Conflict of Interests
The authors declare no conflict of interests.
Alzheimer's disease (AD), a progressive neurodegenerative disease, is the most common type of dementia in the elderly population. Much effort has been investigated in the risk factors of early onset of AD. Accumulating evidences have shown that western sedentary lifestyle may lead to cognitive dysfunction 1. Childhood and adult obesity is increasing dramatically and obese individuals who show early impairment of cognitive performance could have earlier onset of dementia.
As for the treatment of obesity, diet and exercise have been the mainstays of weight management in the obese population, but with limited efficacy 2. Thus, other treatment modalities and medications might be required for some patients. Orlistat, an inhibitor of pancreatic lipases, has been the only remaining anti‐obesity drug till June 2012 when lorcaserin (a selective 5‐HT2C receptor agonist) was approved by the FDA. We initiated this study to determine whether cognitive dysfunction was linked to obesity and anti‐obesity medications.
Diet‐induced obese mouse model was established using 40% high fat diet as previously described 3. We adopted Morris water maze (MWM) to evaluate spatial learning and memory in C57BL/6 mice. Oxidative stress in the mouse hippocampus was assessed by dihydroethidium (DHE) fluorescent staining. Function to adhesion/migrate of bone marrow endothelial progenitor cells (EPCs) and endothelium‐dependent vasodilation of thoracic aortae was evaluated to examine endothelial function in mice 4.
Obese mice exhibited impaired cognitive function in mice, which could be partially restored by lorcaserin or orlistat treatment. Latency of obese mice at the 5th training day was significantly longer than that of control mice; lorcaserin/orlistat significantly shortened the latency in anti‐obesity treated obese mice (Figure 1A). When the hidden platform was removed, mice in obese group spent less time and distance in the original target quadrant compared with the control group; Lorcaserin/orlistat treatment significantly increased percentage of time and distance in the target quadrant in obese mice (Figure 2B). Furthermore, DHE staining assay showed that lorcaserin/orlistat treatment alleviated obesity‐exacerbated oxidative stress in the hippocampus of C57BL/6 mice.
Figure 1.
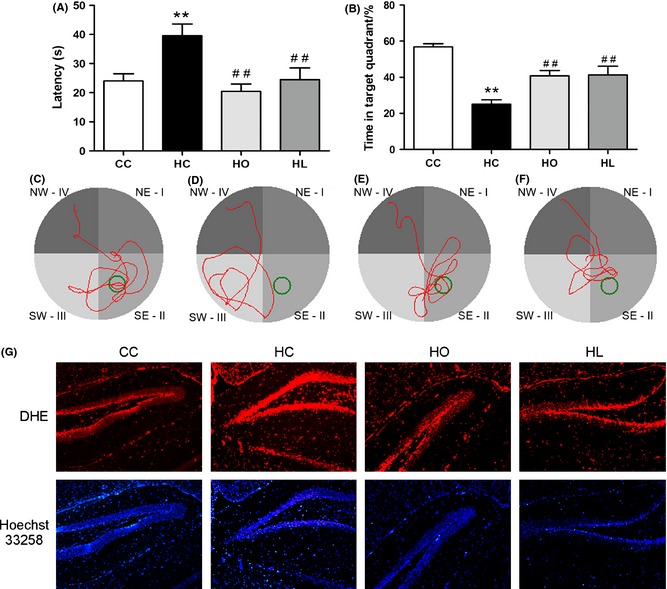
Obesity impaired spatial learning/memory ability in C57BL/6 mice and induced oxidative stress in the hippocampus (A–F). Obese mice showed shorter latency to original platform site (A) and lower percentage of time in target quadrant (B) compared to control (C–F). Representative swimming tracks of control group (C), HFD group (D), orlistat group (E) and lorcaserin group (F). (G) Representative staining of dentate gyri of the hippocampus with DHE and Hoechst 3325. Magnification: 50×. HFD mice exhibited a higher level of the hippocampal ROS compared to control. (CC: control diet; HC: high‐fat diet; HO: high‐fat diet + Orlistat; HL: high‐fat diet + Lorcaserin). Values are means ± SEM. (*P < 0.05, **P < 0.01 vs. control diet, # P < 0.05, ## P < 0.01 vs. High‐fat diet, n = 6, 12 for each group).
Figure 2.
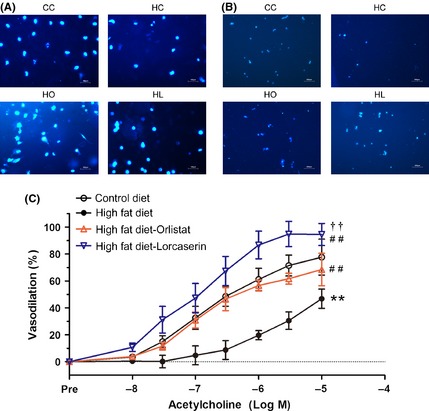
Obesity impaired BM‐EPCs function and endothelial‐dependent vasodilation in C57BL/6 mice. (A). Obesity significantly impaired adhesion ability of BM‐EPCs in mice. Magnification: 100× (B). Obesity significantly impaired migration ability of BM‐EPCs in mice. Magnification: 50× (C). Obesity significantly impaired thoracic aortae endothelium‐dependent vasodilation in mice. (CC: control diet; HC: high‐fat diet; HO: high‐fat diet + Orlistat; HL: high‐fat diet + Lorcaserin.) Values are means ± SEM. (*P < 0.05, **P < 0.01 vs. control diet, ## P < 0.01 vs. high‐fat diet, †† P < 0.01 vs. High‐fat diet + Orlistat, n = 6 for each group).
We also found that dietary obesity significantly impaired EPCs function and the endothelium‐dependent vasodilation of thoracic artery, which could be restored by lorcaserin/orlistat. Lorcaserin/orlistat significantly ameliorated obesity‐impaired BM‐EPCs capacity to adhere (control: 1.00 ± 0.054, HFD: 0.805 ± 0.043, HFD + orlistat: 1.14 ± 0.102, HFD + lorcaserin: 1.23 ± 0.078; P < 0.01, Figure 2A) and migrate (control: 1.00 ± 0.072, HFD: 0.538 ± 0.051, HFD + orlistat: 1.13 ± 0.121, HFD + lorcaserin: 1.38 ± 0.102, P < 0.01, Figure 2B). Endothelium‐dependent vasodilation of mouse artery was significantly impaired by obesity and restored by lorcaserin/orlistat administration (P < 0.01; Figure 2C).
Obese individuals could have decreased life expectancy and increased cardiovascular risks including diabetes, hypertension, coronary artery disease, strokes, and even cancers. Recent studies showed that high‐fat diet intake interferes with hippocampal functioning 5. While studies in humans suggest that obesity is associated with disruptions in cognitive ability, results from animal studies were a little confusing, with some reports describing significant effects of obesity on performance in the Morris maze and others failing to detect effects of obesity on spatial learning. In the basis of previous studies, we conducted this study. It is shown that lorcaserin significantly alleviated obesity‐impaired spatial learning/memory ability in DIO mice. This finding might be clinically significant to lift the life quality of obese Alzheimer's patients.
Accumulating studies are showing that oxidative stress is closely connected to impaired cognitive function 6. It is reported that after prolonged cerebral hypoperfusion, oxidative stress interferes with white matter repair by disrupting renewal mechanisms, thus deteriorating cognitive function 7. Increase of brain oxidative stress in mild cognitive impairment could serve as a predictor of Alzheimer's disease. Moreover, oxidative stress is one of the risk factors of endothelial dysfunction. Obesity is characteristic of aggravated oxidative stress, thus we postulated that obesity might lead to cognitive dysfunction at least partially owing to aggravated oxidative stress.
Cognitive dysfunction is frequently seen in subjects with endothelial impairment 8. It is reported that both type I and type II diabetes mellitus have been associated with reduced performance on numerous domains of cognitive function 9. In research of Alzheimer's disease, a prevailing theory is that vascular disorder is an important pathogenesis. The emerging view is that cerebrovascular dysfunction is a feature not only of cerebrovascular diseases, such as stroke, but also of neurodegenerative conditions, such as AD 8, 10. This article manifested that obese mice with endothelial dysfunction showed impaired cognitive function, providing another evidence of direct link between endothelial dysfunction and cognitive impairment.
In summary, we report that consumption of HFD induced cognitive dysfunction in mice that were susceptible to DIO, which could be restored by lorcaserin/orlistat administration. This work also identifies a potential link between endothelial dysfunction and cognitive dysfunction in obese subjects.
Acknowledgments
This work was supported by the grants from the Natural Science Foundation of Zhejiang (20131813A20).
References
- 1. White CL, Pistell PJ, Purpera MN, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol Dis 2009;35:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yanovski SZ, Yanovski JA. Long‐term drug treatment for obesity: A systematic and clinical review. JAMA 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vickers SP, Jackson HC, Cheetham SC. The utility of animal models to evaluate novel anti‐obesity agents. Br J Pharmacol 2011;164:1248–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen JK, Deng YP, Jiang GJ, Liu YZ, Zhao T, Shen FM. Establishment of tube formation assay of bone marrow‐derived endothelial progenitor cells. CNS Neurosci Ther 2013;19:533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal‐dependent memory and sensitivity to interoceptive signals. Behav Neurosci 2011;125:943–955. [DOI] [PubMed] [Google Scholar]
- 6. Liu YZ, Chen JK, Li ZP, et al. High‐salt diet enhances hippocampal oxidative stress and cognitive impairment in mice. Neurobiol Learn Mem 2014;114:10–15. [DOI] [PubMed] [Google Scholar]
- 7. Miyamoto N, Maki T, Pham LD, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke 2013;44:3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takeda S, Sato N, Uchio‐Yamada K, et al. Diabetes‐accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 2010;107:7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008;29:494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high‐energy diet on hippocampal function and blood‐brain barrier integrity in the rat. J Alzheimers Dis 2010;21:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]


