Key Points
Question
Are individuals with major depressive disorder who are not taking medication characterized by lower dopamine transporter levels within the brain reward system compared with psychiatrically healthy control participants?
Findings
In this cross-sectional study that analyzed positron emission tomography in 25 individuals with major depressive disorder and 23 healthy controls and postmortem data in 15 individuals with major depressive disorder and 14 healthy controls, major depressive disorder was linked to lower dopamine transporter levels in the dorsal striatum. In the imaging data, this dysfunction was exacerbated by more episodes of depression.
Meaning
Decreased dopamine transporter availability might represent a compensatory downregulation because of low dopaminergic signaling within mesolimbic pathways.
Abstract
Importance
Major depressive disorder (MDD) might involve dopamine (DA) reductions. The DA transporter (DAT) regulates DA clearance and neurotransmission and is sensitive to DA levels, with preclinical studies (including those involving inescapable stressors) showing that DAT density decreases when DA signaling is reduced. Despite preclinical data, evidence of reduced DAT in MDD is inconclusive.
Objective
Using a highly selective DAT positron emission tomography (PET) tracer ([11C] altropane), DAT availability was probed in individuals with MDD who were not taking medication. Levels of DAT expression were also evaluated in postmortem tissues from donors with MDD who died by suicide.
Design, Setting, and Participants
This cross-sectional PET study was conducted at McLean Hospital (Belmont, Massachusetts) and Massachusetts General Hospital (Boston) and enrolled consecutive individuals with MDD who were not taking medication and demographically matched healthy controls between January 2012 and March 2014. Brain tissues were obtained from the Douglas-Bell Canada Brain Bank. For the PET component, 25 individuals with current MDD who were not taking medication and 23 healthy controls recruited from McLean Hospital were included (all provided usable data). For the postmortem component, 15 individuals with depression and 14 healthy controls were considered.
Intervention
PET scan.
Main Outcomes and Measures
Striatal and midbrain DAT binding potential was assessed. For the postmortem component, tyrosine hydroxylase and DAT levels were evaluated using Western blots.
Results
Compared with 23 healthy controls (13 women [56.5%]; mean [SD] age, 26.49 [7.26] years), 25 individuals with MDD (19 women [76.0%]; mean [SD] age, 26.52 [5.92] years) showed significantly lower in vivo DAT availability in the bilateral putamen and ventral tegmental area (Cohen d range, −0.62 to −0.71), and both reductions were exacerbated with increasing numbers of depressive episodes. Unlike healthy controls, the MDD group failed to show an age-associated reduction in striatal DAT availability, with young individuals with MDD being indistinguishable from older healthy controls. Moreover, DAT availability in the ventral tegmental area was lowest in individuals with MDD who reported feeling trapped in stressful circumstances. Lower DAT levels (and tyrosine hydroxylase) in the putamen of MDD compared with healthy controls were replicated in postmortem analyses (Cohen d range, −0.92 to −1.15).
Conclusions and Relevance
Major depressive disorder, particularly with recurring episodes, is characterized by decreased striatal DAT expression, which might reflect a compensatory downregulation due to low DA signaling within mesolimbic pathways.
This cross-sectional study explores dopamine transporter availability in individuals with major depressive disorder who were not taking medication and levels of dopamine transporter expression in postmortem tissues from donors with major depressive disorder who died by suicide.
Introduction
Despite decades of research, the molecular underpinnings of major depressive disorder (MDD) remain incompletely understood. Among various targets, dopamine (DA) has received considerable attention, particularly owing to its role in motivation, which is affected in MDD.1 Growing data point to blunted DA transmission in MDD. The most direct evidence stems from DA depletion studies, which have described rapid increases in depressive symptoms after catecholamine depletion in remitted MDD.2,3,4 Second, functional magnetic resonance imaging studies have shown that MDD is associated with reduced reward-associated activation within DA-rich regions, including the ventral (nucleus accumbens [NAc]) and dorsal (caudate and putamen) striatum.5,6 Notably, such blunting could be normalized by a pharmacological challenge hypothesized to transiently increase DA signaling,7 suggesting that blunted striatal responses might be linked to hypodopaminergic mechanisms. Third, animal models relevant to depression reliably induce anhedonic phenotypes and mesolimbic DA abnormalities.1,8
Neurobiologically, these preclinical models trigger a long-lasting downregulation of mesolimbic DA pathways8 and reduced levels of the DA transporter (DAT).9 Reduced DAT levels, interpreted as reflecting compensatory DAT downregulation owing to blunted DA transmission, have been reported in the NAc, caudate, or putamen of animals exposed to chronic stressors.9,10,11 Reduced striatal DAT levels have been also described in rats bred for increased vulnerability to depression.12
The DAT, which is localized on the membrane of presynaptic terminals, plays a central role in regulating the intensity and duration of dopaminergic transmission in the synaptic cleft by reuptaking DA into presynaptic cells in the striatum and midbrain (eg, ventral tegmental area [VTA]). In addition to regulating the clearance of extracellular striatal DA, the DAT modulates the signal-to-noise ratio of DA neurotransmission and affects presynaptic DA levels.13 Critically, DAT is regulated by extracellular DA levels. Specifically, DA synthesis depletion reduces striatal DAT density and function,14,15 highlighting compensatory DAT downregulation to adjust to reduced DA concentrations. Similarly, DAT downregulation was seen in surviving midbrain DA neurons after the loss of striatal DA terminals.16 Collectively, these findings highlight plastic changes in DAT levels depending on striatal DA availability.
Despite these preclinical data, evidence from human studies is inconclusive. In vivo evidence from positron emission tomography (PET) or single-photon emission computed tomography (SPECT) studies in MDD has been inconsistent. A recent meta-analysis revealed no significant associations.17 However, 11 of the included 12 studies (91.7%) used SPECT, which has poorer spatial resolution compared with PET. Moreover, some of the tracers used (eg, [123I]β-CIT) have incomplete specificity for DAT vs the serotonin transporter, which complicates interpretations. This meta-analysis revealed considerable heterogeneity across studies, and it is unclear whether clinical heterogeneity contributed to inconsistencies. Similarly, to our knowledge, few human postmortem studies have investigated DAT in MDD18 and none have assessed striatal DAT levels in MDD.
Our goal was to address these limitations by using a highly selective DAT tracer ([11C]altropane) in individuals with MDD who were not taking medication and demographically matched healthy controls. Compared with other DAT tracers, altropane offers several advantages, including a rapid and specific kinetics in DA-rich striatal regions and high selectivity for DAT.19,20 Moreover, clinical heterogeneity was addressed by only including individuals with MDD who were not taking medication (mostly nonsmokers) who were characterized using clinical scales that captured constructs conceptually associated with DA, including anhedonia and the perception of entrapment. The latter was selected because of a convergence of preclinical and clinical findings. Preclinically, depression-like responses21,22 and DAT downregulation1,8 have been observed in stressful circumstances in which escape is blocked. These data have been complemented by human findings that chronic stressors characterized by the perception of being trapped in inescapable situations are particularly depressogenic23,24 and are prospectively associated with depression onset25 and reoccurrence.26 Finally, DAT expression was evaluated in postmortem tissues from donors with MDD for independent corroboration. We hypothesized that, compared with healthy controls, MDD would be characterized by lower striatal DAT density, reflecting a compensatory downregulation due to chronically reduced DA signaling.
Methods
PET Study
Participants
Participants included 23 healthy controls and 25 individuals with MDD who were not taking medication (Table). Because DAT expression declines with age,30 the age range was restricted to 18 to 45 years. Participants provided written informed consent according to a protocol approved by the Partners Healthcare institutional review board. Eligibility was established using the structured clinical interview for the DSM-IV31 (eMethods and eTable 1 in the Supplement). At screening, participants were administered several clinical scales, including the 17-item Hamilton Depression Inventory.28
Table. Sociodemographic and Clinical Data for 23 Healthy Controls and 25 Participants With MDD.
| Characteristic | Participants, Mean (SD) | Statistics | P Value | |
|---|---|---|---|---|
| Control (n = 23) | MDD (n = 25) | |||
| Age, ya | 26.49 (7.26) | 26.52 (5.92) | t = −0.02 | >.98 |
| Sex ratio (male/female) | 10/13 | 6/19 | χ2 = 2.05 | >.15 |
| Luteal, No. (%)b | 5 (41.7) | 6 (31.6) | χ2 = 0.33 | >.55 |
| Follicular, No. (%) | 7 (58.3) | 13 (68.4) | NA | NA |
| Education, y | 15.67 (1.93) | 16.40 (2.42) | t = −1.14 | >.25 |
| White, No. (%) | 14 (60.9) | 18 (72.0) | χ2 = 0.67 | >.40 |
| Never married, No. (%) | 17 (73.9) | 23 (92.0) | χ2 = 2.82 | >.09 |
| Average caffeine consumption, mg/d | 116.93 (95.86) | 126.78 (93.62) | t = −0.36 | >.72 |
| Caffeine consumed 24 h before the PET session, mg/d | 77.55 (120.84) | 103.60 (101.73) | t = −0.80 | >.42 |
| Current smokers, No. (%)c | 2 (9.5) | 3 (12.5) | χ2 = 1.00 | >.75 |
| Income, $, No. (%)d | NA | NA | χ2 = 0.93 | >.25 |
| <50 000 | 13 (56.5) | 16 (69.6) | NA | NA |
| 50 000-100 000 | 8 (34.8) | 6 (26.1) | ||
| >100 000 | 2 (8.7) | 1 (4.3) | ||
| Age of MDD onset, y | NA | 16.46 (4.71) | NA | NA |
| Lifetime MDEe | ||||
| 1 MDE | NA | 3 | NA | NA |
| 2-3 MDEs | NA | 8 | ||
| ≥5 MDEs | NA | 13 | ||
| HRSD | 0.55 (1.01) | 17.91 (3.79) | t = −20.79 | <.001 |
| BDI-IIa | 0.48 (1.31) | 25.80 (8.65) | t = −8.90 | <.001 |
| SHAPSa | 20.74 (5.54) | 33.20 (4.11) | t = −13.89 | <.001 |
| EESa | 1.44 (2.15) | 19.64 (8.53) | t = −9.94 | <.001 |
Abbreviations: BDI-II, Beck Depression Inventory-II27; EES, External Entrapment Scale22; HRSD, Hamilton Rating Scale for Depression28; MDD, major depressive disorder; MDE, major depressive episode; NA, not applicable; PET, positron emission tomography; SHAPS, Snaith Hamilton Pleasure Scale.29
Variable assessed at the PET session.
Missing for 1 healthy control.
Missing for 2 healthy controls and 1 participant with MDD.
Missing for 2 participants with MDD.
Missing for 1 participant with MDD.
Procedure
Approximately 10 mCi of [11C] altropane was injected intravenously (MDD: mean [SD]. 9.00 [0.47]; controls: mean, [SD], 9.19 [0.34]; P > .13; range, 8.27-10.22 mCi) and serial PET images were acquired. After the scan, participants filled out various questionnaires, including the Beck Depression Inventory-II (BDI-II27) and the Snaith Hamilton Pleasure Scale (SHAPS29), to assess levels of depressive symptoms and anhedonia, respectively, as well as the External Entrapment Scale22 (eMethods in the Supplement). Data regarding the apparatus and the data analyses are described in eMethods in the Supplement.
Statistics
A multivariate analysis of covariance with hemisphere (left and right), striatal region (caudate, putamen, and NAc; eFigure 1 in the Supplement) and group (MDD and healthy controls) as factors and age as a covariate was run on binding potential (BPND). For the VTA, BPND values were averaged across the hemispheres, and entered in an analysis of covariance with group as a factor (covariate, age). Analyses were also repeated excluding age as a covariate and using partial volume corrected (PVC) data.32,33
For regions showing group differences in BPND, correlation analyses were performed with 3 clinical scales (BDI, SHAPS, and Entrapment Scale) and the number of lifetime major depressive episodes (MDEs). For number of MDEs, because the distribution was skewed toward the right, we rebinned data by categorizing participants with MDD who reported 1 MDE, between 2 and 4 MDEs, and 5 or more MDEs.34 For these analyses, healthy controls were included (MDE = 0), and Spearman rank correlations were performed. For the clinical scales, Pearson correlations were run in the MDD group only. All correlation analyses were conducted using age-residualized BPND values. Statistical significance was set at P < .05.
Postmortem Study
Frozen tissue blocks containing the striatum from 15 individuals with depression and 14 psychiatrically healthy controls were obtained from the Douglas-Bell Canada Brain Bank (eMethods in the Supplement). All individuals with depression died by suicide and psychiatrically healthy controls died of natural or accidental causes. The cause of death for each participant was assessed by the Quebec Coroner’s office.
Results
Imaging Studies
Groups were well matched with respect to sociodemographic variables, menstrual cycle, and caffeine consumption (Table).27,28,29 At screening, the MDD group had a mean (SD) 17-item Hamilton Rating Scale for Depression score of 17.91 (3.79; range: 12-28), indicating, on average, moderate depression; at the PET session, the MDD group reported a mean (SD) BDI-II score of 25.80 (8.65), also indicating moderate depression. Four participants with MDD (16.0%) had a current comorbidity of social phobia (secondary to MDD) and 1 participant with MDD (4.0%) had a current comorbidity of dysthymic disorders. Forty of 45 participants (88.9%) were nonsmokers (smoking status was missing for 2 healthy controls [8.7%] and 1 participant with MDD [4.0%]).
Group Differences in Striatal and Midbrain DAT BPND
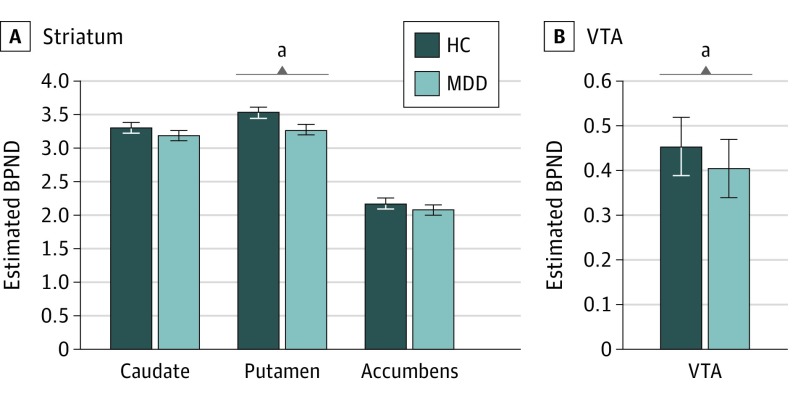
A group (healthy controls and MDD) × region (caudate, putamen, and NAc) × hemisphere multivariate analysis of covariance (covariate, age) yielded a significant main effect of region (Wilks λ [2,44] = 34.36; P < .001) because of significantly higher BPND in the putamen than caudate, which in turn had significantly higher BPND than the NAc (all pairwise Bonferroni-corrected simple effects: P < .00003). The effect of age (covariate) was also significant (F1,45 = 7.83; P < .01) because of decreasing striatal BPND with increasing age. Critically, the group × region interaction (Wilks λ2,44 = 4.15; P < .02) was significant (Figure 1). Bonferroni-corrected simple effects revealed that, compared with controls, the MDD group had significantly lower BPND in the bilateral putamen (P < .03; Cohen d = −0.66), whereas groups did not differ in the caudate (d = −0.30) and NAc (d = −0.23) (P > 0.28). When excluding age as a covariate, the group × region interaction (F2,45 = 4.20; P < .02) and reduced bilateral putamen BPND in MDD (P < .03; Cohen d = −0.62) remained, indicating that age did not influence the group × region interaction. Groups also differed in putamen BPND when using PVC data (t [46] = 2.04; P < .047; d = −0.59).
Figure 1. Dopamine Transporter (DAT) Availability in Major Depressive Disorder (MDD).
Age-residualized DAT availability (as assessed by binding potential [BPND]) in the healthy control (HC; n = 23) and MDD (n = 25) groups for striatal regions (A) and the ventral tegmental area (VTA) (B).
aP < .05.
For the VTA, analyses including (F1,48 = 6.04; P < .02; d = −0.71) or excluding (t [46] = 2.45; P < .02; d = −0.71) age as a covariate revealed a main effect of group due to overall lower BPND in MDD than controls (Figure 1B). However, this group difference was not seen when using PVC data (t [46] = 1.18; P > .24). For the putamen and VTA, all significant effects were confirmed when analyses were rerun only in nonsmokers (eResults in the Supplement).
Association With Number of Lifetime MDE
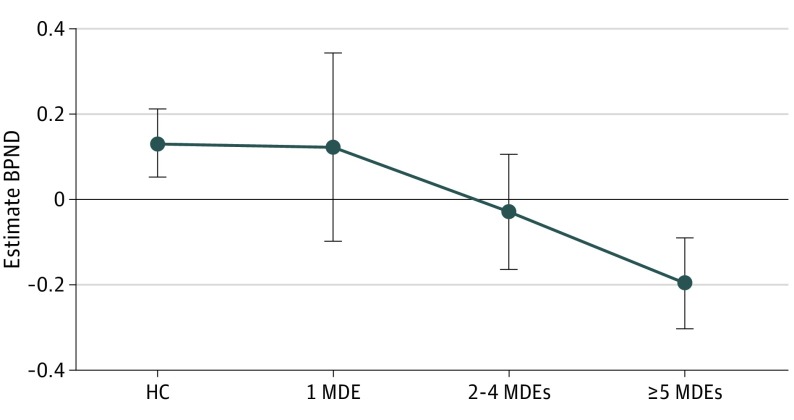
Among the participants with MDD, 3 (12.0%) reported 1 MDE, 8 (32.0%) had experienced between 2 and 4 MDEs, and 13 (52.0%) had experienced 5 or more MDEs. Spearman rank correlations among all participants (including healthy controls) indicated that, for the putamen (ρ = −0.36; P < .01; Figure 2) and the VTA (ρ = −0.40; P < .01), age-residualized BPND was negatively correlated with the numbers of lifetime MDEs. Because number of MDEs and age could be strongly related, hierarchical regressions entering age in the first step were performed (eResults in the Supplement). For both the putamen and VTA, number of MDEs continued to predict raw (non–age-residualized) BPND (putamen, ΔR2 = 0.111; P < .02; VTA, ΔR2 = 0.125; P < .01). The association of age with striatal and midbrain DAT BPND is described in eResults in the Supplement.
Figure 2. Dopamine Transporter (DAT) Availability as a Function of the Number of Major Depressive Episodes.
Age-residualized DAT availability (as assessed by binding potential [BPND]) in the mean (averaged across left and right) putamen as a function of the lifetime number of major depressive episodes (MDEs). HC indicates healthy control.
Association With Clinical Symptoms
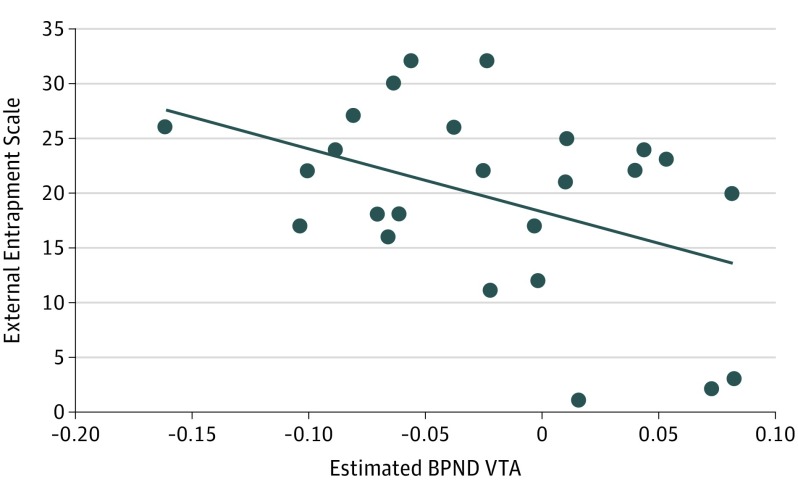
Owing to the group findings reported above, correlations were performed between (1) mean age-residualized BPND in the putamen or VTA and (2) scores on 3 clinical scales (SHAPS, BDI, and Entrapment scale), leading to a total of 6 tests (Bonferroni correction: P < .05/6 = .0083). Contrary to hypotheses, no correlations emerged for SHAPS scores (rs <0.38; P > .06). Mean age-residualized VTA BPND was negatively associated with scores on the External Entrapment Scale (r = −0.43; P < .03), indicating that an increasing perception of entrapment was associated with lower VTA BPND (Figure 3). Highlighting the specificity of this finding, External Entrapment Scale scores predicted VTA BPND even when entering BDI and SHAPS scores in the first step of a hierarchical regression (ΔR2 = 0.193; ΔF1,21 = 6.31; P < .02).
Figure 3. Dopamine Transporter (DAT) Availability as a Function of Perceived Entrapment.
Scatterplot between external entrapment scale score and mean (averaged across left and right) ventral tegmental area (VTA) DAT availability within the major depressive disorder group. BPND indicates binding potential.
Human Postmortem Studies
In light of the PET findings, Western blots on putamen were used to measure DAT expression (the VTA was unavailable). Owing to the hypothesized role of the NAc in MDD, Western blot analyses were also performed on the NAc (eResults in the Supplement). Depressed and control groups did not differ in age, sex ratio, postmortem time interval, or pH values (eTable 1 in the Supplement).
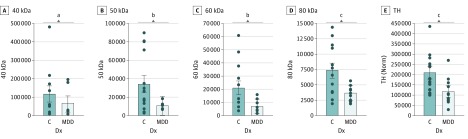
In the MDD group, tyrosine hydroxylase (TH) expression was significantly lower in the putamen (P = .007; d = −1.11) (Figure 4; eTable 2 in the Supplement). The expression of the mature form of DAT (80 kDa)35,36 was significantly decreased in MDD (P = .005; d = −1.20). In addition, DAT 50 kDa (P = .02; d = −0.99) and 60 kDa (P = .01; d = −0.99), thought to represent intermediate glycosylated forms, were also significantly decreased. In contrast, the putative DAT precursor (40 kDa) did not differ. For TH and DAT, none of the covariates significantly affected group effects. Only the TH and 80 kDa group differences survived a Bonferroni correction (P < .05/5 = 0.01).
Figure 4. Dopamine Transporter (DAT) and Tyrosine Hydroxylase (TH) Expression in Postmortem Analyses.
Dopamine transporter and TH expression levels are decreased in the putamen of people with major depressive disorder (MDD). Box blots show the expression levels (mean and SE) for DAT (40, 50, 60, and 80 kDa) (A-D) and TH (60 kDa) (E). Protein levels are expressed as a normalized signal in fluorescence arbitrary units (LI-COR Odyssey CLx scanner). C indicates control group; Dx, diagnosis; Norm, normalized signal.
aNot significant (P > .05).
bSignificant (P = .05 to P = .01).
cHighly significant (P = .01 to P = .001).
Discussion
Dopamine dysfunction has been implicated in MDD, but in vivo evidence has been elusive.1,17 Abundant evidence indicates that DAT plays a pivotal role in synaptic DA regulation and reflects the integrity and function of the DA system.13 Because of evidence that stress-associated animal models of depression and pharmacological manipulations depleting DA lead to reduced DAT levels, interpreted as reflecting a compensatory downregulation to regulate DA levels, we hypothesized that individuals with MDD who were not taking medication would show blunted striatal and midbrain DAT availability. We further hypothesized that such dysfunction would be greatest in individuals with MDD who reported elevated anhedonic symptoms and perceived entrapment (a construct associated with helplessness; eMethods in the Supplement). Several findings emerged.
First, compared with controls, the MDD group had significantly lower DAT availability in the bilateral putamen and VTA. Notably, putamen and VTA availability were inversely associated with the lifetime number of MDEs. Although this latter observation is novel, prospective studies are needed to determine whether these findings represent a cumulative effect or potential premorbid marker of increased recurrence risk. Second, unlike controls, the MDD group failed to show age-associated declines in DAT availability. Third, contrary to our hypotheses, striatal and midbrain DAT availability was not moderated by anhedonic symptoms. However, in the VTA, a negative association between external entrapment scores and VTA DAT availability emerged (eResults in the Supplement). Thus, individuals with MDD who reported being trapped in putatively inescapable circumstances showed the lowest VTA DAT availability. These findings are intriguing, particularly considering prior reports that external entrapment scores prospectively predicted depression37 and MDD reoccurrence26 12 to 16 months later. Fourth, a corroboration of reduced putamen DAT in MDD emerged in postmortem analyses.
The current findings of lower striatal DAT availability in MDD agree with a prior PET study,38 but findings have been inconsistent.17 However, 11 of the 12 studies (91.7%) included in a prior meta-analysis,17 as well as 14 of 15 studies (93.3%) included in a recent literature review,39 used SPECT and suboptimal DAT tracers. Notably, the only PET study38 reported reduced DAT availability in the putamen in a small MDD group (n = 9). In addition to showing a 28-fold selectivity for DAT over the serotonin transporter (vs 1:1 for [123I]β-CIT and 3:1 for TRODAT19,20), altropane accumulates within 30 minutes almost exclusively to DA-rich striatal regions,40 and the putamen:cerebellum ratio is 120:1, highlighting an exceptional degree of binding selectivity.40 We speculate that using this highly selective PET tracer allowed us to more reliably probe DAT function in unmedicated MDD. Our findings fit prior reports of negative associations between dorsal striatal DAT availability and depressive symptoms in patients with MDD41 and Parkinson disease.42 Moreover, recovery from MDD has been associated with an increase in midbrain DAT availability,43 and 6-week treatment with escitalopram increased striatal DAT availability by 20%.41
Strengths and Limitations
The strengths of this study are that all individuals with MDD were unmedicated, had minimal degrees of comorbidity, and most were nonsmokers (21 of 24 [87.5%]). Focusing on an unmedicated sample is particularly important because of evidence that even brief treatment with selective serotonin reuptake inhibitors led to a 10% to 20% increase in striatal DAT availability.44
A novel finding is that, in MDD, the perception of being trapped in inescapable circumstances was associated with lower bilateral VTA availability. This link is intriguing, particularly in light of abundant evidence indicating that (1) exposure to stressful situations in which escape is blocked suppresses approach behavior and downregulates mesolimbic DA pathways1,8 and (2) stressful life events characterized by entrapment are particularly depressogenic.29,32,33,34 However, because this correlation finding did not survive correction for multiple comparisons, replication is warranted.
Although PET imaging cannot pinpoint the source of DAT downregulation, increased inflammation and oxidative stress might be candidate mechanisms. Based on preclinical findings,16,45 it is possible that increased inflammation and/or oxidative stress contributes to reducing DA signaling, which could eventually result in compensatory DAT downregulation. Consistent with this, mounting evidence points to increased inflammation in MDD,46 and inflammation acutely decreases striatal DAT expression levels.47 Moreover, and replicating prior findings,30 DAT availability was negatively associated with age among the control, but not MDD, group. Notably, participants with MDD younger than the median (SD) age (21.72 [1.48] years) were indistinguishable from healthy controls (32.09 [6.70] years) and individuals with MDD (30.94 [4.86] years) older than the median age. Importantly, comparatively younger and older individuals with MDD did not differ in their number of lifetime MDEs (eFigure 2 in the Supplement) and age was not associated with depressive (Hamilton Rating Scale for Depression and BDI) or anhedonic (SHAPS) symptoms, indicating that these variables did not confound findings. Larger samples should evaluate whether the current findings reflect accelerated aging in MDD.48 Because oxidative stress has been implicated in aging49 and DA uptake has been found to be inhibited by oxidative stress,50,51 it is possible that the current striatal and midbrain DAT downregulation might be due to increased oxidative stress in MDD. Two additional, and not reciprocally exclusive, potential mechanisms are suggested by the postmortem results. First, a disruption of DAT expression may be due to altered posttranslational modifications leading to a decrease of its active, mature form.35,36 Our postmortem results show that levels of the immature DAT form were not altered in donors with MDD, whereas increasingly glycosylated forms up to the active 80 kDa form were decreased. These findings suggest that a disruption of glycosylation mechanisms may lead to decreased DAT activity. Notably, the reduction of mature (glycosylated) forms of DAT has been described in surviving DA neurons after a loss of striatal DA terminals due to an oxidative injury in models of Parkinson disease.16 Second, concurrent TH and DAT level decreases in MDD fit the possibility that DA terminals within the putamen may be reduced in MDD. If so, the result would be an overall decrease of DA tone. This possibility may be at odds with results showing normal levels of the immature forms of DAT, suggesting that dopaminergic terminals are still present in depression but express an immature, inactive form of DAT. However, it is plausible that both mechanisms may be involved and contribute to our findings. This possibility will be tested in future studies.
Despite strengths, including the inclusion of participants not taking medication, the use of a highly selective PET tracer, groups that were well matched for variables that could affect DA (eg, age, menstrual phase, smoking status, and caffeine use), and replication of the main finding in postmortem analyses, limitations should be emphasized. First, the MDD imaging sample was relatively young and moderately depressed; thus, it is unclear whether the current findings will replicate in different samples. This limitation is, at least partially, mitigated by parallel findings in postmortem studies, comprised of older donors with severe MDD that was likely to have led to suicide. Although results from postmortem studies cannot exclude that suicide, rather than MDD, is associated with DAT and TH level decreases, the convergence between imaging and postmortem results suggests otherwise. In addition, in the PET sample, suicidal ideation did not affect results (eResults in the Supplement). Second, information on pharmacological treatment for the participants included in postmortem studies was limited to toxicology data. It is possible that long-term treatment may affect DAT and TH expression. Again, the similarities between postmortem and imaging findings, the latter including participants not taking medication, mitigate this concern. Third, our cross-sectional design prevented us from testing whether DAT downregulation is a potential cause or consequence of recurrence risk. Fourth, group differences in the VTA were not confirmed when using PVC data, indicating that caution should be used when interpreting VTA findings. Finally, it is unclear why BPND differences emerged in the dorsal but not ventral striatum, although it is important to emphasize that, in humans, DAT expression is highest in the dorsal striatum and weakest in the NAc.52
Conclusions
These findings provide convergent evidence from in vivo molecular imaging and postmortem assays that MDD is characterized by DAT downregulation, likely reflecting a compensatory downregulation due to blunted DA signaling within reward-associated pathways, particularly with increasing numbers of prior MDEs.
eMethods. Expanded Methodology
eResults. Expanded Results
eDiscussion. Expanded Discussion
eFigure 1. (A) Masks showing caudate (Cd, yellow), putamen (Pt, red), and nucleus accumbens (NAc, green) region-of-interest. (B) Masks showing the ventral tegmental area (VTA, blue) region-of-interest.
eFigure 2. (a) Scatterplot between age (at the day of the PET scan) and DAT availability (as assessed by binding potential (BPND)) in the bilateral putamen for the healthy control (gray triangles) and MDD (black dots) groups.
eFigure 3. Examples of Western blots for DAT and TH from control and MDD subjects.
eTable 1. Demographic and clinical information for the subject cohort included in the post-mortem study.
eTable 2. Results from human postmortem studies showing raw data, significance values and effect sizes (Cohen’s d values) for DAT and TH.
References
- 1.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393-423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasler G, Fromm S, Carlson PJ, et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65(5):521-531. doi: 10.1001/archpsyc.65.5.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasler G, Luckenbaugh DA, Snow J, et al. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry. 2009;66(3):201-205. doi: 10.1016/j.biopsych.2009.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homan P, Neumeister A, Nugent AC, Charney DS, Drevets WC, Hasler G. Serotonin versus catecholamine deficiency: behavioral and neural effects of experimental depletion in remitted depression. Transl Psychiatry. 2015;5(3):e532-e532. doi: 10.1038/tp.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64-73. doi: 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702-710. doi: 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Admon R, Kaiser RH, Dillon DG, et al. Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry. 2017;174(4):378-386. doi: 10.1176/appi.ajp.2016.16010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36(1):79-89. doi: 10.1016/j.neubiorev.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 9.Lucas LR, Celen Z, Tamashiro KL, et al. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449-457. doi: 10.1016/j.neuroscience.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 10.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19(7):1863-1874. doi: 10.1111/j.1460-9568.2004.03286.x [DOI] [PubMed] [Google Scholar]
- 11.Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108-115. doi: 10.1016/j.brainres.2007.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao X, Paré WP, Tejani-Butt S, Paré WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):913-919. doi: 10.1016/S0278-5846(03)00150-7 [DOI] [PubMed] [Google Scholar]
- 13.Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016;6(3):123-148. doi: 10.1016/j.baga.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol. 1996;298(1):27-30. doi: 10.1016/0014-2999(95)00770-9 [DOI] [PubMed] [Google Scholar]
- 15.Han S, Rowell PP, Carr LA. D2 autoreceptors are not involved in the down-regulation of the striatal dopamine transporter caused by alpha-methyl-p-tyrosine. Res Commun Mol Pathol Pharmacol. 1999;104(3):331-338. [PubMed] [Google Scholar]
- 16.Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, et al. The dopamine transporter is differentially regulated after dopaminergic lesion. Neurobiol Dis. 2010;40(3):518-530. doi: 10.1016/j.nbd.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 17.Li Z, He Y, Tang J, Zong X, Hu M, Chen X. Molecular imaging of striatal dopamine transporters in major depression—a meta-analysis. J Affect Disord. 2015;174:137-143. doi: 10.1016/j.jad.2014.11.045 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald ML, Kassir SA, Underwood MD, Bakalian MJ, Mann JJ, Arango V. Dysregulation of striatal dopamine receptor binding in suicide. Neuropsychopharmacology. 2017;42(4):974-982. doi: 10.1038/npp.2016.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischman AJ, Bonab AA, Babich JW, et al. [(11)C, (127)I] altropane: a highly selective ligand for PET imaging of dopamine transporter sites. Synapse. 2001;39(4):332-342. doi: [DOI] [PubMed] [Google Scholar]
- 20.Madras BK, Meltzer PC, Liang AY, Elmaleh DR, Babich J, Fischman AJ. Altropane, a SPECT or PET imaging probe for dopamine neurons: I. dopamine transporter binding in primate brain. Synapse. 1998;29(2):93-104. doi: [DOI] [PubMed] [Google Scholar]
- 21.Dixon AK, Fisch HU, Huber C, Walser A. Ethological studies in animals and man, their use in psychiatry. Pharmacopsychiatry. 1989;22(suppl 1):44-50. doi: 10.1055/s-2007-1014624 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P, Allan S. The role of defeat and entrapment (arrested flight) in depression: an exploration of an evolutionary view. Psychol Med. 1998;28(3):585-598. doi: 10.1017/S0033291798006710 [DOI] [PubMed] [Google Scholar]
- 23.Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol Med. 1995;25(1):7-21. doi: 10.1017/S003329170002804X [DOI] [PubMed] [Google Scholar]
- 24.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164(10):1521-1529. doi: 10.1176/appi.ajp.2007.06091564 [DOI] [PubMed] [Google Scholar]
- 25.Broadhead JC, Abas MA. Life events, difficulties and depression among women in an urban setting in Zimbabwe. Psychol Med. 1998;28(1):29-38. doi: 10.1017/S0033291797005618 [DOI] [PubMed] [Google Scholar]
- 26.Sturman ED, Mongrain M. Entrapment and perceived Status in graduate students experiencing a recurrence of major depression. Can J Behav Sci. 2008;40(3):505-519. doi: 10.1037/0008-400X.40.3.185 [DOI] [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99-103. doi: 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- 30.Spencer TJ, Biederman J, Madras BK, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059-1061. doi: 10.1016/j.biopsych.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM-IV-TR Axis I Disorders-Non-patient Edition (SCID-I/NP, 11/2002 revision). New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 32.Greve DN, Salat DH, Bowen SL, et al. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334-343. doi: 10.1016/j.neuroimage.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greve DN, Svarer C, Fisher PM, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225-236. doi: 10.1016/j.neuroimage.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treadway MT, Waskom ML, Dillon DG, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77(3):285-294. doi: 10.1016/j.biopsych.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14(2):43-49. doi: 10.1016/0165-6147(93)90029-J [DOI] [PubMed] [Google Scholar]
- 36.Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, et al. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol Dis. 2009;36(3):494-508. doi: 10.1016/j.nbd.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 37.Griffiths AW, Wood AM, Maltby J, Taylor PJ, Tai S. The prospective role of defeat and entrapment in depression and anxiety: a 12-month longitudinal study. Psychiatry Res. 2014;216(1):52-59. doi: 10.1016/j.psychres.2014.01.037 [DOI] [PubMed] [Google Scholar]
- 38.Meyer JH, Krüger S, Wilson AA, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12(18):4121-4125. doi: 10.1097/00001756-200112210-00052 [DOI] [PubMed] [Google Scholar]
- 39.Nikolaus S, Mamlins E, Hautzel H, Müller H-W. Acute anxiety disorder, major depressive disorder, bipolar disorder and schizophrenia are related to different patterns of nigrostriatal and mesolimbic dopamine dysfunction. Rev Neurosci. 2018;0(0). doi: 10.1515/revneuro-2018-0037 [DOI] [PubMed] [Google Scholar]
- 40.Madras BK, Gracz LM, Meltzer PC, et al. Altropane, a SPECT or PET imaging probe for dopamine neurons: II. Distribution to dopamine-rich regions of primate brain. Synapse. 1998;29(2):105-115. doi: [DOI] [PubMed] [Google Scholar]
- 41.Rominger A, Cumming P, Brendel M, et al. Altered serotonin and dopamine transporter availabilities in brain of depressed patients upon treatment with escitalopram: A [123 I]β-CIT SPECT study. Eur Neuropsychopharmacol. 2015;25(6):873-881. doi: 10.1016/j.euroneuro.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 42.Hesse S, Meyer PM, Strecker K, et al. Monoamine transporter availability in Parkinson’s disease patients with or without depression. Eur J Nucl Med Mol Imaging. 2009;36(3):428-435. doi: 10.1007/s00259-008-0979-7 [DOI] [PubMed] [Google Scholar]
- 43.Laasonen-Balk T, Viinamäki H, Kuikka JT, Husso-Saastamoinen M, Lehtonen J, Tiihonen J. 123I-beta-CIT binding and recovery from depression. A six-month follow-up study. Eur Arch Psychiatry Clin Neurosci. 2004;254(3):152-155. doi: 10.1007/s00406-004-0458-5 [DOI] [PubMed] [Google Scholar]
- 44.Merens W, Booij L, Van Der Does AJ. Residual cognitive impairments in remitted depressed patients. Depress Anxiety. 2008;25(6):E27-E36. doi: 10.1002/da.20391 [DOI] [PubMed] [Google Scholar]
- 45.Felger JC, Mun J, Kimmel HL, et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38(11):2179-2187. doi: 10.1038/npp.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151. doi: 10.1007/7854_2012_211 [DOI] [PubMed] [Google Scholar]
- 47.Lai YT, Tsai YP, Cherng CG, et al. Lipopolysaccharide mitigates methamphetamine-induced striatal dopamine depletion via modulating local TNF-α and dopamine transporter expression. J Neural Transm (Vienna). 2009;116(4):405-415. doi: 10.1007/s00702-009-0204-2 [DOI] [PubMed] [Google Scholar]
- 48.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327-338. doi: 10.1002/da.20686 [DOI] [PubMed] [Google Scholar]
- 49.Barja G. Free radicals and aging. Trends Neurosci. 2004;27(10):595-600. doi: 10.1016/j.tins.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 50.Berman SB, Zigmond MJ, Hastings TG. Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem. 1996;67(2):593-600. doi: 10.1046/j.1471-4159.1996.67020593.x [DOI] [PubMed] [Google Scholar]
- 51.Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum. Eur J Pharmacol. 1997;334(1):111-114. doi: 10.1016/S0014-2999(97)01175-8 [DOI] [PubMed] [Google Scholar]
- 52.González-Hernández T, Barroso-Chinea P, De La Cruz Muros I, Del Mar Pérez-Delgado M, Rodríguez M. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J Comp Neurol. 2004;479(2):198-215. doi: 10.1002/cne.20323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Expanded Methodology
eResults. Expanded Results
eDiscussion. Expanded Discussion
eFigure 1. (A) Masks showing caudate (Cd, yellow), putamen (Pt, red), and nucleus accumbens (NAc, green) region-of-interest. (B) Masks showing the ventral tegmental area (VTA, blue) region-of-interest.
eFigure 2. (a) Scatterplot between age (at the day of the PET scan) and DAT availability (as assessed by binding potential (BPND)) in the bilateral putamen for the healthy control (gray triangles) and MDD (black dots) groups.
eFigure 3. Examples of Western blots for DAT and TH from control and MDD subjects.
eTable 1. Demographic and clinical information for the subject cohort included in the post-mortem study.
eTable 2. Results from human postmortem studies showing raw data, significance values and effect sizes (Cohen’s d values) for DAT and TH.