Key Points
Question
Can ligating the effluent veins first during surgery reduce the dissemination of tumor cells in patients with non–small cell lung cancer?
Findings
In this randomized clinical trial of 86 patients randomized to receive vein-first vs artery-first ligation during surgery, incremental change of circulating tumor cells was observed in 65% of patients in the artery-first group and 31.6% of patients in the vein-first group. Five-year overall survival, disease-free survival, and lung cancer–specific survival for the patients in the vein-first group were significantly better than those in the artery-first group.
Meaning
Ligating the effluent veins first during operation may be more in line with cancer treatment principles and should be recommended for lung cancer surgery.
This study combines a randomized clinical trial with a data registry analysis to compare outcomes associated with vein-first vs artery-first sequences of blood vessel ligation during surgery on the dissemination of tumor cells and survival outcomes in patients with non–small cell lung cancer.
Abstract
Importance
It is important to develop a surgical technique to reduce dissemination of tumor cells into the blood during surgery.
Objective
To compare the outcomes of different sequences of vessel ligation during surgery on the dissemination of tumor cells and survival in patients with non–small cell lung cancer.
Design, Setting, and Participants
This multicenter, randomized clinical trial was conducted from December 2016 to March 2018 with patients with non–small cell lung cancer who received thoracoscopic lobectomy in West China Hospital, Daping Hospital, and Sichuan Cancer Hospital. To further compare survival outcomes of the 2 procedures, we reviewed the Western China Lung Cancer database (2005-2017) using the same inclusion criteria.
Interventions
Vein-first procedure vs artery-first procedure.
Main Outcomes and Measures
Changes in folate receptor–positive circulating tumor cells (FR+CTCs) after surgery and 5-year overall, disease-free, and lung cancer–specific survival.
Results
A total of 86 individuals were randomized; 22 patients (25.6%) were younger and 64 (74.4%) older than 60 years. Of these, 78 patients were analyzed. After surgery, an incremental change in FR+CTCs was observed in 26 of 40 patients (65.0%) in the artery-first group and 12 of 38 (31.6%) in the vein-first group (P = .003) (median change, 0.73 [interquartile range (IQR), −0.86 to 1.58] FU per 3 mL vs −0.50 [IQR, −2.53 to 0.79] FU per 3 mL; P = .006). Multivariate analysis confirmed that the artery-first procedure was a risk factor for FR+CTC increase during surgery (hazard ratio [HR], 4.03 [95% CI, 1.53-10.63]; P = .005). The propensity-matched analysis included 420 patients (210 with vein-first procedures and 210 with artery-first procedures). The vein-first group had significantly better outcomes than the artery-first group for 5-year overall survival (73.6% [95% CI, 64.4%-82.8%] vs 57.6% [95% CI, 48.4%-66.8%]; P = .002), disease-free survival (63.6% [95% CI, 55.4%-73.8%] vs 48.4% [95% CI, 40.0%-56.8%]; P = .001), and lung cancer–specific survival (76.4% [95% CI, 67.6%-85.2%] vs 59.9% [95% CI, 50.5%-69.3%]; P = .002). Multivariate analyses revealed that the artery-first procedure was a prognostic factor of poorer 5-year overall survival (HR, 1.65 [95% CI, 1.07-2.56]; P = .03), disease-free survival (HR, 1.43 [95% CI, 1.01-2.04]; P = .05) and lung cancer–specific survival (HR = 1.65 [95% CI, 1.04-2.61]; P = .03).
Conclusions and Relevance
Ligating effluent veins first during surgery may reduce tumor cell dissemination and improve survival outcomes in patients with non–small cell lung cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT03436329
Introduction
Surgery is the most preferred treatment of choice for many solid tumors, such as non–small cell lung cancer, esophageal cancer, and hepatocellular carcinoma. However, even after curative resection, approximately 50% of patients may develop local recurrence or distant metastases within 3 years.1,2,3,4 Recently, some studies have demonstrated that the presence of circulating tumor cells (CTCs) in peripheral blood can be a surrogate biomarker of prospective recurrence and prognosis.5,6,7,8 Circulating tumor cells are released from the primary tumor into the bloodstream and have the potential to spread to distant sites and develop into micrometastatic deposits.9 Numerous studies have demonstrated that surgical manipulation could promote the dissemination of tumor cells into the circulation.10,11,12,13,14 A surgical technique, named no-touch isolation, has been reported to reduce intraoperative shedding of tumor cells into the circulation in colorectal cancer,15,16,17,18 hepatocellular carcinoma,19,20 and pancreatic cancer.21,22,23 In addition, another surgical technique that may prevent the dissemination of tumor cells into the bloodstream is to ligate the effluent veins first during surgery.24 As reported by McCulloch et al,25 tumor cells can be detected in effluent venous blood during surgery. In addition, vascular invasion within the tumor is also common in lung cancer, which might be responsible for the high incidence of hematogenous spread of tumor cells.26,27,28 Surgical manipulations of lung cancer may squeeze the tumor and further promote the spread of tumor cells into circulation.13,14 The potential risk of tumor cell dissemination can theoretically be minimized if the effluent veins were ligated first (via the vein-first [V-first] technique). However, this technical concept has not yet been widely accepted as a standard of surgical oncology in current guidelines owing to a lack of sufficient evidence. Previous studies evaluating the association between the sequence of vessel ligation and tumor cells dissemination have shown conflicting results.14,29,30,31 Therefore, we conducted a prospective, multicenter, randomized clinical trial to evaluate whether the sequence of vessel ligation can affect the dissemination of tumor cells into the circulation during surgery.
Methods
Randomized Clinical Trial
This trial was carried out in accordance with Consolidated Standards of Reporting Trials reporting guidelines.32 The full trial protocol is available in Supplement 1.
Study Design and Participants
This is a prospective, multicenter, randomized clinical trial that has been registered at ClinicalTrials.gov. This study was designed to enroll patients with non–small cell lung cancer who underwent thoracoscopic lobectomy from December 1, 2016, to March 31, 2018, in West China Hospital, Daping Hospital, and Sichuan Cancer Hospital. We included patients older than 18 years who planned to undergo thoracoscopic lobectomy and who had tumors larger than 2 cm. Patients with clinical stage I to II disease were included. Patients were excluded if they had received a wedge resection of the lesion prior to the standard lobectomy. All the patient records and samples were gathered in the 3 hospitals.
The institutional review board of all the participating hospitals approved the study. Written informed consent was obtained from each patient.
Randomization and Masking
Patients were randomly assigned in a 1:1 ratio according to either the V-first surgical procedure (which involves ligating the pulmonary vein first) and artery-first (A-first) procedure (which involves ligating the pulmonary artery first) via computer-generated randomized numbers, and both the laboratory investigators and the patients were masked as to the allocation schedule. Sealed and numbered envelopes that contained the allocated group for each patient were prepared and opened at the beginning of each surgery.
Sample Size
Sample size was calculated based on a preliminary study. The preliminary results showed that the increasing of folate receptor–positive circulating tumor cells (FR+CTCs) after surgery were observed in 40% of patients in the V-first group and 80% of patients in the A-first group. We expected that there would be at least 10% differences in the rate of increase of the FR+CTCs between the 2 groups. To achieve a statistical power of 80% and a 2-sided type I error of 5%, 36 patients were needed in each group. With an assumption of an approximate 10% dropout rate, our aim was to enroll 80 patients total.
Procedures
Before randomization and surgery, patients usually received a physical examination, laboratory tests, chest and upper abdominal computed tomographic (CT) scan, brain magnetic resonance imaging, single-photon emission computed tomography bone scan, or positron emission tomography–CT as an alternative to try to locate distant metastasis. Generally, surgical resection is not considered for patients with distant metastasis.
Thoracoscopic lobectomy was performed with the standard technique, with the only difference being the sequence of vessel ligation. For the V-first technique, the pulmonary veins in the hilum of pulmonary lobes were dissected and transected first, followed by the bronchus and pulmonary artery branches, leaving the fissure for last. This was described as the single-direction thoracoscopic lobectomy.33 For the A-first technique, all arteries were to be completely ligated before venous interruption. Taking the right upper lobectomy as an example, there were often multiple arteries that required ligation first. The apico-anterior trunk of the right upper artery was fully exposed from the anterior aspect of hilum, and a stapler was used to divide this branch. Then the upper lobe of lung was pulled forward, and the upper lobar bronchus was dissected through the posterior approach. After the upper lobar bronchus was transected, the posterior ascending branch of the right upper artery was exposed and ligated. Finally, the remaining right upper lobe veins were transected. All operations were carried out by certified thoracic surgeons who had at least 3 years of experience in thoracoscopic lobectomy. Three milliliters of peripheral blood was sampled from radial artery in all the enrolled patients before and after surgery, using an EDTA anticoagulant vacuum tube (BD Diagnostics). Preoperative blood samples were collected from patients before making the incision. Postoperative blood samples were harvested immediately after the chest was closed. Samples were then stored at 4°C and processed within 24 hours.
Detection of CTCs
All CTCs were analyzed and quantified using the FR+CTCs Detection Kit (Geno Biotech Co Ltd), which has been approved by the China Food and Drug Administration.34 The enrichment of CTCs was initially achieved by lysing erythrocytes, followed by immunomagnetic depletion of leukocytes from the whole blood. Then, the FR+CTCs in each sample were quantified by ligand-targeted polymerase chain reaction, as published previously.34 A self-referenced CTC unit (denoted FU) derived from standard curve was used to indicate the abundance of FR+CTCs in 3 mL of peripheral blood. A serial of standards containing oligonucleotides (10−14 to 10−9 M, corresponding to 2 to 2 × 105 CTC units/3 mL blood) are used for FR+CTC quantification.34
Outcome Assessment
The pathological stage of each patient was defined according to the 8th edition of TNM classification of the International Association for the Study of Lung Cancer.35 The primary end points were the changes of FR+CTCs levels before and after surgery. Secondary end points included intraoperative blood loss and operative time, postoperative drainage time, postoperative hospital stay, and overall complications. For patients without preoperative or postoperative blood samples, the detection of FR+CTC levels was not possible. Thus, we did not include these patients in the final analysis. The survival outcomes were not available in this prospective study because of an insufficient follow-up period. Thus, we conducted an additional retrospective analysis of a lung cancer registry using the same inclusion and exclusion criteria as the prospective study to evaluate the survival outcomes of patients undergoing V-first and A-first lobectomy procedures.
Retrospective Analysis of the Lung Cancer Registry
Study Design and Cohort Population
The retrospective study was conducted with the data collected from a prospectively maintained database (Western China Lung Cancer Database) in the Department of Thoracic Surgery of West China Hospital. We screened all the patients with non–small cell lung cancer who underwent thoracoscopic lobectomy from September 1, 2005, to December 31, 2017, with the same inclusion criteria and exclusion criteria as the prospective study. Data of this registry were automatically obtained from the hospital information system. Two full-time research assistants were responsible for checking the correctness of clinical information and follow-up data. Two researchers (L.L. and C.G.) were responsible for overseeing the registry. A structured template of medical records was used in the database. The operative records required the research assistants to record the surgical procedures in as much detail as possible, including the incision distribution, the sequence of vessels and bronchus interruption, the methods of intervention for each vessel and bronchus (ie, ligation with clips or transection with a stapler), and additional data points. After recorders completed each note, a senior physician (Q.P.) would check it to ensure the accuracy of the record. We grouped patients into the V-first or A-first group according to the operative records.
Propensity Score Matching
To minimize the effects of potential confounding factors, a propensity-matched analysis was applied. We included as many variables as possible in the propensity score model in an effort to maximally inform the propensity of the dependent variable. The variables included age, sex, year of surgery, smoking status, tumor size, tumor location, histological type, pathologic TNM stage, and postoperative adjuvant therapy. Then, we calculated a propensity score for each patient using results of this model, regardless of statistical significance of the independent variables in the model. Finally, patients were matched 1:1 without replacement using a nearest-neighbor approach with caliper restrictions. Propensity score matching was performed using the psmatch2 routine in Stata version 12.0 (StataCorp).
Outcomes Assessment
The pathological stage of these patients were defined according to the 7th edition of TNM classification of the International Association for the Study of Lung Cancer.36 Patients’ clinical characteristics, tumor characteristics, surgical information, and survival outcomes were collected from the lung cancer registry. Primary outcomes were the 5-year overall survival (OS), disease-free survival (DFS), and lung cancer specific survival (LCSS). Secondary outcomes were intraoperative blood loss, operative time, postoperative drainage time, postoperative hospital stay, and overall complications (followed by the Clavien-Dindo Classification of Surgical Complications).37
All patients visited the surgical outpatient office 1 month after surgery for follow-up assessment. Patients were then evaluated every 3 to 6 months during the first 2 years, at 6-month intervals in the next 3 years, and annually thereafter. During routine follow-up visits, physical examinations, chest and abdominal CT scans, and brain CT or magnetic resonance imaging scans were performed. A single-photon emission tomography CT bone scan was performed once a year, and a positron emission tomography–CT scan was also conducted if necessary. Telephone follow-up was conducted if an outpatient follow-up visit was not feasible. Follow-up was conducted with each patient until their death or May 2018, if the individual remained alive.
Statistical Analysis
Continuous data were presented as medians with interquartile ranges (IQRs) and compared using Mann-Whitney U test in cases of nonnormal distribution. Categorical data were presented as counts and percentages and compared with the χ2 test or the Fisher exact test, according to the cell size examined among groups. All analyses were performed according to intention-to-treat principle for prospective studies. Logistic regression was performed to examine the risk factors associated with the change of FR+CTC levels during surgery. The sequence of vessel ligation and other known and significant factors, such as age, sex, tumor size, tumor location, tumor stage, and more, were introduced to estimate the risk of the increment of FR+CTC via univariate analysis. Then, we entered the remaining factors that were found to be associated with the increment of FR+CTC into the multivariate analysis. Survival curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Factors potentially affecting the survival were assessed by univariable and multivariable Cox regression analysis with the similar methods with the prospective study. Statistical analyses were performed using SPSS Statistics version 20 (IBM Corporation) and GraphPad Prism version 5 (GraphPad Software Inc). A P value less than .05 was considered to be statistically significant.
Results
Randomized Clinical Trial
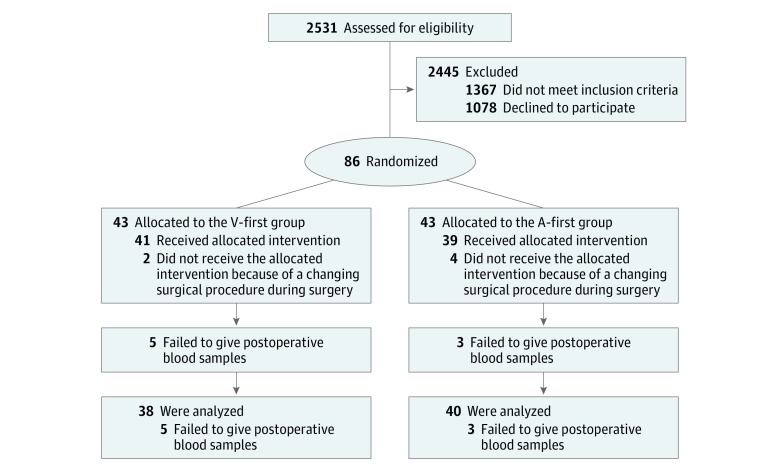
A total of 86 patients were enrolled and randomly assigned to the V-first group or the A-first group. Of these patients, 35 (40.7%) were women, and 51 (59.3%) were men. Eight patients were not included in the final analysis because postoperative blood samples were not collected, and 6 patients whose surgical procedures were changed during surgery were included in an intent-to-treat analysis (Figure 1). Baseline demographic data are reported in Table 1, and perioperative outcomes are in eTable 1 in Supplement 2. Patients’ baseline characteristics were comparable between the 2 groups; for example, in each group, 11 of 43 patients (25.6%) were younger than 60 years, and 32 (74.4%) were older than 60 years.
Figure 1. Flow Diagram of Enrollment and Randomization in the Randomized Clinical Trial.
A-first indicates the group in whom arteries were ligated first; V-first, the group in whom veins were ligated first.
Table 1. Baseline Demographic and Clinical Characteristics of the Enrolled Patients in the Randomized Clinical Trial.
| Characteristics | Patients, No. (%) | |
|---|---|---|
| Vein-First Group (n = 43) | Artery-First Group (n = 43) | |
| Age, y | ||
| <60 | 11 (25.6) | 11 (25.6) |
| ≥60 | 32 (74.4) | 32 (74.4) |
| Sex | ||
| Male | 25 (58.1) | 26 (60.5) |
| Female | 18 (41.9) | 17 (39.5) |
| Comorbidity | ||
| No | 17 (39.5) | 21 (48.8) |
| Yes | 26 (60.5) | 22 (51.2) |
| Current or former smoking | ||
| No | 23 (53.5) | 24 (55.8) |
| Yes | 20 (46.5) | 19 (44.2) |
| Family cancer history | ||
| No | 37 (86.0) | 39 (90.7) |
| Yes | 6 (14.0) | 4 (9.3) |
| Tumor location | ||
| Right lobe | 27 (62.8) | 28 (65.1) |
| Left lobe | 16 (37.2) | 15 (34.9) |
| Tumor size, cm | ||
| <3 | 23 (53.5) | 23 (53.5) |
| ≥3 | 20 (46.5) | 20 (46.5) |
| Histological type | ||
| Adenocarcinoma | 31 (72.1) | 29 (67.4) |
| Nonadenocarcinoma | 12 (27.9) | 14 (32.6) |
| Pathological TNM stage | ||
| I | 22 (51.2) | 21 (48.8) |
| II | 10 (23.3) | 10 (23.3) |
| III | 9 (20.9) | 12 (27.9) |
| IV | 2 (4.6) | 0 (0.0) |
Malignant pleural nodules were unexpectedly found in 2 patients in the V-first group during surgery. The planned thoracoscopic lobectomy was performed after consulting and reaching a unanimous consensus with patient’s family members in these cases.
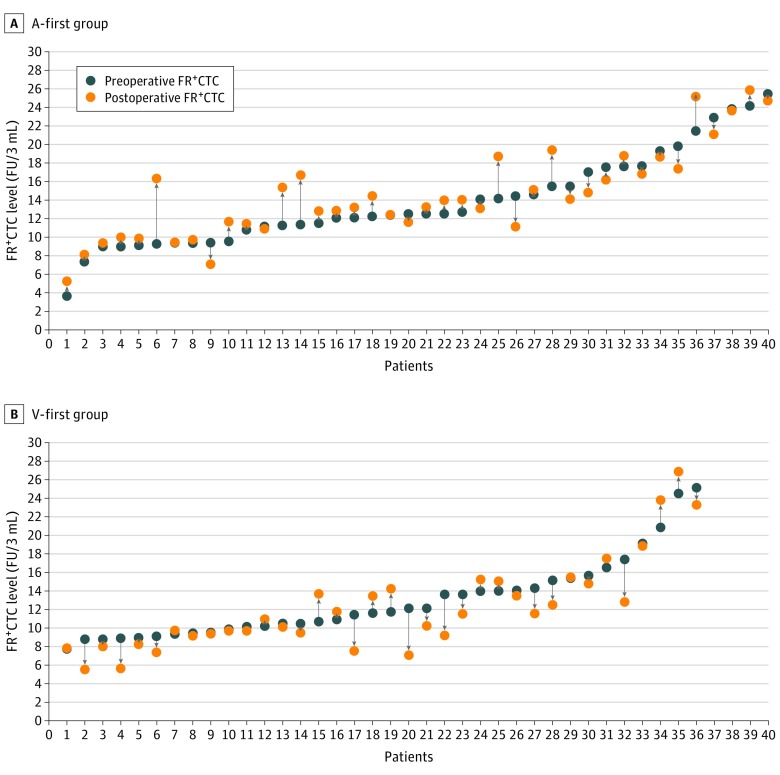
After surgery, incremental change of FR+CTCs was observed in 26 of 40 patients (65.0%) in the A-first group and 12 of 38 patients (31.6%) in the V-first group (P = .003; Figure 2). The median changes of FR+CTCs levels were 0.73 (IQR, −0.86 to 1.58) FU per 3 mL in the A-first group and −0.50 (IQR, −2.53 to 0.79) FU per 3 mL in the V-first group (P = .006). The percentage of the changes of FR+CTC levels were 6.2% for the A-first group and −4.2% for the V-first group (P = .002; eFigure 1 in Supplement 2). In addition, multivariate analysis showed that the A-first procedure was an independent risk factor for the increment of FR+CTC during surgery (hazard ratio [HR], 4.03 [95% CI, 1.53-10.63]; P = .005; eTable 2 in Supplement 2).
Figure 2. Folate Receptor—Positive Circulating Tumor Cell (FR+CTC) Levels Before and After Surgery.
The plot shows the preoperative (blue dot) and postoperative (orange dot) FR+CTC levels of each patient in the artery-first (A-first) group (A) and the vein-first (V-first) group (B). In the A-first group, patient 7 had a preoperative FR+CTC level of 9.31 FU per 3 mL and a postoperative FR+CTC level of 9.41 FU per 3 mL, and patient 19 had a preoperative FR+CTC level of 12.29 FU per 3 mL and a postoperative FR+CTC level of 12.39 FU per 3 mL. In the V-first group, patient 1 had a preoperative FR+CTC level of 7.75 FU per 3 mL and a postoperative FR+CTC level of 7.72 FU per 3 mL, and patient 29 had a preoperative FR+CTC level of 15.36 FU per 3 mL and a postoperative FR+CTC level of 15.38 FU per 3 mL. Data for patients 37 and 38 of the V-first group are not shown; patient 37 had a preoperative FR+CTC level of 33.32 FU per 3 mL and a postoperative FR+CTC level of 30.83 FU per 3 mL, and patient 38 had a preoperative FR+CTC level of 35.51 FU per 3 mL and a postoperative FR+CTC level of 32.19 FU per 3 mL.
Retrospective Analysis of the Lung Cancer Registry
There were 9208 patients registered in the Western China Lung Cancer Database until December 31, 2017. A total of 1691 patients were screened out and analyzed (210 patients in the A-first group and 1481 patients in the V-first group; eFigure 2 in Supplement 2). Baseline characteristics and perioperative outcomes of these patients were shown in eTable 3 and eTable 4 in Supplement 2. The median follow-up time was 31 (IQR, 16-54) months. The 5-year OS rates were 65.0% (95% CI, 61.7%-68.3%) and 57.6% (95% CI, 48.4-66.8%) in the V-first and A-first groups, respectively (P = .03). The 5-year DFS rates were 52.2% (95% CI, 48.9%-55.5%) and 48.4% (95% CI, 40.0%-56.8%) in the V-first and A-first groups, respectively (P = .05). The 5-year LCSS rates were 67.4% (95% CI, 64.3%-70.5%) and 59.9% (95% CI, 50.5%-69.3%) in the V-first and A-first groups, respectively (P = .04) (eFigure 3 in Supplement 2).
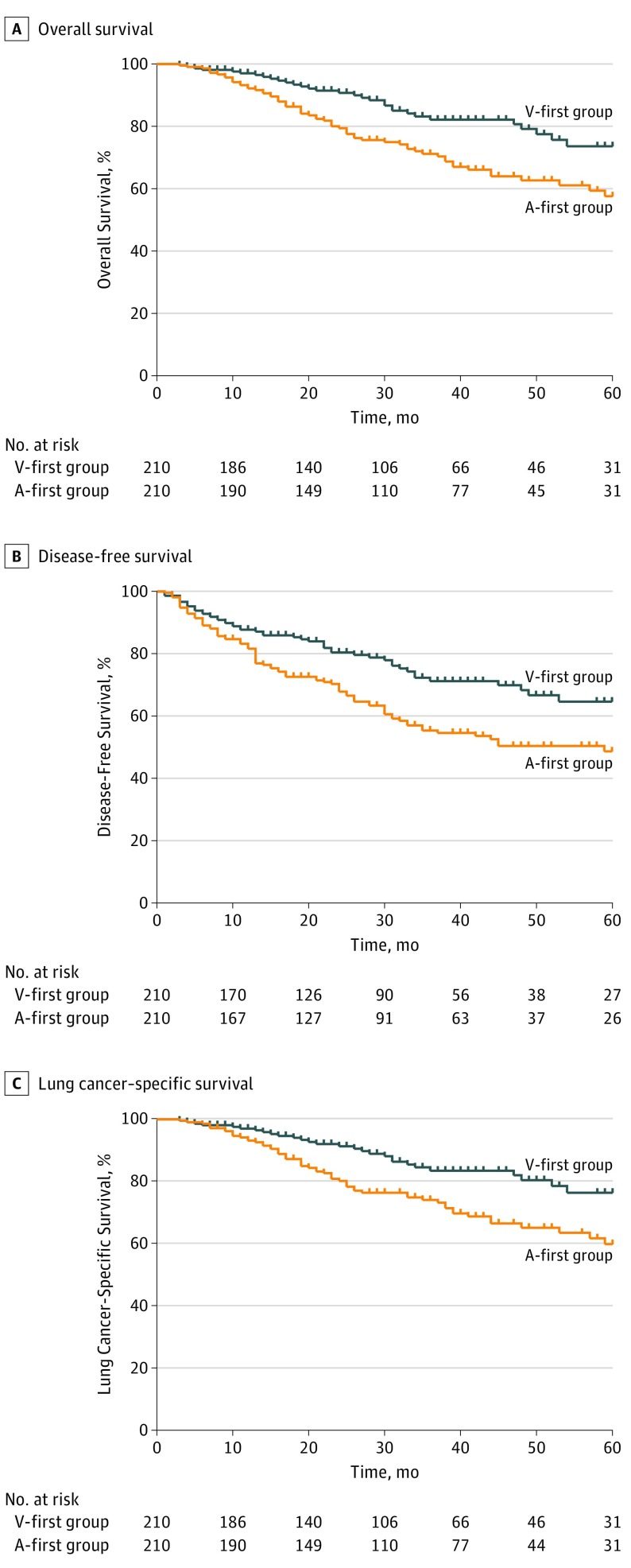
After propensity matching, 210 pairs were matched. Of the 420 patients, 187 (44.5%) were women and 233 (55.5%) were men. Patients’ baseline characteristics and perioperative outcomes were shown in eTable 5 and eTable 6 in Supplement 2. The median follow-up period was 30 (IQR, 16-47) months. The 5-year OS rates were 73.6% (95% CI, 64.4%-82.8%) and 57.6% (95% CI, 48.4-66.8%) in the V-first group and the A-first group, respectively (P = .002; Figure 3A). The 5-year DFS rates were 64.6% (95% CI, 55.4%-73.8%) and 48.4% (95% CI, 40.0%-56.8%) in the V-first and A-first groups, respectively (P = .001; Figure 3B). The 5-year LCSS rates were 76.4% (95% CI, 67.6%-85.2%) and 59.9% (95% CI, 50.5%-69.3%) in the V-first and A-first groups, respectively (P = .002; Figure 3C). Subgroup analyses according to TNM stage indicated that patients who received V-first lobectomy experienced higher 5-year OS, DFS, and LCSS than those who had undergone A-first lobectomy in stage I and II diseases. However, these results were not observed in patients with stage III disease (eFigure 4 in Supplement 2).
Figure 3. Kaplan-Meier Survival Estimates.
A, Kaplan-Meier survival estimates for 5-year overall survival patients undergoing vein-first lobectomy (V-first group) or artery-first lobectomy (A-first group) (hazard ratio, 1.95 [95% CI, 1.27-2.99]; P = .002). B, Estimates for 5-year disease-free survival (hazard ratio, 1.76 [95% CI, 1.24-2.49]; P = .001) and C, Estimates for 5-year lung cancer-specific survival (hazard ratio, 1.98 [95% CI, 1.26-3.11]; P = .003) in propensity score–matched patients in the V-first and A-first groups.
Univariate analysis indicated that the A-first procedure was associated with worse 5-year OS (HR, 1.95 [95% CI, 1.27-2.99]; P = .002), DFS (HR, 1.76 [95% CI, 1.24-2.49]; P = .001), and LCSS (HR, 1.98 [95% CI, 1.26-3.11]; P = .003; Table 2). A tumor size larger than 3 cm (OS: HR, 1.83 [95% CI, 1.21-2.75]; P = .004; DFS: HR, 1.81 [95% CI, 1.29-2.53]; P = .001; LCSS: HR, 1.79 [95% CI, 1.17-2.74]; P = .008), stage II disease (OS: HR, 2.23 [95% CI, 1.26-3.96]; P = .006; DFS: HR, 1.75 [95% CI, 1.06-2.91]; P = .03; LCSS: HR, 2.82 [95% CI, 1.55-5.11]; P = .001), and stage III disease (OS: HR, 4.02 [95% CI, 2.57-6.30]; P < .001; DFS: HR, 4.18 [95% CI, 2.90-6.00]; P < .001; LCSS: HR, 5.07 [95% CI, 3.14-8.18]; P < .001) were also associated with worse outcomes at 5 years.
Table 2. Univariate and Multivariate Cox-Regression Analyses of Factors That May be Associated With 5-Year Overall Survival, Disease-Free Survival, and Lung Cancer–Specific Survival in the Retrospective Analysis of the Lung Cancer Registry.
| Covariates | Overall Survival | Disease-Free Survival | Lung Cancer–Specific Survival | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Univariate Analysis | ||||||
| Age, y | ||||||
| <60 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥60 | 1.05 (0.70-1.59) | .81 | 0.80 (0.57-1.14) | .22 | 0.96 (0.62-1.49) | .85 |
| Sex | ||||||
| Male | 1.14 (0.76-1.72) | .53 | 0.95 (0.68-1.32) | .74 | 1.02 (0.67-1.57) | .92 |
| Female | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Comorbidity | ||||||
| No | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1.05 (0.69-1.61) | .82 | 0.90 (0.63-1.28) | .56 | 0.94 (0.60-1.47) | .77 |
| Current or former smoking | ||||||
| No | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1.21 (0.81-1.82) | .35 | 0.91 (0.65-1.27) | .58 | 1.11 (0.73-1.69) | .64 |
| Tumor location | ||||||
| Left lobe | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Right lobe | 1.06 (0.70-1.60) | .79 | 1.08 (0.76-1.53) | .66 | 1.05 (0.68-1.63) | .82 |
| Sequence of vessel ligation | ||||||
| Vein-first | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Artery-first | 1.95 (1.27-2.99) | .002 | 1.76 (1.24-2.49) | .001 | 1.98 (1.26-3.11) | .003 |
| Tumor size, cm | ||||||
| <3 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥3 | 1.83 (1.21-2.75) | .004 | 1.81 (1.29-2.53) | .001 | 1.79 (1.17-2.74) | .008 |
| Histological type | ||||||
| Adenocarcinoma | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Nonadenocarcinoma | 1.21 (0.78-1.87) | .39 | 1.01 (0.70-1.46) | .96 | 1.18 (0.75-1.87) | .47 |
| Pathological TNM stage | ||||||
| I | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| II | 2.23 (1.26-3.96) | .006 | 1.75 (1.06-2.91) | .03 | 2.82 (1.55-5.11) | .001 |
| III | 4.02 (2.57-6.30) | <.001 | 4.18 (2.90-6.00) | <.001 | 5.07 (3.14-8.18) | <.001 |
| Postoperative adjuvant therapy | ||||||
| No | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1.00 (0.66-1.50) | .99 | 1.13 (0.81-1.57) | .49 | 1.14 (0.75-1.75) | .54 |
| Multivariate analysis | ||||||
| Sequence of vessel ligation | 1.65 (1.07-2.56) | .03 | 1.43 (1.01-2.04) | .05 | 1.65 (1.04-2.61) | .03 |
| Tumor size ≥3 cm | 1.39 (0.90-2.13) | .14 | 1.51 (1.06-2.15) | .02 | 1.26 (0.81-1.98) | .31 |
| Pathological TNM stage | ||||||
| II | 1.97 (1.09-3.57) | .03 | 1.53 (0.91-2.58) | .11 | 2.57 (1.39-4.77) | .003 |
| III | 3.45 (2.17-5.49) | <.001 | 3.65 (2.50-5.31) | <.001 | 4.45 (2.72-7.29) | <.001 |
Abbreviation: NA, not applicable.
In the multivariate analysis, A-first procedure (hazard ratio [HR], 1.65 [95% CI, 1.07-2.56]; P = .03), stage II disease (HR, 1.97 [95% CI, 1.09-3.57]; P = .03), and stage III disease (HR, 3.45 [95% CI, 2.17-5.49]; P < .001) were independent prognostic factors for poorer 5-year OS (Table 2). Receiving an A-first procedure (HR, 1.43 [95% CI, 1.01-2.04]; P = .05), having a tumor size larger than 3 cm (HR, 1.51 [95% CI, 1.06-2.15]; P = .02), and having stage III disease (HR, 3.65 [95% CI, 2.50-5.31]; P < .001) were independent prognostic factors for worse 5-year DFS (Table 2). The A-first procedure (HR, 1.65 [95% CI, 1.04-2.61]; P = .03), stage II disease (HR, 2.57 [95% CI, 1.39-4.77]; P = .003), and stage III disease (HR, 4.45 [95% CI, 2.72-7.29]; P < .001) were independent prognostic factors for poorer 5-year LCSS (Table 2).
Discussion
To our knowledge, the present study is the first prospective randomized clinical trial to compare V-first and A-first surgical techniques by means of examining FR+CTC in the peripheral blood. The V-first technique was shown to result in reduced dissemination of tumor cells into the circulation during surgery and potential survival benefits for patients with non–small cell lung cancer. These better outcomes may be attributed to the reduction in manipulation of the tumor-bearing pulmonary lobe and the avoidance of squeezing tumor cells into circulation during surgery. With the V-first procedure, the most superficial pulmonary veins at the hilum are dissected and transected first, followed by dissecting the bronchus and pulmonary artery branches. This procedure eliminates the operation of repeated squeezes and turning over of the tumor-bearing lobe during surgery. Moreover, once the effluent vein is blocked, tumor cells should be less likely to enter the blood stream.
In fact, the V-first technique has been described to deal with solid neoplasms in certain anatomic regions, such as the stomach and lungs, in the 1950s.38 This surgical concept was summarized from the findings of vascular invasion by tumors and the demonstration of tumor cells in the venous blood in patients undergoing cancer surgery.39 The V-first technique for lung resection surgery has been suggested by several surgeons.29,30 However, some surgeons still insisted that continuing pulmonary arterial flow into a vein-ligated lobe would result in the loss of intravascular volume. On the other hand, the A-first technique may have the advantage of preventing unnecessary blood loss in the resected lobe. For these reasons, Miller et al39 conducted a study to measure the pulmonary arterial blood flow influenced by pulmonary vein ligation using radioactive macro-aggregated serum albumin and found that pulmonary arterial flow to a vein-ligated pulmonary lobe ceased almost immediately with the application of venous occlusion. A study conducted by Yellin et al40 showed that the sequence of vessel ligation had no effect on the amount of blood retained in the resected lobe. Taken together, the V-first technique would not increase unnecessary blood loss during surgery, and blood loss should not be the reason for limiting the application of this surgical technique.
In the present study, FR+CTCs in peripheral blood decreased after surgery in the V-first group. Such a decrease may be explained by some potential reasons. First, after ligating the effluent vein, the route of tumor cells entering the bloodstream was blocked immediately. Second, the immune cells, such as natural killer cells and macrophages, may eliminate tumor cells in the circulation.41 On the other hand, in cases of ligating the pulmonary artery first, FR+CTC in peripheral blood increased after surgery. The increment of CTCs after surgery may increase the chance of recurrence.42 In the present prospective study, however, we failed to calculate the survival outcomes of the 2 groups because of the insufficient follow-up period. To further compare the survival outcomes of the 2 surgical procedures, we conducted a propensity-matched analysis of a lung cancer registry. The results revealed improved survival outcomes in patients undergoing V-first lobectomy. Taken together, the V-first technique may be more in line with the principles of cancer surgery, with the outcome of reducing the dissemination of tumor cells during surgery. Such a surgical concept may affect not only patients having lung cancer surgery but also those who undergoing surgery for other malignant conditions. However, whether the advantages of this technique for patients with lung cancer can be extrapolated to those with other malignant conditions should be subjected to further evaluation.
Limitations
There are some limitations in the current study. First, the sample size was small. Only 78 patients were included for the final analyses in the randomized clinical trial, and only 420 patients were included after propensity matching in the retrospective analysis. Second, although we calculated OS, DFS, and LCSS in the retrospective analysis, survival outcomes in the clinical trial were not available owing to the insufficient follow-up period. Third, given the retrospective nature of the lung cancer registry, it is possible that patients may have been placed in the wrong category, even though we had taken steps to minimize this. Thus, further prospective studies with larger sample sizes and adequate postoperative surveillance are encouraged to assess the benefits of the V-first technique.
Conclusions
In this study, ligating effluent veins first resulted in reduced dissemination of tumor cells into circulation and may have improved survival outcomes in patients undergoing lung cancer surgery. Such a surgical concept may be more in line with cancer treatment principles than current practice and should be recommended for cancer surgery. The current study sheds light on the choice of the sequence of blood vessel ligation during lung cancer surgery.
Trial Protocol.
eTable 1. Perioperative outcomes of the enrolled patients in the randomized controlled trial
eTable 2. Univariate and multivariate analyzes of factors that may increase the levels of FR+CTC
eTable 3. Baseline demographic and clinical characteristics of patients before propensity score matching in the retrospective analysis of the lung cancer registry
eTable 4. Comparison on perioperative outcomes of patients before propensity score matching in the retrospective analysis of the lung cancer registry
eTable 5. Baseline demographic and clinical characteristics of patients after propensity score matching in the retrospective analysis of the lung cancer registry
eTable 6. Comparison on perioperative outcomes of patients after propensity score matching in the retrospective analysis of the lung cancer registry
eFigure 1. The percentage of the changes of FR+CTC level during surgery in the A-first group and the V-first group.
eFigure 2. Study flow diagram of the enrolled patients and propensity matching in the lung cancer registry.
eFigure 3. Kaplan-Meier survival estimates for (A) 5-year overall survival (OS), (B) 5-year disease-free survival (DFS) and (C) 5-year lung cancer-specific survival (LCSS) in patients undergoing vein-first lobectomy or artery-first lobectomy. (HR, hazard ratio)
eFigure 4. Kaplan-Meier survival estimates for 5-year overall survival (OS), disease-free survival (DFS) and lung cancer-specific survival (LCSS) in patients with different stage diseases undergoing vein-first lobectomy or artery-first lobectomy.
Data Sharing Statement.
References
- 1.Demicheli R, Fornili M, Ambrogi F, et al. . Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7(4):-. doi: 10.1097/JTO.0b013e31824a9022 [DOI] [PubMed] [Google Scholar]
- 2.Bhatti I, Patel M, Dennison AR, Thomas MW, Garcea G. Utility of postoperative CEA for surveillance of recurrence after resection of primary colorectal cancer. Int J Surg. 2015;16(pt A):123-128. doi: 10.1016/j.ijsu.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Gil E, Joh JW, Park HC, Yu JI, Jung SH, Kim JM. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol. 2015;13:227. doi: 10.1186/s12957-015-0637-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margonis GA, Gani F, Buettner S, et al. . Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford). 2016;18(11):872-878. doi: 10.1016/j.hpb.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, et al. . Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781-791. doi: 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 6.Wülfing P, Borchard J, Buerger H, et al. . HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12(6):1715-1720. doi: 10.1158/1078-0432.CCR-05-2087 [DOI] [PubMed] [Google Scholar]
- 7.Pierga JY, Bidard FC, Mathiot C, et al. . Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004-7010. doi: 10.1158/1078-0432.CCR-08-0030 [DOI] [PubMed] [Google Scholar]
- 8.Krebs MG, Sloane R, Priest L, et al. . Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29(12):1556-1563. doi: 10.1200/JCO.2010.28.7045 [DOI] [PubMed] [Google Scholar]
- 9.Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623-631. doi: 10.1038/nrc3820 [DOI] [PubMed] [Google Scholar]
- 10.Weitz J, Kienle P, Lacroix J, et al. . Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4(2):343-348. [PubMed] [Google Scholar]
- 11.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232(1):58-65. doi: 10.1097/00000658-200007000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazono F, Takao S, Natsugoe S, et al. . Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am J Surg. 1999;177(6):475-479. doi: 10.1016/S0002-9610(99)00086-0 [DOI] [PubMed] [Google Scholar]
- 13.Sawabata N, Okumura M, Utsumi T, et al. . Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg. 2007;55(5):189-192. doi: 10.1007/s11748-007-0101-2 [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Tanaka F, Yoneda K, et al. . Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18(6):775-783. doi: 10.1093/icvts/ivu048 [DOI] [PubMed] [Google Scholar]
- 15.Turnbull RB Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166(3):420-427. doi: 10.1097/00000658-196709000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull RB Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. CA Cancer J Clin. 1968;18(2):82-87. doi: 10.3322/canjclin.18.2.82 [DOI] [PubMed] [Google Scholar]
- 17.Sales JP, Wind P, Douard R, Cugnenc PH, Loric S. Blood dissemination of colonic epithelial cells during no-touch surgery for rectosigmoid cancer. Lancet. 1999;354(9176):392. doi: 10.1016/S0140-6736(99)92164-5 [DOI] [PubMed] [Google Scholar]
- 18.Takii Y, Shimada Y, Moriya Y, et al. ; Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group . A randomized controlled trial of the conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colorectal cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn J Clin Oncol. 2014;44(1):97-100. doi: 10.1093/jjco/hyt156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232(1):25-31. doi: 10.1097/00000658-200007000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244(2):194-203. doi: 10.1097/01.sla.0000225095.18754.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirota M, Kanemitsu K, Takamori H, et al. . Pancreatoduodenectomy using a no-touch isolation technique. Am J Surg. 2010;199(5):e65-e68. doi: 10.1016/j.amjsurg.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 22.Kuroki T, Eguchi S. No-touch isolation techniques for pancreatic cancer. Surg Today. 2017;47(1):8-13. doi: 10.1007/s00595-016-1317-5 [DOI] [PubMed] [Google Scholar]
- 23.Gall TM, Jacob J, Frampton AE, et al. . Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014;149(5):482-485. doi: 10.1001/jamasurg.2013.3643 [DOI] [PubMed] [Google Scholar]
- 24.Kurusu Y, Yamashita J, Hayashi N, Mita S, Fujino N, Ogawa M. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg. 1998;116(1):107-113. doi: 10.1016/S0022-5223(98)70248-X [DOI] [PubMed] [Google Scholar]
- 25.McCulloch P, Choy A, Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet. 1995;346(8986):1334-1335. doi: 10.1016/S0140-6736(95)92345-4 [DOI] [PubMed] [Google Scholar]
- 26.Roberts TE, Hasleton PS, Musgrove C, Swindell R, Lawson RA. Vascular invasion in non-small cell lung carcinoma. J Clin Pathol. 1992;45(7):591-593. doi: 10.1136/jcp.45.7.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodendorf MO, Haas V, Laberke HG, Blumenstock G, Wex P, Graeter T. Prognostic value and therapeutic consequences of vascular invasion in non-small cell lung carcinoma. Lung Cancer. 2009;64(1):71-78. doi: 10.1016/j.lungcan.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 28.Funaki S, Sawabata N, Abulaiti A, et al. . Significance of tumour vessel invasion in determining the morphology of isolated tumour cells in the pulmonary vein in non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;43(6):1126-1130. doi: 10.1093/ejcts/ezs553 [DOI] [PubMed] [Google Scholar]
- 29.Song PP, Zhang W, Zhang B, Liu Q, Du J. Effects of different sequences of pulmonary artery and vein ligations during pulmonary lobectomy on blood micrometastasis of non-small cell lung cancer. Oncol Lett. 2013;5(2):463-468. doi: 10.3892/ol.2012.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge MJ, Shi D, Wu QC, Wang M, Li LB. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol. 2006;132(4):248-256. doi: 10.1007/s00432-005-0059-3 [DOI] [PubMed] [Google Scholar]
- 31.Toufektzian L, Attia R, Polydorou N, Veres L. Does the sequence of pulmonary vasculature ligation have any oncological impact during an anatomical lung resection for non-small-cell lung cancer? Interact Cardiovasc Thorac Surg. 2015;20(2):260-264. doi: 10.1093/icvts/ivu361 [DOI] [PubMed] [Google Scholar]
- 32.Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Che G, Pu Q, et al. . A new concept of endoscopic lung cancer resection: single-direction thoracoscopic lobectomy. Surg Oncol. 2010;19(2):e71-e77. doi: 10.1016/j.suronc.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Zhou F, Li X, et al. . Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10(8):1163-1171. doi: 10.1097/JTO.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 35.Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 36.Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions . The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714. doi: 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 37.Clavien PA, Barkun J, de Oliveira ML, et al. . The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 38.Aylwin JA. Avoidable vascular spread in resection for bronchial carcinoma. Thorax. 1951;6(3):250-267. doi: 10.1136/thx.6.3.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GE Jr, Smeloff EA. Physiologic basis of preliminary venous ligation in the surgery of pulmonary neoplasms. Am J Surg. 1969;118(6):921-924. doi: 10.1016/0002-9610(69)90258-X [DOI] [PubMed] [Google Scholar]
- 40.Yellin A, Sadetzki S, Simansky DA, Refaely Y, Chetrit A, Paley M. The sequence of vessel interruption during lobectomy: does it affect the amount of blood retained in the lobe? Eur J Cardiothorac Surg. 2007;31(4):711-713. doi: 10.1016/j.ejcts.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 41.Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14(3):155-167. doi: 10.1038/nrclinonc.2016.144 [DOI] [PubMed] [Google Scholar]
- 42.Sawabata N, Funaki S, Hyakutake T, Shintani Y, Fujiwara A, Okumura M. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood? Surg Today. 2016;46(12):1402-1409. doi: 10.1007/s00595-016-1318-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Perioperative outcomes of the enrolled patients in the randomized controlled trial
eTable 2. Univariate and multivariate analyzes of factors that may increase the levels of FR+CTC
eTable 3. Baseline demographic and clinical characteristics of patients before propensity score matching in the retrospective analysis of the lung cancer registry
eTable 4. Comparison on perioperative outcomes of patients before propensity score matching in the retrospective analysis of the lung cancer registry
eTable 5. Baseline demographic and clinical characteristics of patients after propensity score matching in the retrospective analysis of the lung cancer registry
eTable 6. Comparison on perioperative outcomes of patients after propensity score matching in the retrospective analysis of the lung cancer registry
eFigure 1. The percentage of the changes of FR+CTC level during surgery in the A-first group and the V-first group.
eFigure 2. Study flow diagram of the enrolled patients and propensity matching in the lung cancer registry.
eFigure 3. Kaplan-Meier survival estimates for (A) 5-year overall survival (OS), (B) 5-year disease-free survival (DFS) and (C) 5-year lung cancer-specific survival (LCSS) in patients undergoing vein-first lobectomy or artery-first lobectomy. (HR, hazard ratio)
eFigure 4. Kaplan-Meier survival estimates for 5-year overall survival (OS), disease-free survival (DFS) and lung cancer-specific survival (LCSS) in patients with different stage diseases undergoing vein-first lobectomy or artery-first lobectomy.
Data Sharing Statement.