Key Points
Question
Are measurements of physiological stenosis severity associated with angina-limited exercise time in patients with stable angina and coronary stenosis?
Findings
In this cohort study, anatomical stenosis characteristics were not significantly associated with angina-limited exercise time. Conversely, fractional flow reserve, instantaneous wave-free ratio, hyperemic stenosis resistance, and coronary flow reserve were all associated with angina-limited exercise time.
Meaning
In a selected group of patients with severe, single-vessel stable angina, fractional flow reserve, instantaneous wave-free ratio, hyperemic stenosis resistance, and coronary flow reserve were all modestly correlated with angina-limited exercise time to varying degrees; notwithstanding the limited sample size, no clear association was demonstrated between anatomical stenosis severity and angina-limited exercise time.
This cohort study assesses the association between all commonly-used indices of physiological stenosis severity and angina-limited exercise time in patients with stable angina.
Abstract
Importance
Physiological stenosis assessment is recommended to guide percutaneous coronary intervention (PCI) in patients with stable angina.
Objective
To determine the association between all commonly used indices of physiological stenosis severity and angina-limited exercise time in patients with stable angina.
Design, Setting, and Participants
This cohort study included data (without follow-up) collected over 1 year from 2 cardiac hospitals. Selected patients with stable angina and physiologically severe single-vessel coronary artery disease presenting for clinically driven elective PCI were included.
Exposures
Fractional flow reserve (FFR), instantaneous wave-free ratio (iFR), hyperemic stenosis resistance (HSR), and coronary flow reserve (CFR) were measured invasively. Immediately after this, patients maximally exercised on a catheter-table–mounted supine ergometer until they developed rate-limiting angina. Subsequent PCI was performed in most patients, followed by repeat maximal supine exercise testing.
Main Outcomes and Measures
Associations between FFR, iFR, HSR, CFR, and angina-limited exercise time were assessed using linear regression and Pearson correlation coefficients. Additionally, the associations between the post-PCI increment in exercise time and baseline FFR, iFR, HSR, and CFR were assessed.
Results
Twenty-three patients (21 [91.3%] of whom were male; mean [SD] age, 60.6 [8.1] years) completed the pre-PCI component of the study protocol. Mean (SD) stenosis diameter was 74.6% (10.4%). Median (interquartile range [IQR]) values were 0.54 (0.44-0.72) for FFR, 0.53 (0.38-0.83) for iFR, 1.67 (0.84-3.16) for HSR, and 1.35 (1.11-1.63) for CFR. Mean (SD) angina-limited exercise time was 144 (77) seconds. Anatomical stenosis characteristics were not significantly associated with angina-limited exercise time. Conversely, FFR (R2 = 0.27; P = .01), iFR (R2 = 0.46; P < .001), HSR (R2 = 0.39; P < .01), and CFR (R2 = 0.16; P < .05) were all associated with angina-limited exercise time. Twenty-one patients (19 [90.5%] of whom were male; mean [SD] age, 60.1 [8.2] years) competed the full protocol of PCI, post-PCI physiological assessment, and post-PCI maximal exercise. After PCI, the median (IQR) FFR rose to 0.91 (0.85-0.96), median (IQR) iFR to 0.98 (0.94-0.99), and median (IQR) CFR to 2.73 (2.50-3.12), while the median (IQR) HSR fell to 0.16 (0.06-0.37) (P < .001 for all). The post-PCI increment in exercise time was most significantly associated with baseline iFR (R2 = 0.26; P = .02).
Conclusions and Relevance
In a selected group of patients with severe, single-vessel stable angina, FFR, iFR, HSR, and CFR were all modestly correlated with angina-limited exercise time to varying degrees. Notwithstanding the limited sample size, no clear association was demonstrated between anatomical stenosis severity and angina-limited exercise time.
Introduction
Physiology-guided revascularization is recommended by treatment guidelines,1 primarily owing to reductions in clinical events demonstrated in randomized clinical trials. However, in clinical practice, most percutaneous coronary interventions (PCI) for stable angina are performed for symptomatic and not prognostic benefit. Despite this, the association between physiological stenosis severity and angina-limited exercise capacity is poorly understood.
Recently, we reported a study that used supine exercise during invasive coronary catheterization to determine the association of PCI with exercise hemodynamics in patients with stable angina.2 In this study, we have reported a separate analysis to determine the association between angina-limited exercise time and fractional flow reserve (FFR), instantaneous wave–free ratio (iFR), hyperemic stenosis ratio (HSR), and coronary flow reserve (CFR). Additionally, we tested whether any of these indices were associated with the change in maximal exercise time assessed immediately after PCI.
Methods
Study Population
Selected patients with stable angina and physiologically severe single-vessel coronary artery disease presenting for clinically driven elective PCI were recruited from 2 cardiac centers. Data were collected from December 2016 to December 2017. All participants gave written informed consent in accordance with the protocol approved by the London Central regional ethics committee.
Catheterization and Exercise Protocol
The catheterization and exercise protocol have previously been described2 and are detailed in the eMethods and eFigure 1 in the Supplement. In brief, all patients performed an incremental exercise protocol while simultaneous coronary pressure-flow measurements were made in the target vessel using a Combowire (Philips Volcano Corporation). Patients exercised until the development of angina (defined as chest pain or rate-limiting shortness of breath). The time from the start of exercise to the onset of angina (ETA) was recorded, with each patient blinded to their pre-PCI exercise time.
Stenting was then performed according to standard clinical practice. After stenting, for most patients, the Combowire was reintroduced to the same intracoronary position as it had previously occupied. All aforementioned stages of the pre-PCI study protocol were then repeated, including the incremental exercise protocol.
Data Analysis
We calculated FFR, iFR, HSR, and CFR offline. Details are in eTable 1 in the Supplement.
Statistical Analysis
Linear regression analysis and Pearson correlation coefficients were used to investigate the association between ETA and patient characteristics, anatomic stenosis characteristics, and FFR, iFR, HSR, and CFR. Tests for nonlinearity were performed to validate this approach and exclude the need for modeling using restricted cubic splines. Log transformation of HSR values was performed to permit linear regression analysis. Applicable tests were 2 tailed, and P values less than .05 were considered statistically significant. All analyses were performed using R version 3.2.1 (R Foundation).
Results
Study Population
Baseline characteristics of the pre-PCI study population are summarized in eTable 2 in the Supplement. Twenty-three patients (21 male [91.3%]; mean [SD] age, 60.6 [8.1] years) completed the pre-PCI component of the study protocol. Twenty-one of these patients (19 male [90.5%]; mean [SD] age, 60.1 [8.2] years) competed the full study protocol, inclusive of post-PCI physiological assessment and post-PCI maximal exercise. Stenosis and procedural characteristics are summarized in the Table. Vessel-specific data are summarized in eTable 3 in the Supplement.
Table. Overall Anatomical and Physiological Stenosis Characteristics.
| Characteristic | Result |
|---|---|
| Target vessel, No. | |
| Left anterior descending artery | 14 |
| Circumflex artery | 5 |
| Right coronary artery | 4 |
| Stenosis location, No. | |
| Proximal | 13 |
| Mid | 8 |
| Distal | 2 |
| Diameter stenosis by quantitative coronary angiography, mean (SD) | 74.6 (10.4) |
| Stenosis length, mm, mean (SD) | 10.7 (3.9) |
| Pre–percutaneous coronary intervention, median (IQR) | |
| FFR | 0.54 (0.44-0.72) |
| iFR | 0.53 (0.38-0.83) |
| CFR | 1.35 (1.11-1.63) |
| HSR | 1.67 (0.84-3.16) |
| Stent length, mm, mean (SD) | 23 (8.3) |
| Stent diameter, mm, mean (SD) | 3.4 (0.5) |
| Stent postdilatation, No./Total No. (%) | 19/23 (83) |
| Post–percutaneous coronary intervention, median (IQR) | |
| FFR | 0.91 (0.85-0.96)a |
| iFR | 0.98 (0.94-0.99)a |
| CFR | 2.73 (2.50-3.12)a |
| HSR | 0.16 (0.06-0.37)a |
| ΔFFR | 0.34 (0.21-0.42)a |
| ΔiFR | 0.25 (0.09-0.54)a |
| ΔCFR | 1.28 (0.74-1.50)a |
| ΔHSR | −1.37 (−2.38 to −2.08)a |
Abbreviations: Δ, change; CFR, coronary flow reserve; FFR, fractional flow reserve; HSR, hyperemic stenosis resistance; iFR, instantaneous wave-free ratio; IQR, interquartile range.
Significant difference before vs after percutaneous coronary intervention; all P < .001.
Symptoms and Exercise Capacity
Mean (SD) exercise time before PCI was 144 (77) seconds (a mean [SD] of 4.3 [1.2] metabolic equivalents). After PCI, exercise time increased to a mean (SD) of 219 (69) seconds (an increase of 75 [95% CI, 31-120] seconds; P < .001 for the difference in exercise time).
Associations With Angina-Limited Exercise Time
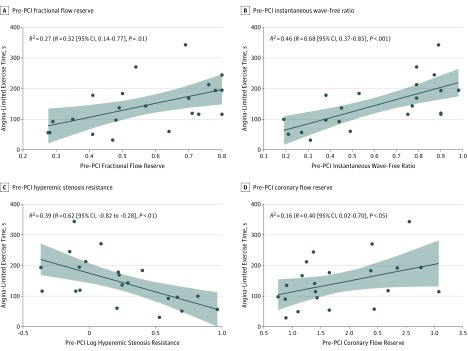
Univariate linear regression results between ETA, patient characteristics, anatomical characteristics, and physiological stenosis characteristics are displayed in eTable 4 in the Supplement. Patient characteristics were not significantly associated with ETA. Similarly, anatomical stenosis characteristics did not correlate with ETA (eFigure 2 in the Supplement). Conversely, correlations between ETA and pre-PCI coronary physiology index included an R2 of 0.27 (R = 0.52 [95% CI, 0.14-0.77]; P = .01) for FFR, an R2 of 0.46 (R = 0.68 [95% CI, 0.37-0.85]; P < .001) for iFR, an R2 of 0.39 (R = −0.62 [95% CI, −0.82 to 0.28]; P = .003) for HSR, and an R2 of 0.16 (R = 0.40 [95% CI, 0.02-0.70]; P = .048 for CFR (Figure 1).
Figure 1. Association Between Angina-Limited Exercise Time and Pre–Percutaneous Coronary Intervention (PCI) Values.
Scatterplots of the association between angina-limited exercise time and pre-PCI fractional flow reserve (A), instantaneous wave-free ratio (B), hyperemic stenosis resistance (C), and coronary flow reserve (D) values.
Associations With Observed Change in Exercise Time After PCI
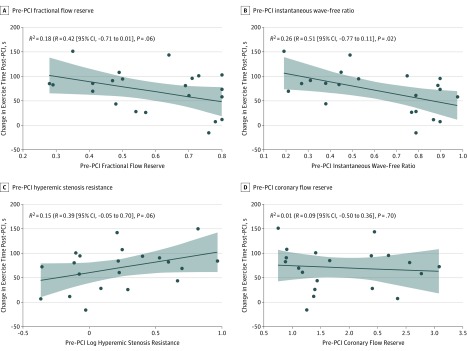
The correlation between the change in exercise time after PCI and iFR was significant (R2 = 0.26; R = −0.51 [95% CI, −0.77 to −0.11]; P = .02). Other correlations for pre-PCI coronary physiology index values were nonsignificant (FFR: R2 = 0.18; R = −0.42 [95% CI, −0.72 to −0.01]; P = .06; HSR, R2 = 0.15; R = 0.39 [95% CI, −0.05 to −0.70]; P = .08; CFR, R2 = 0.01; R = −0.09 [95% CI, −0.50 to 0.36]; P = .70; Figure 2).
Figure 2. Association Between the Change in Exercise Time After Percutaneous Coronary Intervention (PCI) and Pre-PCI Values.
Scatterplots of the association between the change in exercise time after PCI and pre-PCI fractional flow reserve (A), instantaneous wave-free ratio (B), hyperemic stenosis resistance (C), and coronary flow reserve (D) values.
Discussion
We found that, in selected patients with stable angina and physiologically severe single-vessel coronary artery disease, neither patient nor anatomical stenosis characteristics were associated with angina-limited exercise time. Second, conversely, FFR, iFR, HSR, and CFR were all associated with angina-limited exercise time. Third, iFR was most closely associated with the improvement in exercise capacity observed after PCI.
Physiological Stenosis Severity and Angina-Limited Exercise Time
Because the extraction of oxygen is already near its maximum in the resting state, the increase in myocardial oxygen demand during exercise must principally be met by the augmentation of coronary blood flow.3 By quantifying the transstenotic pressure ratio during maximal hyperemia4 or the wave-free period of diastole at rest,5 respectively, both FFR and iFR provide pressure-based estimates of coronary flow (and thus mechanistic rationale for their association with angina-limited exercise time). Additionally, the association between iFR and ETA, which was the strongest of all tested associations, may result from the ability of iFR to quantify microcirculatory vasodilator capacity6 and its close association with hyperemic coronary flow velocity.7,8
Physiological Stenosis Severity and the Change in Exercise Capacity After PCI
Within the present study, and in contrast with the physiology-stratified analysis of the Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial,9 pre-PCI FFR and iFR were associated with the improvement in exercise time after PCI. A number of reasons may explain this difference. Unlike in ORBITA, all patients in the present study were aware they had undergone successful PCI and therefore may have had the greatest willingness to exert themselves maximally. Second, the mean (SD) number of anti-anginal medications per patient in the present study were significantly fewer (1.4 [0.7] per patient) than in the ORBITA study (2.9 [1.1] per patient).
Clinical Implications
Compared with anatomy alone, coronary physiology provides superior ischemia detection,10 improved clinical patient outcomes when used to guide myocardial revascularization11 and, as demonstrated in this study, proof of concept that physiological measurements of stenosis severity are associated with angina-limited exercise time in selected patients with stable angina and severe coronary stenosis.
Limitations
We recruited predominantly male patients with physiologically severe, focal, single-vessel coronary artery disease. Accordingly, the generalizability of these findings to wider populations is limited. Furthermore, the small sample size of this invasive study cohort limits more detailed exploration of the (unadjusted-for) patient characteristics that may confound the association between a coronary lesion and angina-limited exercise time.
Although the reproducibility of physiological indices has been previously reported,12 intracoronary flow measurements are technically demanding to perform. This may negatively bias the association between angina-limited exercise capacity and HSR or CFR compared with FFR or iFR. However, only high-quality Doppler flow data were included for analysis.
Lastly, this study did not blind patients to the presence of PCI. Although patients were blinded to their pre-PCI exercise time, the observed improvement in exercise time post-PCI must be considered to be inclusive of a combination of the physical and placebo effects of PCI, as well as statistical effects, such as regression to the mean. Future invasive exercise studies should aim to incorporate a sham-PCI control arm to provide greater insight.
Conclusions
In a selected group of patients with physiologically severe, single-vessel stable angina, FFR, iFR, CFR, and HSR were all associated with angina-limited exercise time to varying degrees. Conversely, notwithstanding the limited sample size, no clear association was demonstrated between anatomical stenosis severity and angina-limited exercise time.
eMethods. Methods
eFigure 1. Experimental apparatus and study protocol
eTable 1. Calculation of physiologic indices
eTable 2. Baseline characteristics
eTable 3. Per vessel anatomical and physiological stenosis characteristics
eTable 4. Univariate linear regression of baseline characteristics and anatomical stenosis characteristics on angina-limited exercise time
eFigure 2. The relationship between angina-limited exercise time and anatomical stenosis severity
References
- 1.Neumann F-J, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 2.Cook CM, Ahmad Y, Howard JP, et al. . Impact of percutaneous revascularization on exercise hemodynamics in patients with stable coronary disease. J Am Coll Cardiol. 2018;72(9):970-983. doi: 10.1016/j.jacc.2018.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88(3):1009-1086. doi: 10.1152/physrev.00045.2006 [DOI] [PubMed] [Google Scholar]
- 4.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354-1367. doi: 10.1161/01.CIR.87.4.1354 [DOI] [PubMed] [Google Scholar]
- 5.Sen S, Escaned J, Malik IS, et al. . Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59(15):1392-1402. doi: 10.1016/j.jacc.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Nijjer SS, de Waard GA, Sen S, et al. . Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian-Dutch-English (IDEAL) collaborators. Eur Heart J. 2016;37(26):2069-2080. doi: 10.1093/eurheartj/ehv626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook CM, Jeremias A, Petraco R, et al. . FFR/iFR discordance in angiographically intermediate coronary stenoses: an analysis using Doppler-derived coronary flow measurements. JACC Cardiovasc Interv. 2017;10(24):2514-2524. doi: 10.1016/j.jcin.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petraco R, van de Hoef TP, Nijjer S, et al. . Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity–Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7(4):492-502. doi: 10.1161/CIRCINTERVENTIONS.113.000926 [DOI] [PubMed] [Google Scholar]
- 9.Al-Lamee R, Howard JP, Shun-Shin MJ, et al. . Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease: physiology-stratified analysis of ORBITA. Circulation. 2018;138(17):1780-1792. doi: 10.1161/CIRCULATIONAHA.118.033801 [DOI] [PubMed] [Google Scholar]
- 10.Christou MAC, Siontis GCM, Katritsis DG, Ioannidis JPA. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol. 2007;99(4):450-456. doi: 10.1016/j.amjcard.2006.09.092 [DOI] [PubMed] [Google Scholar]
- 11.Tonino PAL, De Bruyne B, Pijls NHJ, et al. ; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi: 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 12.Davies JE, Whinnett ZI, Francis DP, et al. . Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113(14):1768-1778. doi: 10.1161/CIRCULATIONAHA.105.603050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods
eFigure 1. Experimental apparatus and study protocol
eTable 1. Calculation of physiologic indices
eTable 2. Baseline characteristics
eTable 3. Per vessel anatomical and physiological stenosis characteristics
eTable 4. Univariate linear regression of baseline characteristics and anatomical stenosis characteristics on angina-limited exercise time
eFigure 2. The relationship between angina-limited exercise time and anatomical stenosis severity