanoma within the French multicentric prospective cohort MelBase. Patients treated with first-line immunotherapies, targeted therapies, or chemotherapy were included. Patients with unknown primary or de novo metastatic melanoma were not included. Data were analyzed from March 1, 2013, to December 1, 2017.
Main Outcomes and Measures
The date of primary excision and time to first distant recurrence, progression-free survival, and overall survival were collected. Cox proportional hazards regression models were planned to assess the association between time to first distant recurrence and progression-free survival or overall survival, which was evaluated in terms of hazard ratio (HR). Time to recurrence was analyzed both as a continuous and categorical variable (<12 months, 12-24 months, and >24 months).
Results
A total of 638 patients (272 women and 366 men; median age, 64 years [interquartile range, 52-73 years]) were included in the study. The median time from primary excision to first distant recurrence was 25 months (interquartile range, 12-55 months). There was no evidence of association of the time to recurrence with progression-free survival, both when analyzed as a continuous variable (HR, 0.99; 95% CI, 0.99-1.01) or after categorization (12-24 months: HR, 0.75; 95% CI, 0.56-1.02; >24 months: HR, 0.62; 95% CI; 0.47-1.01). There was no evidence of association of the time to recurrence with overall survival, both when analyzed as a continuous variable (HR, 0.99; 95% CI, 0.98-1.02) or after categorization (12-24 months: HR, 0.76; 95% CI, 0.54-1.07; >24 months: HR, 0.61; 95% CI, 0.54-1.03). Those results remained nonsignificant after stratification by treatment.
Conclusions and Relevance
In the MelBase cohort, time to recurrence of metastatic melanoma appears not to be associated with progression-free survival or overall survival.
This cohort study examines whether progression of metastatic disease and survival are associated with the time to the first distant recurrence of melanoma.
Key Points
Question
Is the course of metastatic melanoma associated with the time to the first distant recurrence?
Findings
This cohort study of 638 adults in the French cohort MelBase revealed no evidence of an association of the time to recurrence with progression-free survival or overall survival, both when analyzed as a continuous variable or after categorization (<12 months, 12-24 months, and >24 months).
Meaning
The timing of advanced disease appeared not to be associated with the time to first distant recurrence of metastatic melanoma in this cohort study.
Introduction
In the past decade, the prognosis of advanced melanoma has been improved by new therapeutic agents such as immunotherapies and targeted therapies. In practice, clinicians rely partially on dynamic signals to evaluate metastatic disease kinetics that drive their therapeutic choices.1,2 Although the kinetics of metastatic disease seem to be correlated with patient survival, the first relapse is not predictable, and data from the literature on the topic are controversial.3,4,5,6 We hypothesized that the progression of the metastatic disease would be associated with the time from primary excision to the first distant recurrence of melanoma.
Methods
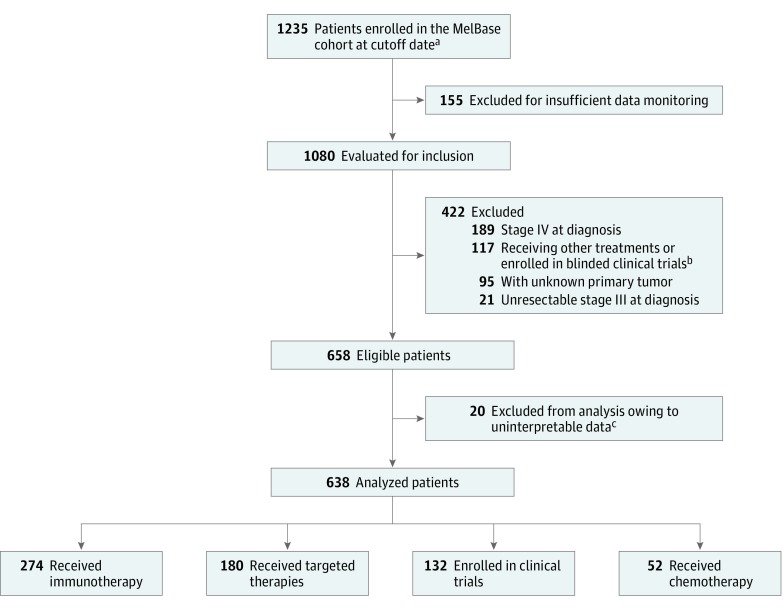
We conducted our study within the French multicentric cohort MelBase, which is dedicated to the prospective follow-up in 26 participating centers of adults with unresectable stage III or IV melanoma at the time of declaration of metastasis. In this study, we included 1235 patients enrolled in MelBase from March 1, 2013, to September 1, 2017. Patients treated with first-line immunotherapies, targeted therapies, chemotherapy, or those who were participating in open clinical trials with at least 3 months of follow-up were included. Patients receiving combination treatment were excluded. Patients with unknown primary tumor or with a diagnosis of de novo unresectable stage III or IV cancer were excluded because no data on time to first recurrence were available. Among the 1235 patients in the MelBase cohort in September 2017, a total of 1080 patients receiving first-line therapy were evaluated for inclusion (155 were excluded for insufficient data monitoring). As shown in Figure 1, 422 patients were excluded because they did not meet the enrollment criteria (unknown primary tumor, n = 95; unresectable stage III cancer, n = 21; unresectable stage IV cancer, n = 189; and undergoing other treatments or participating in blinded clinical trials, n = 117). Twenty additional patients were excluded because they had uninterpretable data (missing date of primary excision or of first distant recurrence). The dates of primary excision, first distant recurrence, treatment initiation, disease progression, and death of the 638 patients were collected and analyzed in December 2017. In a prespecified statistical analysis plan, Cox proportional hazards regression models were planned to assess the association between time to first distant recurrence (TFDR) and progression-free survival (PFS) and overall survival (OS). The association between TFDR and PFS and OS was evaluated in terms of hazard ratio (HR) and analyzed as a continuous variable (by observed time-to-event) and as a categorical variable (<12 months, 12-24 months, and >24 months). Cox proportional hazards regression models were also used to assess any interaction with other disease factors (Breslow thickness, presence of primary ulceration, and American Joint Committee on Cancer stage) and to stratify the results on treatment. The MelBase protocol was approved by French ethics committee (Comité de Protection des Personnes, Île-de-France XI, No. 12027, 2012) and registered in the National Institutes of Health clinical trials database (NCT02828202). Written informed consent was obtained from all patients.
Figure 1. Study Flowchart.
aThe last patient was included in the study in September 2017.
bPatients excluded for other treatments: ipilimumab-nivolumab combination (n = 69), other combination therapy (n = 16), blinded clinical trials (n = 32).
cMissing date of primary excision or of first distant recurrence.
Results
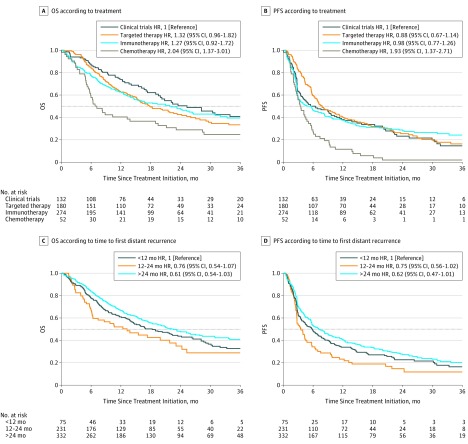
A total of 638 patients were included in this study, who were treated with first-line immunotherapies (n = 274), targeted therapies (n = 180), chemotherapy (n = 52), or who were participating in open clinical trials (n = 132). The patient characteristics at the time of primary excision and at the treatment baseline are reported in the Table. The median Breslow thickness was 3.0 mm (interquartile range, 1.6-5.2 mm). The median time from primary excision to first distant recurrence was 25 months (interquartile range, 12-55 months) and the median time from primary excision to treatment initiation was 27 months (interquartile range, 14-57 months). There was no evidence of an association between TFDR and PFS when TFDR was studied as a continuous variable (HR, 0.99; 95% CI, 0.99-1.01) or when it was analyzed as a categorical variable (12-24 months: HR, 0.75; 95% CI, 0.56-1.02; >24 months: HR, 0.62; 95% CI, 0.47-1.01). Likewise, there was no evidence of an association between TFDR and OS when TFDR was studied as a continuous variable (HR, 0.99; 95% CI, 0.98-1.02) or when it was analyzed as a categorical variable (12-24 months: HR, 0.76; 95% CI, 0.54-1.07; >24 months: HR, 0.61; 95% CI, 0.54-1.03). Figure 2 shows Kaplan-Meier curves of PFS and OS according to treatments and PFS and OS according to time to recurrence analyzed as a categorical variable. Cox proportional hazards regression models showed no interaction between TFDR and Breslow thickness (>2 mm: HR, 0.99; 95% CI, 0.99-1.01; ≤2 mm: HR, 0.99; 95% CI, 0.99-1.01; P = .06 for interaction), and no interaction with the American Joint Committee on Cancer stage at diagnosis (stage I or II: HR, 1.16; 95% CI, 0.94-1.19; stage III: HR, 1.02; 95% CI, 0.97-1.09; P = .88 for interaction). Those results remained nonsignificant after stratification by treatment.
Table. Patient Characteristics at Primary Excision and Treatment Initiation.
| Patient Characteristic | No. (%) |
|---|---|
| At Primary Excision (N = 638) | |
| Age, median (IQR), y | 64 (52-73) |
| Female | 272 (42.6) |
| AJCC stage | |
| 0 | 20 (3.1) |
| I | 147 (23.0) |
| II | 278 (43.6) |
| III | 193 (30.3) |
| Breslow thickness, median (IQR), mm | 3.0 (1.6-5.2) |
| Ulceration | 271 (42.5) |
| Primary localization | |
| Head and neck | 95 (14.9) |
| Lower limb | 156 (24.5) |
| Upper limb | 88 (13.8) |
| Trunk | 242 (37.9) |
| Mucosal | 30 (4.7) |
| Other | 25 (3.9) |
| Time from excision to first distant recurrence, median (IQR), mo | 25 (12-55) |
| At Treatment Initiation (N = 638) | |
| Time from excision to treatment initiation, median (IQR), mo | 27 (14-57) |
| Eastern Cooperative Oncology Group performance status | |
| 0-1 | 586 (91.8) |
| 2-3 | 52 (8.2) |
| BRAF status | |
| Mutated | 295 (46.2) |
| Wild-type | 340 (53.3) |
| Lactate dehydrogenase level at or above upper limit of normal | 313 (49.1) |
| AJCC stage | |
| III | 15 (2.4) |
| IV | 623 (97.6) |
| Metastatic stage | |
| M0 | 13 (2.0) |
| M1a | 63 (9.9) |
| M1b | 147 (23.0) |
| M1c | 415 (65.0) |
| Brain metastasis | 128 (20.1) |
| Immunotherapies | |
| Ipilimumab | 104 (16.3) |
| Nivolumab | 63 (9.9) |
| Pembrolizumab | 107 (16.8) |
| Targeted therapies | |
| Dabrafenib | 34 (5.3) |
| Vemurafenib | 87 (13.6) |
| Clinical trials | 59 (9.2) |
| Chemotherapy | |
| Dacarbazine | 22 (3.4) |
| Fotemustine | 9 (1.4) |
| Temozolomide | 8 (1.3) |
| Clinical trials | 13 (2.0) |
Abbreviations: AJCC, American Joint Committee on Cancer; IQR, interquartile range.
Figure 2. Overall Survival and Progression-Free Survival According to Treatment and Time to Recurrence.
A, Overall survival according to treatment. B, Progression-free survival according to treatment. C, Overall survival according to the time to recurrence (<12 months, 12-24 months, or >24 months). D, Progression-free survival according to the time to recurrence (<12 months, 12-24 months, or >24 months). HR indicates hazard ratio.
Discussion
In our cohort, TFDR was not found to be associated with PFS or OS, regardless of whether it was analyzed as a continuous variable or a categorical variable. The association of the time interval between the primary excision and first distant relapse with survival has been debated in the literature, and contrasting opinions persist. Studies from the 1980s have shown that the time from primary excision to distant recurrence is correlated with patient survival.3,4 In the study by Karakousis et al,4 patients with stage IIIB recurrence had a longer OS when TFDR was greater than 1 year. Patients with stage IV recurrence had a longer OS when TFDR was greater than 2 years (P < .05). In the study by Balch et al,3 OS was increased only for patients who relapsed more than 1 year after diagnosis (P = .02). In the study by Cohn-Cedermark et al,5 a disease-free interval greater than 5 years reached statistical significance in the multivariate analyses (P < .01), although it appeared to be less significant in the univariate analysis (P = .16). In the study by Crowley and Seigler,6 survival was independent of TFDR, which is in accordance with our results. Comparisons of these findings are difficult not only owing to variations in the definitions of the disease-free interval but also because OS and PFS have improved during the past decade with the use of targeted therapies and immunotherapies. Our study, which involved only 52 patients who were treated with chemotherapy, is hardly comparable with previous studies.
However, our findings confirm more recent data concerning the unpredictability of cancer relapse. Different models of cancer evolution demonstrated that the physiopathological mechanisms underlying metastatic progression could not be extracted from relapse data in many cancers, including melanoma.7,8 Moreover, biology of the switch from dormant cells to an active metastatic process is largely unknown and the factors underlying this switch are only beginning to be discovered.9,10 Permissive metastatic niches seem to play an important role in this switch, but it is unclear to what extent and under what circumstances this supportive microenvironment underlies micrometastatic dormancy.11
Limitations
Although the MelBase cohort enrolls patients with stage IV or unresectable stage III cancer at the time of declaration of metastasis, the dates of primary excision and of the iterative relapses are collected retrospectively. Moreover, in most patients, we lack the estimation of the start of disease from the patient’s point of view. Indeed, the data concerning the date when the patient first noticed the skin lesion are available for only 62.1% of the patients (396 of 638). This is the reason why we used the date of primary excision as the earlier event to evaluate TFDR.
Conclusions
The timing of advanced disease was not associated with the disease-free interval in our cohort. Now that immunotherapies and targeted therapies have been approved in the adjuvant setting for patients with stage III disease, it would be interesting to analyze recurrence-free survival and PFS in relapsing patients who previously received adjuvant therapies.
References
- 1.Grob JJ, Long GV, Schadendorf D, Flaherty K. Disease kinetics for decision-making in advanced melanoma: a call for scenario-driven strategy trials. Lancet Oncol. 2015;16(13):e522-e526. doi: 10.1016/S1470-2045(15)00003-0 [DOI] [PubMed] [Google Scholar]
- 2.Heppt MV, Tietze JK, Reinholz M, et al. Disease kinetics but not disease burden is relevant for survival in melanoma of unknown primary tumor. Discov Med. 2015;20(110):231-237. [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Murad TM, Smith JW, Maddox WA, Durant JR. A multifactorial analysis of melanoma, IV: prognostic factors in 200 melanoma patients with distant metastases (stage III). J Clin Oncol. 1983;1(2):126-134. doi: 10.1200/JCO.1983.1.2.126 [DOI] [PubMed] [Google Scholar]
- 4.Karakousis CP, Temple DF, Moore R, Ambrus JL. Prognostic parameters in recurrent malignant melanoma. Cancer. 1983;52(3):575-579. doi: [DOI] [PubMed] [Google Scholar]
- 5.Cohn-Cedermark G, Månsson-Brahme E, Rutqvist LE, Larsson O, Singnomklao T, Ringborg U. Metastatic patterns, clinical outcome, and malignant phenotype in malignant cutaneous melanoma. Acta Oncol. 1999;38(5):549-557. doi: 10.1080/028418699431122 [DOI] [PubMed] [Google Scholar]
- 6.Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127(11):1303-1308. doi: 10.1001/archsurg.1992.01420110045011 [DOI] [PubMed] [Google Scholar]
- 7.Willis L, Graham TA, Alarcón T, Alison MR, Tomlinson IPM, Page KM. What can be learnt about disease progression in breast cancer dormancy from relapse data? PLoS One. 2013;8(5):e62320. doi: 10.1371/journal.pone.0062320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner-Klein M, Scheitler S, Hoffmann M, et al. Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat Commun. 2018;9(1):595. doi: 10.1038/s41467-017-02674-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merlino G, Herlyn M, Fisher DE, et al. The state of melanoma: challenges and opportunities. Pigment Cell Melanoma Res. 2016;29(4):404-416. doi: 10.1111/pcmr.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611-622. doi: 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750-764. doi: 10.1016/j.cell.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]