Summary
Aims
Cerebrovascular white matter lesion (WML) is a major subtype of cerebral small vessel disease. Clinical drugs are not available for WML. We investigated whether peroxisome proliferator‐activated receptor‐γ agonist pioglitazone, with properties of vascular protection and antiinflammation, exerts beneficial effect in hypertensive WML rats.
Methods
Stroke‐prone renovascular hypertensive rats (RHRSP) were treated with pioglitazone for 12 weeks. Morris water maze experiment was conducted to assess cognition. WML was observed by Luxol fast blue staining. Smooth muscle actin‐alpha, collagen I, collagen IV, glial fibrillary acidic protein, and ionized calcium‐binding adaptor molecule‐1 were evaluated by immunohistochemistry. Interleukin‐1 beta (IL‐1β) and tumor necrosis factor alpha (TNF‐α) in brain and soluble intercellular adhesion molecule‐1 (sICAM‐1) in serum were detected.
Results
Pioglitazone significantly attenuated WML in corpus callosum, caudate putamen, external capsule, and internal capsule. Cognitive impairment in RHRSP was ameliorated by pioglitazone. Pioglitazone attenuated arteriolar remodeling and reduced sICAM‐1 level in serum. Pioglitazone decreased the proliferation of microglia and astrocyte and lowered the expression of proinflammatory cytokines IL‐1β and TNF‐α in the white matter.
Conclusions
Long‐term treatment of pioglitazone has beneficial effect on hypertension‐induced WML and cognition decline, which may partly through its effect on attenuation of arteriolar remodeling, endothelial activation, and brain inflammation.
Keywords: Hypertensive white matter lesion, Inflammation, Peroxisome proliferator‐activated receptor‐γ, Pioglitazone, Small vessel disease
Introduction
Cerebrovascular white matter lesion (WML), a major subtype of cerebral small vessel disease (SVD), is characterized by diffuse areas of hypodensities on CT scans or hyperintensities on T2‐weighted MRI scans in periventricular or subcortical white matter. Hypertension and age are key risk factors for WML 1, 2. The probable mechanisms responsible are chronic hypoperfusion 3, changes in glial cells 4, disruption of blood‐brain barrier 5, and even gene mutation 6. Arteriosclerosis is observed in most of the patients with WML 7 and considered to play a critical role in the pathogenesis of WML. Structural alterations of small arteries with reduction in cerebral blood flow cause chronic ischemia which leads to WML. Glial cells are activated following ischemia 4. Growing evidence shows that glial activation is involved in the evolution of WML 8, 9. These imply that suppression of vascular remodeling and antiinflammation might be beneficial to prevent pathogenesis of WML.
In the vascular system, peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) is expressed in endothelium cells and vascular smooth muscle cells. Activation of PPAR‐γ exerts protective effect on some vascular diseases 10. PPAR‐γ agonists are found to attenuate structural changes of vessels, improve endothelial function, and prevent upregulation of proinflammatory cytokines in angiotensin II‐induced hypertensive rats 11. PPAR‐γ agonists also showed antiinflammatory properties in diseases of the central nervous system such as stroke 12, 13, Parkinson's disease 14, and spinal cord injury 15. As arteriosclerosis and inflammation are involved in the pathogenesis of WML, we presume that PPAR‐γ agonists may have a protective effect on WML. In the present study, the stroke‐prone renovascular hypertensive rats (RHRSP) were used as an applicable animal model to determine whether PPAR‐γ activator pioglitazone, with the vascular protective and antiinflammatory properties, exerted beneficial effect on hypertensive WML.
Methods
Animals and Drug Administration
All experiments were approved by the Ethics Committee for Animal Research at Sun Yat‐sen University (Guangzhou, China) and were conducted under the institutional guidelines for animal experimentation. A total of 42 male Sprague–Dawley rats weighing 80–100 g were used to establish the RHRSP model, as described by Zeng et al. 16. Briefly, under anesthesia with 10% chloral hydrate (3 mL/kg, intraperitoneal injection), rats underwent a median longitudinal incision on abdominal skin and the renal arteries were exposed. Ring‐shaped silver clips (0.3 mm in diameter) were placed around the roots of both right and left renal arteries. Additional 6 sham‐operated rats (SHAM) were used as controls. At postoperative week 8, RHRSP were randomly divided into two groups, vehicle‐treated RHRSP (RHRSP/veh) and pioglitazone‐treated RHRSP (RHRSP/pio). RHRSP/pio were treated with pioglitazone hydrochloride tablets daily (10 mg/kg; Hangzhou, China) by gastric gavage for 12 weeks. RHRSP/veh and sham‐operated rats were treated with saline. At postoperative week 20, 6 sham‐operated rats, 15 RHRSP/veh, and 15 RHRSP/pio underwent the next experiment. The remaining 12 rats were excluded from the experiment due to death. Systolic blood pressure (SBP) was measured at postoperative weeks 8 (before drug treatment), 10, 12, 14, 16, 18, and 20 by the tail‐cuff method (ML866 Powerlab 4/30; ADInstruments Pty Ltd, Sydney, NSW, Australia).
Morris Water Maze Test
Morris water maze (MWM) test was employed at postoperative week 20. All rats underwent pretraining the day before the test. Rats were allowed to swim freely in a pool (120 cm diameter) for 60 seconds. At the training block, rats were placed in water from four positions sequentially and latency of escaping onto the platform was recorded (maximum swimming time 60 second). The same training was conducted consecutively for 5 days. On the sixth day, the platform was removed. Number of times of crossing the former platform was recorded for 60 seconds. Data were analyzed from videotape recording (MWM, DMS‐2; Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Peking, China).
Histopathology
At postoperative week 20, after a deep anesthesia with 10% chloral hydrate (5 mL/kg, intraperitoneal injection), nine rats in RHRSP/veh and RHRSP/pio and three rats in SHAM were perfused transcardially with 0.9% saline, followed by 4% formaldehyde in phosphate‐buffered saline (PBS; 0.1 mol/L, pH 7.4). The brain was removed and tissue block was postfixed for 24–48 h at 4°C. Brain blocks were embedded in paraffin and cut into 5‐um sections. Hematoxylin and eosin staining was used to observe the overall morphology, and Luxol fast blue staining was used to examine the nerve fibers and myelin. They were stained according to the standard procedures.
Immunohistochemistry
For immunohistochemistry, the sections were incubated with 3% hydrogen peroxide for 15 min and bovine serum albumin for 1 h and then stained with the following primary antibodies: α ‐smooth muscle actin (α‐SMA) (1:200; Abcam, Cambridge, MA, USA), collagen IV (1:250; Abcam), collagen I (1:250; Sigma, St. Louis, MO, USA), glial fibrillary acidic protein (GFAP, 1:100; Boster, Wuhan, China), and ionized calcium‐binding adaptor molecule‐1 (IBa‐1) (1:100; Abcam). Thereafter, they were incubated with secondary antibody. The products of the immunoreaction were visualized in 3,3′‐diaminobenzidine tetrahydrochloride. PBS 0.01 mol/L replaced the primary antibody in negative controls.
Enzyme‐Linked Immunosorbent Assay
Six rats in RHRSP/veh and RHRSP/pio and three rats in SHAM were anesthetized and perfused transcardially with 0.9% saline. The white matter tissues were dissected and homogenized to separate the supernatant. Blood samples were obtained from the tail vein. Activity of interleukin‐1 beta (IL‐1β) and tumor necrosis factor alpha (TNF‐α) in white matter supernatant and activity of soluble intercellular adhesion molecule‐1 (sICAM‐1) in the blood serum were measured using enzyme‐linked immunosorbent assay (ELISA) kits (IL‐1β and TNF‐α ELISA kits were obtained from Multiscience, Zhejiang, China; sICAM‐1 ELISA kit was obtained from Uscn Life Science Inc., Wuhan, China).
Morphometric Analysis
All parameters were investigated in the white matter regions: corpus callosum, caudate putamen, external capsule, and internal capsule. Severity of WML was graded in accordance with previous reports 4: grade 0 = normal, grade 1 = disarrangement of the nerve fibers, grade 2 = formation of marked vacuoles, and grade 3 = disappearance of myelinated fibers.
Arterioles with an external diameter between 15 and 80 μm were analyzed to assess the small vessel pathology. The arterioles with long axis:short axis ratio <1.5 were chosen as suitable vessels for the study. To assess vascular remodeling, the ratio of immunoreactive area to the luminal area for α‐SMA, collagen I, and collagen IV was calculated using Image J software (National Institutes of Health, Bethesda, MD, USA) using on‐screen measurements.
To assess capillary density, collagen IV immunopositive capillaries were counted in a predefined area (0.0675 mm2). Three areas from each rat were selected for counting. Any brown‐staining capillary that was clearly separate from adjacent vessels was considered as a countable capillary. Capillary number was determined using Image J software.
Statistical Analysis
All data were expressed as mean ± SEM and analyzed using IBM SPSS Version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). SBP and escape latency in MWM were compared by repeated‐measure ANOVA. The severity of WML and percentage of immunoreaction for α‐SMA, collagen I, and collagen IV were analyzed by the nonparametric Kruskal–Wallis test and subsequently with Mann–Whitney U‐test. Other data were analyzed by one‐way ANOVA followed by least significance difference t‐test. P < 0.05 was considered to be statistically significant.
Results
Effect of Pioglitazone on Blood Pressure
At postoperative week 20, the mortality rate was 34.78% (8/23) in vehicle‐treated RHRSP and 21.05% (4/19) in pioglitazone‐treated RHRSP. No rat died in sham‐operated group. At postoperative week 8, the average SBP of the vehicle‐treated RHRSP and pioglitazone‐treated RHRSP was 152.84 ± 4.28 and 147.31 ± 4.38 mmHg before drug administration. The SBP was significantly higher in the RHRSP than in the sham‐operated rats throughout the treatment (P < 0.001). There was no significant difference in SBP between vehicle‐treated RHRSP and pioglitazone‐treated RHRSP throughout the treatment (P > 0.05) (Figure 1).
Figure 1.
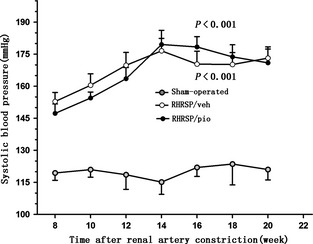
Systolic blood pressure in the three groups of rats during the treatment period. Data were presented as mean pressure ± SEM. n = 15 in RHRSP/veh and RHRSP/pio, n = 6 in sham‐operated rats. P < 0.001 in RHRSP/veh and RHRSP/pio compared with sham‐operated rats, respectively. RHRSP, stroke‐prone renovascular hypertensive rats; RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
Effect of Pioglitazone on WML
Vehicle‐treated RHRSP showed obvious WML. Demyelination, fracture of fibers, and marked vacuoles were observed in corpus callosum, caudate putamen, and external capsule of vehicle‐treated RHRSP. Pioglitazone significantly ameliorated WML with less rarefaction in the white matter (Figure 2). Grading scores for WML were lower in the pioglitazone‐treated RHRSP than in the vehicle‐treated RHRSP in corpus callosum, caudate putamen, external capsule, and internal capsule (Table 1).
Figure 2.
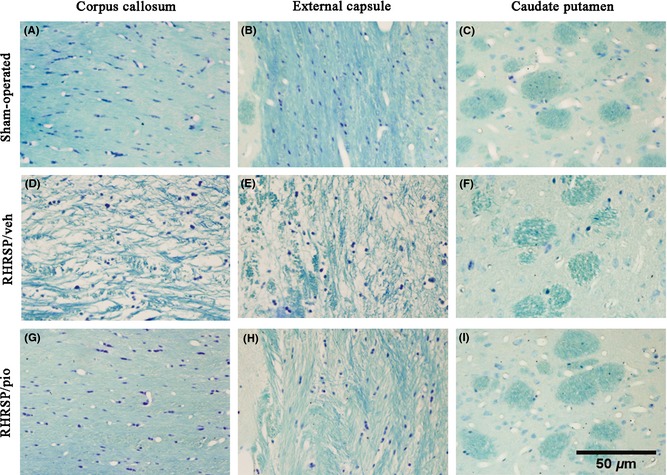
Photomicrographs of Luxol fast blue staining of the white matter. Nerve fibers were neat in sham‐operated rats (A, B, C). Marked leukoaraiosis was observed in vehicle‐treated RHRSP (D, E, F). Pioglitazone‐treated RHRSP showed significantly less WML (G, H, I). RHRSP, stroke‐prone renovascular hypertensive rats; WML, white matter lesion; RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
Table 1.
Grading scores for WML
| n | Corpus callosum | Caudate putamen | External capsule | Internal capsule | |
|---|---|---|---|---|---|
| Sham‐operated | 3 | 0.33 ± 0.21* | 0.00 ± 0.00* | 0.33 ± 0.21* | 0.00 ± 0.00 |
| RHRSP/veh | 9 | 2.60 ± 0.13 | 1.60 ± 0.13 | 2.40 ± 0.21 | 0.60 ± 0.19 |
| RHRSP/pio | 9 | 0.50 ± 0.13* | 0.75 ± 0.12* | 0.25 ± 0.13* | 0.00 ± 0.00* |
WML, white matter lesion; RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone. *P < 0.05 versus the RHRSP/veh.
Effect of Pioglitazone on Cognition
As shown in Figure 3, escape latencies were decreased following the 5‐day training sessions in all rats. However, vehicle‐treated RHRSP had longer escape latencies than sham‐operated rats (P < 0.01), indicating learning deficit in RHRSP. Pioglitazone treatment significantly shortened escape latencies in RHRSP (P < 0.001). The average times of crossing the hidden platform were 4.56 ± 0.41 in the pioglitazone‐treated RHRSP and 5.20 ± 0.51 in the sham‐operated rats versus 2.33 ± 0.73 in the vehicle‐treated RHRSP (P < 0.05). These results indicated memory impairment in RHRSP and the protective effect by pioglitazone.
Figure 3.
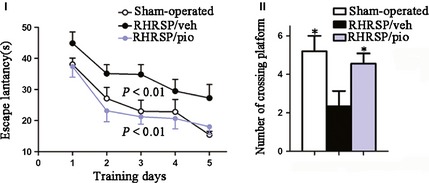
I Escape latency in the morris water maze test. II The times of crossing hidden platform in the morris water mazetest. n = 15 in RHRSP/veh and RHRSP/pio, n = 6 in sham‐operated rats. P < 0.01 RHRSP/veh compared with sham‐operated rats. P < 0.001 RHRSP/veh compared with RHRSP/pio. *P < 0.05 compared with RHRSP/veh. RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
Effect of Pioglitazone on Small Vessel Lesions
As shown in Figure 4I, obvious small vessel remodeling was seen in vehicle‐treated RHRSP. In thickened small vessels, increase of positive immunoreactive area for α‐SMA, collagen I, and collagen IV was observed, demonstrating the hyperplasia or hypertrophy of smooth muscle cells (SMCs) and the accumulation of extracellular matrix in RHRSP. The immunoreactive area / lumen area ratio for α‐SMA (1.99 ± 0.16 vs. 0.49 ± 0.05), collagen I (0.94 ± 0.10 vs. 0.51 ± 0.06), and collagen IV (1.42 ± 0.12 vs. 0.55 ± 0.04) was significantly greater in vehicle‐treated RHRSP than those in sham‐operated rats. Pioglitazone significantly ameliorated arteriolar wall thickening. Pioglitazone‐treated RHRSP showed significantly less expression of α‐SMA (0.59 ± 0.04 vs. 1.99 ± 0.16), collagen I (0.55 ± 0.12 vs. 0.94 ± 0.10), and collagen IV (0.57 ± 0.05 vs. 1.42 ± 0.12) than the vehicle‐treated RHRSP. As shown in Figure 5, the number of capillaries was 16.67 ± 0.82, 11.67 ± 0.46, and 12.44 ± 0.51 per 0.0675 mm2 in sham‐operated rats, vehicle‐treated RHRSP, and pioglitazone‐treated RHRSP, respectively. A reduction in capillaries density was observed in the vehicle‐treated RHRSP, compared with sham‐operated rats. Although it is not significant (P > 0.05), pioglitazone attenuated the microvascular rarefaction in RHRSP.
Figure 4.
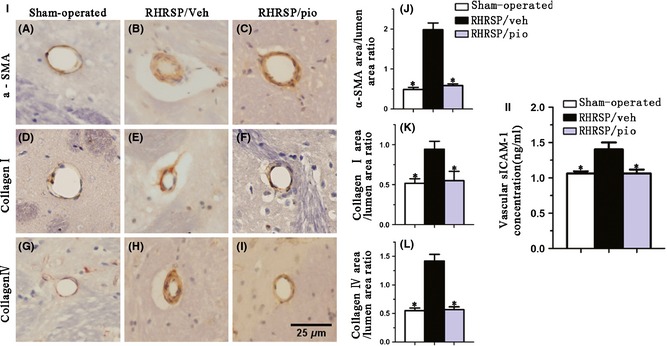
I, Immunohistochemical staining for α‐SMA (A, B, C), collagen I (D, E, F), and collagen IV (G, H, I) in small vessels. Bar graphs indicate ratios of immunoreactive area to luminal area for α‐SMA (J), collagen I (K), and collagen IV (L). II, Assessment of expression of vascular sICAM‐1 through ELISA. Data are presented as mean ± SEM. *P < 0.05 versus the RHRSP/veh. α‐SMA, smooth muscle actin‐alpha; sICAM‐1, soluble intercellular adhesion molecule‐1; RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
Figure 5.
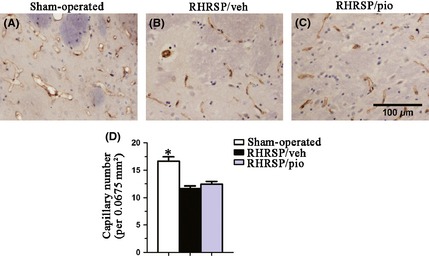
Immunohistochemical staining for collagen IV‐positive capillaries. Bar graphs indicate number of capillaries per 0.0675 mm2. Data are presented as mean ± SEM. *P < 0.05 versus the RHRSP/veh. RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
The expression of sICAM‐1 showed a 1.3‐fold (1.41 ± 0.10 vs. 1.06 ± 0.03 ng/mL) increase in the vehicle‐treated RHRSP than in the sham‐operated rats. The increase in the level of sICAM‐1 in RHRSP was significantly attenuated by pioglitazone (1.41 ± 0.10 vs. 1.06 ± 0.06 ng/mL) (Figure 4II).
Effect of Pioglitazone on Brain Inflammation
In corpus callosum of vehicle‐treated RHRSP, GFAP‐immunopositive astrocyte and IBa‐1‐immunopositive microglia were activated, and expressions of IL‐1β and TNF‐α were 1.3‐fold (95.28 ± 17.95 vs. 72.30 ± 12.30 pg/mL) and 1.5‐fold (12.49 ± 1.11 vs. 8.17 ± 1.59 pg/mL), respectively, than the sham‐operated rats (Figure 6). Pioglitazone treatment significantly attenuated the activation of astrocyte and microglia in RHRSP and subsequently lowered the increasing level of IL‐1β (95.28 ± 17.95 vs. 50.01 ± 10.95 pg/mL) and TNF‐α (12.49 ± 1.11 vs. 5.86 ± 1.25 pg/mL) in the white matter (Figure 6).
Figure 6.
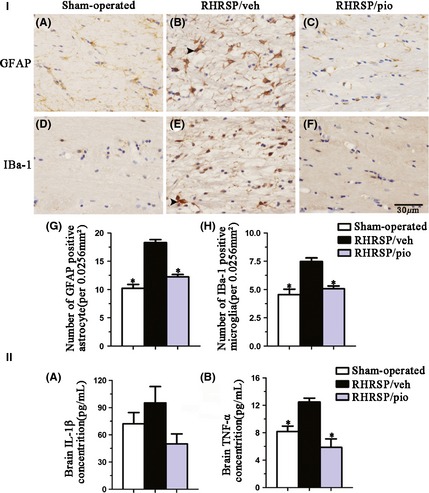
I, Immunohistochemistry staining for GFAP (A, B, C) and IBa‐1 (D, E, F). Bar graphs indicate number of astrocyte (G) and microglia (H) per 0.0256 mm2. II, Bar graphs indicate brain IL‐1β (A) and TNF‐α (B) level. n = 6 in RHRSP/veh and RHRSP/pio, n = 3 in sham‐operated rats. Arrows indicate activated gliocytes. *P < 0.05 versus RHRSP/veh. GFAP, glial fibrillary acidic protein; IBa‐1, ionized calcium‐binding adaptor molecule‐1; IL‐1β, interleukin‐1 beta; TNF, tumor necrosis factor; RHRSP, stroke‐prone renovascular hypertensive rats; RHRSP/veh, RHRSP treated with vehicle; RHRSP/pio, RHRSP treated with pioglitazone.
Discussion
The present study indicates that long‐term treatment of pioglitazone attenuated vascular remodeling, endothelial dysfunction, and brain inflammation as well as stimulated angiogenesis in RHRSP. Furthermore, pioglitazone provided beneficial effect on hypertensive WML and cognition decline without affecting arterial blood pressure.
The PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events) found that pioglitazone reduced macrovascular events in patients with type 2 diabetes 17. Further animal experiment examined the effect of pioglitazone on incidental stroke caused by hypertension and found that pioglitazone significantly protected against hypertension‐induced cerebrovascular injury and stroke by its multiple effects 13. Even though pioglitazone is widely investigated in preventing macrovascular events 12, 13, 18, few is known about its effects in cerebral small vessel disease. Our study focused on the effect of pioglitazone on WML, one of the major subtypes of SVD, and found that pioglitazone prevented the occurrence and progression of hypertensive WML, as evidenced by attenuation of demyelination, fracture of fibers, and marked vacuoles in the white matter. This founding is in agreement with previous study that activation of PPAR‐γ by telmisartan ameliorated WML in mice with chronic cerebral hypoperfusion 19. The pathogenesis and pathology of WML in RHRSP is very similar to that of human WML. Therefore, the observed beneficial effect of pioglitazone on WML highlights it as a potential candidate for SVD therapy.
The effect of pioglitazone on cognition was investigated by morris water maze test. RHRSP showed significant cognitive impairment resulting from long‐term hypertension. Pioglitazone treatment significantly attenuated the acquisition and retention deficits in RHRSP. PPAR‐γ activation was found to improve hippocampus cognition in Alzheimer's disease through its effect of inflammation suppression and amyloid‐β clearance 20. However, in hypertensive rats, the cognition decline is not only associated with hippocampus, but white matter and cerebral cortex 21. Evidence has shown that cognitive deficit resulting from high blood pressure is probably mainly mediated by WML 22. Therefore, we supposed that pioglitazone restored the cognitive function in hypertensive rats may be partly by attenuation of WML.
In previous studies, treatment of PPAR‐γ agonists was reported to show small but significant reduction in blood pressure levels 23. However, in hypertensive patients or animal models not associated with metabolic syndrome, the effect of PPAR‐γ agonist on blood pressure is still controversial 13, 24, 25. In the present study, pioglitazone at a dose of 10 mg/kg did not alter the blood pressure levels in RHRSP. The discrepancy may be explained by difference in diseases, animal models, dose of glitazone, or time of treatment. Moreover, the consistency of blood pressure in RHRSP/veh and RHRSP/pio suggested that pioglitazone prevented cerebralvascular injury and white matter changes independently of blood pressure.
Vascular remodeling was believed to be ascribed to media and basement membrane thickening, collagen deposition, and lumen narrowing 26. In our study, immunohistochemistry for α‐SMA, collagen I, and collagen IV showed the structural changes of artery in RHRSP, which are markers for smooth muscle cells, vessel wall scaring, and basement membrane, respectively. Previous studies also showed these changes in hypertensive animal model or humans 7, 26. It was proved that small‐artery structural alteration is a potent predictor of cardiocerebrovascular events 27, 28. Pioglitazone markedly inhibited small vessel remodeling, as evidenced by decrease of α‐SMA, collagen I, and collagen IV in arteriolar wall. Pioglitazone treatment also significantly attenuated vascular endothelial activation with reduction of sICAM‐1 level, which was supposed to play a causative role in the pathogenesis of WML 29. However, Pioglitazone did not prevent microvascular rarefaction in RHRSP. Previous study showed that pioglitazone prevented microvascular rarefaction and improved vascular function in the nitric oxide synthase inhibitor (L‐NAME)‐induced hypertensive rats 30. In our study, we observed a tendency of increase in capillary number of pioglitazone‐treated rats, although the data did not reach significant difference. We supposed that the short duration of pathological state (20 weeks after hypertension) and time of drug administration may count for the difference. Moreover, pioglitazone ameliorated chronic inflammation in white matter of RHRSP, which was demonstrated by suppression of the astrocyte and microglial activation, decrease of inflammatory cytokines IL‐1β and TNF‐α level. PPAR‐γ agonists were reported to prevent inflammation in transient focal ischemia 12 or traumatic spinal cord injury 15. But our study has provided evidence that PPAR‐γ agonists also attenuated chronic inflammation in hypertensive disease. Our study showed that pioglitazone ameliorated WML, endothelial activation, arteriolar remodeling, and inflammation and promoted neovascularization. As small vessel pathology and glial activation 4, 31 have been well accepted involving the evolution of WML, it was presumed that the beneficial effect of pioglitazone on WML might be attributed to the attenuation of cerebral vascular injury and inflammation. However, this assumption needs to be studied in the further research.
In summary, the present study found that pioglitazone ameliorated WML and cognition decline in hypertensive rats, which may be partly contributed to its effect on attenuation of endothelial activation, arteriolar remodeling, and inflammation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was supported by grants from National Nature Science Foundation of China (No. 81000514), Natural Science Foundation of Guangdong Province (No. S2013010015840), and the hospital funding “support plan for young talents in the First affiliated Hospital of Sun Yat‐sen University”.
References
- 1. O'Sullivan M. Leukoaraiosis. Pract Neurol 2008;8:26–38. [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pantoni L. Pathophysiology of age‐related cerebral white matter changes. Cerebrovasc Dis 2002;13(Suppl 2):7–10. [DOI] [PubMed] [Google Scholar]
- 4. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol 1994;87:484–492. [DOI] [PubMed] [Google Scholar]
- 5. Topakian R, Barrick TR, Howe FA, Markus HS. Blood‐brain barrier permeability is increased in normal‐appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–197. [DOI] [PubMed] [Google Scholar]
- 6. DeStefano AL, Atwood LD, Massaro JM, et al. Genome‐wide scan for white matter hyperintensity: the Framingham Heart Study. Stroke 2006;37:77–81. [DOI] [PubMed] [Google Scholar]
- 7. Huang YH, Zhang WW, Lin L, et al. Could changes in arterioles impede the perivascular drainage of interstitial fluid from the cerebral white matter in leukoaraiosis? Neuropathol Appl Neurobiol 2010;36:237–247. [DOI] [PubMed] [Google Scholar]
- 8. Fredriksson K, Kalimo H, Nordborg C, Olsson Y, Johansson BB. Cyst formation and glial response in the brain lesions of stroke‐prone spontaneously hypertensive rats. Acta Neuropathol 1988;76:441–450. [DOI] [PubMed] [Google Scholar]
- 9. Biran V, Joly LM, Heron A, et al. Glial activation in white matter following ischemia in the neonatal P7 rat brain. Exp Neurol 2006;199:103–112. [DOI] [PubMed] [Google Scholar]
- 10. Touyz RM, Schiffrin EL. Peroxisome proliferator‐activated receptors in vascular biology‐molecular mechanisms and clinical implications. Vascul Pharmacol 2006;45:19–28. [DOI] [PubMed] [Google Scholar]
- 11. Diep QN, El MM, Cohn JS, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II‐infused rats: role of peroxisome proliferator‐activated receptor‐gamma. Circulation 2002;105:2296–2302. [DOI] [PubMed] [Google Scholar]
- 12. Tureyen K, Kapadia R, Bowen KK, et al. Peroxisome proliferator‐activated receptor‐gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type‐2 diabetic rodents. J Neurochem 2007;101:41–56. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura T, Yamamoto E, Kataoka K, et al. Pioglitazone exerts protective effects against stroke in stroke‐prone spontaneously hypertensive rats, independently of blood pressure. Stroke 2007;38:3016–3022. [DOI] [PubMed] [Google Scholar]
- 14. Swanson CR, Joers V, Bondarenko V, et al. The PPAR‐gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation 2011;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol 2007;205:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng J, Zhang Y, Mo J, Su Z, Huang R. Two‐kidney, two clip renovascular hypertensive rats can be used as stroke‐prone rats. Stroke 1998;29:1708–1713 1713–1714. [DOI] [PubMed] [Google Scholar]
- 17. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 18. Pratley RE. The PROactive Study: Pioglitazone in the secondary prevention of macrovascular events in patients with type 2 diabetes. Curr Diab Rep 2006;6:45–46. [DOI] [PubMed] [Google Scholar]
- 19. Washida K, Ihara M, Nishio K, et al. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator‐activated receptor‐gamma activation in mice with chronic cerebral hypoperfusion. Stroke 2010;41:1798–1806. [DOI] [PubMed] [Google Scholar]
- 20. Tsukuda K, Mogi M, Iwanami J, et al. Cognitive deficit in amyloid‐beta‐injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator‐activated receptor‐gamma activation. Hypertension 2009;54:782–787. [DOI] [PubMed] [Google Scholar]
- 21. Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep 2013;15:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray AD, Staff RT, McNeil CJ, et al. Brain lesions, hypertension and cognitive ageing in the 1921 and 1936 Aberdeen birth cohorts. Age (Dordr) 2012;34:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarafidis PA, Nilsson PM. The effects of thiazolidinediones on blood pressure levels ‐ a systematic review. Blood Press 2006;15:135–150. [DOI] [PubMed] [Google Scholar]
- 24. Llorens S, Mendizabal Y, Nava E. Effects of pioglitazone and rosiglitazone on aortic vascular function in rat genetic hypertension. Eur J Pharmacol 2007;575:105–112. [DOI] [PubMed] [Google Scholar]
- 25. Hernanz R, Martin A, Perez‐Giron JV, et al. Pioglitazone treatment increases COX‐2‐derived prostacyclin production and reduces oxidative stress in hypertensive rats: role in vascular function. Br J Pharmacol 2012;166:1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CL, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke‐prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5‐ to 21‐week‐old rats. Neuropathol Appl Neurobiol 2011;37:711–726. [DOI] [PubMed] [Google Scholar]
- 27. Rizzoni D, Porteri E, Boari GE, et al. Prognostic significance of small‐artery structure in hypertension. Circulation 2003;108:2230–2235. [DOI] [PubMed] [Google Scholar]
- 28. Mathiassen ON, Buus NH, Sihm I, et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2007;25:1021–1026. [DOI] [PubMed] [Google Scholar]
- 29. Huang Y, Zhang W, Lin L, et al. Is endothelial dysfunction of cerebral small vessel responsible for white matter lesions after chronic cerebral hypoperfusion in rats? J Neurol Sci 2010;299:72–80. [DOI] [PubMed] [Google Scholar]
- 30. Cipolla MJ, Bishop N, Vinke RS, Godfrey JA. PPAR{gamma} activation prevents hypertensive remodeling of cerebral arteries and improves vascular function in female rats. Stroke 2010;41:1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke 1995;26:1415–1422. [DOI] [PubMed] [Google Scholar]


