Abstract
Objectives
Equisetum arvense has long been used in traditional medicines to treat different disorders, including bone pathologies. In this study a hydromethanolic extract of E. arvense was assessed for its effects on human osteoclastogenesis.
Materials and methods
Osteoclast precursors were maintained in non‐stimulated and stimulated (presence of M‐CSF and RANKL) conditions, or in co‐cultures with osteoblasts. Cell cultures were treated with 0.00016–0.5 mg/ml of a hydromethanolic E. arvense extract.
Results
The extract did not affect spontaneous osteoclastogenesis. In osteoclast precursors committed to osteoclastogenesis (stimulated or co‐cultured with osteoblasts), E. arvense caused dose‐dependent inhibitory effect that became statistically significant at concentrations ≥0.004 mg/ml. This was observed using different osteoclast differentiation and activation markers. Cell response was associated with changes in relative contribution of MEK and NFkB signalling pathways, as well as PGE2 production. As there were differences in the response of osteoclast precursors maintained in the presence of inductive factors, or co‐cultured with osteoblastic cells, it seems that E. arvense extract had the ability to modulate osteoclastogenesis, either by acting directly on osteoclast precursor cells, and/or via osteoblasts.
Conclusions
Equisetum appeared to have a negative effect on human osteoclastogenesis, which is in line with its putative beneficial role in pathophysiological conditions associated with increased osteoclastic activity, and might suggest potential utility for treatment with bone regeneration strategies.
Introduction
Equisetum arvense, also known as horsetail, is a pteridophyte plant with aerial branched stems and regular verticilies, and a main stem around 10 mm long and 4 mm in diameter 1, 2. It grows wild and widespread in the northern hemisphere, particularly in Europe, North and Central America 1. It grows in moist places in temperate climates 3. Its putative medicinal properties have been explored since times of the ancient Greeks and Romans, who used it to treat wounds 3, 4. In addition, it has been included in folk remedies for arthritis, kidney troubles, bleeding ulcers, hepatitis, jaundice and tuberculosis 4, 5. Among the living species of this genus, only E. arvense (as ‘Equiseti herba’) is found in several European pharmacopoeias 4.
A variety of studies has shown that E. arvense reveals a variety of potential pharmacological properties that might help understand its wide application in traditional phytoremedies 2, 3, 4. These include its use as antiseptic, anti‐sehypoglicemic, diuretic, anti‐inflammatory, antioxidant, hepatoprotective, vasorelaxant and anti‐nociceptive 1, 2, 3, 4, 5, 6. In addition, it has also been observed that it can affect bone metabolism, helping in the treatment of some bone disorders, such as osteoporosis, and in healing of osteocytic tissue 3, 7, 8, 9, 10. It is thought that this characteristic of E. arvense might be related to its high content of silica, E. arvense being a plant that contains the highest known concentration of this element 11, 12. This property, combined with its antiseptic activity [against several bacterial agents, including the Staphylococcus aureus 6, the main pathogen of bone infections 13], makes E. arvense a potential, tool not only in treatment of some bone metabolic diseases but also in bone regeneration strategies.
Despite its rigid structure, bone is a very dynamic tissue, being continuously remodelled throughout life. Bone metabolism is mainly performed by co‐ordinated action of two cell types, bone‐synthesizing osteoblasts and bone‐resorbing osteoclasts 14, 15. In addition to their established roles in bone metabolic activities, it is known that there is abundant cross‐talk between both cell types, affecting their differentiation stages 15, 16. In this context, osteoblasts are known to be key players in the osteoclastogenic process 16, specially by synthesis of macrophage‐colony stimulating factor (M‐CSF) and receptor activator of nuclear factor‐κB ligand (RANKL), two important factors that promote osteoclastogenesis in vitro 17, 18.
Although there are lines of evidence that point to potential effects of E. arvense on modulation of bone cell activity, this issue is far from being fully elucidated. Recently, we have reported that a hydromethanolic extract of E. arvense had the ability to stimulate cell viability/proliferation, ALP activity and gene expression of some osteoblastic markers, in human bone marrow cell cultures, suggesting a potentially interesting profile regarding bone regeneration and/or bone disorder contexts 19. The aim of this work was to proceed further in the characterization of the E. arvense modulation properties on human bone cells, by evaluating cellular and molecular effects of the same hydromethanolic E. arvense extract, on human osteoclast development. Thus, osteoclast precursor cells from peripheral blood were exposed to the extract of E. arvense in the absence or presence of osteoclastogenic factors (M‐CSF and RANKL). Also, effects of the extract were tested on co‐cultures of osteoclast precursors and human osteoblasts, due to their role also on osteoclastogenesis 15, 16. Cell cultures were assessed for osteoclast differentiation and activity. Involvement of MEK and NFκB signalling pathways and PGE2 synthesis in the observed osteoclastogenic response was also addressed.
Materials and methods
Preparation of hydromethanolic extract of Equisetum arvense
Dried aerial components of E. arvense were minced into smaller particles, introduced into a glass container and compacted. Then, methanol–water (1:1) was added to completely cover the dry plant constituents and all was left for 3 days in a maceration process. Then the biological material was percolated and extracting solution was collected and evaporated in a rotating evaporator operating at 50 °C, under reduced pressure. The recovered solution was reused as extractor liquid, being added to the glass container once more. This process elapsed in a continuous way, and extract yielded between days 4 and 10 was collected. The deconcoction was concentrated to dryness under reduced pressure, and respective residue was dissolved in dimethylsulphoxide (DMSO; Merck, Darmstadt, Germany) to obtain a stock solution of 100 mg/ml that was sterilized by filtration through 0.2 μm Millipore filters, aliquoted and stored at −20 °C.
Total extract was analysed for its silicon (Si) content. Standard solutions, 10–75 ppm, were prepared by appropriate dilution of a 1000 ppm Si stock solution (Riedel de Haen) with NaCl (20 mg/ml). Absorbance of samples was determined at λ = 251.6 nm, using an atomic absorption spectrometer (GBC 904 AA) with an acetylene/nitrous oxide flame. Concentration of Si in the 100 mg/ml extract stock solution was 11.08 ± 0.81 μg/ml.
Cell cultures
Peripheral blood mononuclear cells
Human peripheral blood mononuclear cell (PBMC), used as osteoclast precursor cells, were isolated from blood of healthy male donors, aged 25–35 years, as described previously 20. Briefly, blood was diluted in PBS (1:1), and applied on the surface of Ficoll‐Paque™ PREMIUM (GE Healthcare Bio‐Sciences, Piscataway, USA). After centrifugation at 400 g for 30 min, cells were collected and washed twice in PBS. On average, in the range of 70 × 106 PBMC were obtained for each 100 ml of processed blood. Cells, seeded at 1.5 × 106 PBMC/cm2 were maintained in α‐minimal essential medium (α‐MEM; Gibco ‐ Life Technologies Ltd, Paisly, UK) supplemented with 30% human serum (from the same donor from which PBMC were collected), 100 IU/ml penicillin (Gibco), 2.5 μg/ml streptomycin (Gibco), 2.5 μg/ml amphotericin B (Gibco) and 2 mm l‐glutamine (Sigma‐Aldrich, MO, USA). Cell cultures were performed in the absence (base medium, BM) or presence of recombinant M‐CSF (25 ng/ml) and RANKL (40 ng/ml) 21, (M+R cultures).
Co‐culture of PBMC with human bone marrow osteoblastic cells
Bone marrow was obtained from patients (25–45 years old) undergoing orthopaedic surgery procedures, after informed consent. Human bone marrow osteoblastic cells (hBMC) were cultured in α‐MEM (Gibco)‐containing 10% foetal bovine serum (Gibco), 100 IU/ml penicillin (Gibco), 2.5 μg/ml streptomycin (Gibco), 2.5 μg/ml amphotericin B (Gibco) and 50 μg/ml ascorbic acid (Sigma‐Aldrich). Cultures were maintained in 5% CO2 humidified atmosphere at 37 °C for 10/15 days up to near confluence. Adherent cells were enzymatically detached with 0.04% trypsin and 0.025% collagenase. Co‐cultures were performed as published previously 22. Shortly, the resulting cell suspension was seeded, 104 cell/cm2, and incubated for 24 h in medium as described above. Then, PBMC 1.5 × 106 cells/cm2, were added, and co‐cultures were maintained in culture conditions as described above for PBMC cultures.
Exposure of cultures to Equisetum arvense extract
PBMC cultures and co‐cultures of PBMC + hBMC were exposed to E. arvense extract at concentrations ranging between 0.00016 and 0.5 mg/ml, chosen according to recently published data on effects of identical E. arvense extract on human osteoblastic cells 19. Both types of culture were maintained for 24 h, to allow for cell adhesion, and the extract was added at this stage. Cultures were incubated for 21 days at 37 °C in 5% CO2 humidified atmosphere and were maintained in the same culture conditions as described above for PBMC cultures. Culture medium was replaced once a week and E. arvense extract was renewed at each medium change. As control, cultures were performed in absence of extract, but maintained in presence of the same final concentration of DMSO as extract‐treated cultures. Cultures were characterized at days 7, 14 and 21 for tartrate‐resistant acid phosphatase (TRAP) activity and number of TRAP‐positive multinucleate cells, and at day 21 for presence of actin rings and vitronectin and calcitonin receptors. In addition, cultures exposed to lowest tested concentrations that caused statistically significant inhibitory effects on TRAP activity, and number of TRAP‐positive multinucleate cells – 0.004 mg/ml – were further analysed, at day 21, by RT‐PCR for expression of osteoclast‐related genes and calcium phosphate resorbing ability. Finally, cell cultures were also assessed at days 7, 14 and 21 for intracellular mechanisms involved in response to E. arvense extract.
Characterization of osteoclastogenic response
TRAP activity
TRAP activity was determined by para‐nitrophenilphosphate (pNPP) hydrolysis assay, as described before 23. After being washed twice in PBS, cells were lysed with 0.1% (V/V) Triton X‐100 for 5 min. Cell lysates were incubated in 12.5 mm pNPP, 0.04 m tartaric acid and 0.09 m citrate (pH 4.8) for 1 h at 37 °C. After incubation, the reaction was stopped using 5 m NaOH, and absorbance of samples was determined at 405 nm in an ELISA plate reader (Synergy HT; Biotek, Winooski, USA). Results were normalized to total protein content of cultures, assessed by Bradford's method 24 and expressed as nmol/min/mg of protein.
TRAP‐positive multinucleated cells
Cell cultures were washed twice in PBS and fixed in 3.7% formaldehyde for 10 min. Cells were rinsed in distilled water and stained for TRAP using Acid Phosphatase, Leukocyte (TRAP) kit (Sigma), according to the manufacturer's instructions. Then, cells were incubated for 1 h, at 37 °C, in the dark, with naphtol AS‐BI 0.12 mg/ml, in presence of 6.76 mm tartrate and 0.14 mg/ml fast garnet GBC. Then, cell layers were washed and stained with haematoxilin. TRAP‐positive multinucleate cells were identified and counted for each experimental condition.
Visualization of F‐actin cytoskeleton, vitronectin and calcitonin receptors by confocal laser scanning microscopy
Cell layers were washed twice in PBS, fixed in 3.7% (V/V) para‐formaldehyde for 15 min and permeabilized in 0.1% (V/V) Triton X‐100 for 5 min. Cells were stained for actin with 5 U/ml Alexa Fluor® 647‐Phalloidin (Invitrogen, CA, USA), and for vitronectin (VNR) and calcitonin (CTR) with 50 μg/ml mouse IgGs anti‐VNR and IgGs anti‐CTR (R&D Systems, MN, USA), respectively. Anti‐VNR and anti‐CTR detection was performed with 2 μg/ml Alexa Fluor® 488‐Goat anti‐mouse IgGs.
RT‐PCR analysis
Cell cultures maintained in absence or presence of E. arvense extract, 0.004 mg/ml, were analysed by RT‐PCR for expression of housekeeping gene GAPDH, osteoclast‐related differentiation and activation factors c‐myc and c‐src, respectively, and for osteoclast functional genes TRAP, cathepsin K (CATK), carbonic anhydrase 2 (CA2) and RANK 25. RNA was extracted using Rneasy® Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions, and quantified by UV spectrophotometry at 260 nm. Then, 0.5 μg RNA was reverse transcribed and amplified (25 cycles) using Titan One Tube RT‐PCR System (Roche, Basel, Switzerland), with annealing temperature of 55 °C. Primers used are listed in Table 1. RT‐PCR products were separated by electrophoresis on 1% (w/V) agarose gel and subjected to densitometric analysis using ImageJ 1.41 software, National Institutes of Health, USA. Values were normalized to corresponding GAPDH value of each experimental condition.
Table 1.
Primers used on RT‐PCR analysis of PBMC cultures
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| GAPDH | 5′‐CAGGACCAGGTTCACCAACAAGT‐3′ | 5′‐GTGGCAGTGATGGCATGGACTGT‐3′ |
| c‐myc | 5′‐TACCCTCTCAACGACAGCAG‐3′ | 5′‐TCTTGACATTCTCCTCGGTG‐3′ |
| c‐src | 5′‐AAGCTGTTCGGAGGCTTCAA‐3′ | 5′‐TTGGAGTAGTAGGCCACCAG‐3′ |
| TRAP | 5′‐ACCATGACCACCTTGGCAATGTCTC‐3′ | 5′‐ATAGTGGAAGCGCAGATAGCCGTT‐3′ |
| CATK | 5′‐AGGTTCTGCTGCTACCTGTGGTGAG‐3′ | 5′‐CTTGCATCAATGGCCACAGAGACAG‐3′ |
| CA2 | 5′‐GGACCTGAGCACTGGCATAAGGACT‐3′ | 5′‐AAGGAGGCCACGAGGATCGAAGTT‐3′ |
| RANK | 5′‐TTAAGCCAGTGCTTCACGGG‐3′ | 5′‐ACGTAGACCACGATGATGTCGC‐3′ |
M‐CSF and RANKL quantification
M‐CSF and RANKL quantification of culture medium from PBMC + hBMC co‐cultures was performed using Human M‐CSF Quantikine ELISA Kit (R&D Systems) and sRANKL (total) Human ELISA (Osteoprotegerin Ligand) (BioVendor, Laboratorni medicina a.s., Brno, Czech Republic), respectively, following manufacturers’ instructions. After detection, absorbance of samples was measured at 450 nm in an ELISA plate reader (Synergy HT; Biotek). Results were expressed as ng/ml.
Calcium phosphate resorbing assay
Cells were cultured on BD BioCoat™ Osteologic™ Bone Cell Culture Plates (BD Biosciences, New Jersey, USA) for 21 days in absence or presence of E. arvense extract (0.004 mg/ml). Then, cells were bleached with 6% NaOCl and 5.2% NaCl, following the manufacturer's protocol and remaining calcium phosphate layers were visualized by phase contrast light microscopy. Resorption lacunae were identified and total resorbed area was quantified with ImageJ 1.41 software.
Intracellular signalling mechanisms
Cell cultures, maintained in absence or presence of 0.004 mg/ml E. arvense extract, were treated with different inhibitors of osteoclastogenic‐related signalling pathways. U0126, a MEK pathway inhibitor, was tested at 1 and 10 μm, due to apparently contradictory effects attributed to low doses of this molecule, by different studies 26, 27, 28. PDTC, an NFκB pathway inhibitor, was used at 10 and 100 μm as the lower concentration has been previously described as IC50 for rat osteoclastic differentiation 29. Finally, indomethacin (1 μm) was tested as it blocks PGE2 synthesis, an osteoclastogenic stimulator 30, 31. PBMC cultures and co‐cultures of PBMC + hBMC were performed under identical experimental conditions as those described above, and were assessed for TRAP activity and number of TRAP‐positive multinucleate cells.
Statistical analysis
All data presented in this work were obtained from three separate experiments, using cell cultures from six different donors. Each experiment was performed in triplicate. Quantitative data are presented as mean ± SD. Groups of data were evaluated using two‐way analysis of variance (ANOVA) and no significant differences in pattern of cell behaviour were found. Statistical differences between controls and experimental conditions were assessed using Bonferroni's method. Values of P ≤ 0.05 were considered significant.
Results
TRAP activity and number of TRAP‐positive multinucleate cells
PBMC cultures performed in BM (Fig. 1a) revealed low values of TRAP activity, which increased slightly over the culture period. Presence of E. arvense extract did not affect cell responses, except for highest tested concentrations (0.1 and 0.5 mg/ml) that elicited a significant decrease in TRAP activity.
Figure 1.
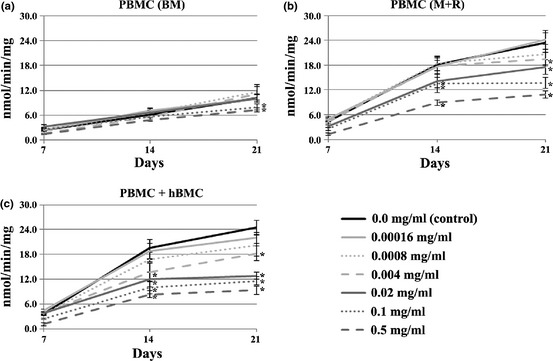
TRAP activity of PBMC cultures. Cell cultures, maintained in base medium (BM, a) or supplemented with M‐CSF and RANKL (M+R, b) and co‐cultures of PBMC + hBMC cells (c), were performed in absence (control) or presence of Equisetum arvense extract. *Significantly different from control cultures.
In the presence of M‐CSF and RANKL (Fig. 1b), PBMC cultures had high TRAP activity, and exposure to extract caused dose‐dependent reduction in enzyme activity. Inhibitory effectsbecame statistically significant at concentrations similar to and higher than 0.004 mg/ml, ranging from 15% (0.004 mg/ml) to 54% (0.5 mg/ml) at day 21.
Exposure to E. arvense extract also caused dose‐dependent reduction in TRAP activity in co‐cultures of PBMC + hBMC (Fig. 1c). However, percentages inhibition were slightly higher than observed in supplemented PBMC cultures, at equal concentrations, varying from 25% (0.004 mg/ml) to 61% (0.5 mg/ml) by day 21.
Globally, number of TRAP‐positive multinucleate cells (Fig. 2) yielded a similar pattern of response to that achieved for TRAP activity.
Figure 2.
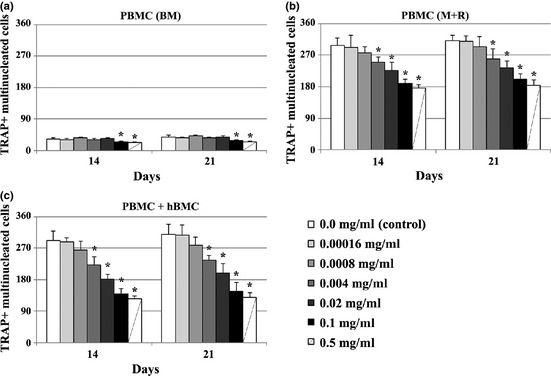
Presence of TRAP+ multinucleated cells in PBMC cultures. Cell cultures, maintained in base medium (BM, a) or supplemented with M‐CSF and RANKL (M+R, b) and co‐cultures of PBMC + hBMC cells (c), were performed in absence (control) or presence of Equisetum arvense extract. *Significantly different from control cultures.
Presence of cells displaying osteoclastic features
Cell density and morphology remained identical at days 14 and 21 of culture (Fig. 3a). Then (day 21), cells were stained for actin, VNR and CTR (Fig. 3b). All cultures revealed presence of cells with characteristic osteoclast features. Nevertheless, relative abundance of these cells was in agreement with results obtained for TRAP determinations. Figure 3c shows representative images of this behaviour, in 21‐day PBMC cultures supplemented with M‐CSF and RANKL, and exposed to 0.00016 and 0.02 mg/ml of the extract.
Figure 3.
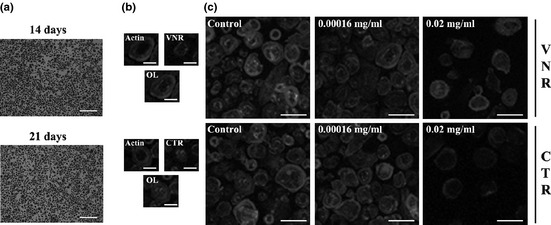
Staining of PBMC cultures treated with the extract. PBMC cultures (M+R) stained for nuclei and TRAP at days 14 and 21 of culture (a). CLSM visualization of PBMC cultures (21 days) supplemented with M‐CSF and RANKL: b – Representative images; c – PBMC cultures: control and exposed to the extract. White bars represent 600 μm (a), 40 μm (b) and 100 μm (c).
Expression of osteoclast‐related genes
PBMC and co‐cultures of PBMC + hBMC were exposed to 0.004 mg/ml of E. arvense extract, and cultures were assessed for expression of c‐myc, s‐src, TRAP, CATK, CA2 and RANK (Fig. 4a). PBMC cultures maintained in BM displayed low expression levels of the analysed genes, and presence of extract did not affect this behaviour significantly. In presence of M‐CSF and RANKL, PBMC expressed higher levels of the tested genes, and exposure to E. arvense extract elicited reduction in expression of all genes (except for CA2 and RANK), between 13% and 49%. Exposure of PBMC + hBMC co‐cultures to E. arvense extract caused a similar effect in expression of osteoclast‐related genes. As observed with TRAP activity, inhibitory effects of the extract were slightly higher than observed for supplemented cultures, between 19% and 53%.
Figure 4.
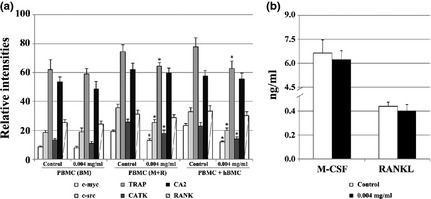
Analysis of the expression of osteoclast‐related genes and M‐CSF and RANKL. PBMC cultures (either maintained in BM or M+R) and PBMC + hBMC co‐cultures, were performed in the absence (control) or presence of Equisetum arvense extract, 0.004 mg/ml (a). RT‐PCR products subjected to densitometric analysis and normalized with value obtained for GAPDH. Quantification of M‐CSF and RANKL present in culture medium of co‐cultures maintained in absence or presence of 0.004 mg/ml Equisetum arvense extract (b). BM, base medium; M+R, M‐CSF + RANKL. *Significantly different from control cultures.
Production of M‐CSF and RANKL
PBMC + hBMC co‐cultures, performed in absence or presence of 0.004 mg/ml E. arvense extract, were analysed for production of M‐CSF and RANKL. For that, pro‐osteoclastogenic growth factors present in culture medium were quantified (Fig. 4b). It was observed that both molecules were present in culture media from co‐cultures, and that M‐CSF concentration was found to be significantly higher than that of RANKL concentration. Supplementation with extract elicited slight reduction in production of both growth factors, although without statistical significance.
Calcium phosphate resorbing ability
PBMC cultures maintained in absence of exogenous osteoclastogenic stimuli elicited but few small resorption lacunae (Fig. 5a) and, thus, a low resorbed area (Fig. 5b). Exposure to 0.004 mg/ml E. arvense extract did not affect this behaviour. Comparatively, of presence of M‐CSF and RANKL, PBMC cultures revealed a significantly higher resorbing ability, that reduced following extract treatment (around 30%). A similar response was observed for PBMC + hBMC co‐cultures, with reduction of approximately 41%.
Figure 5.
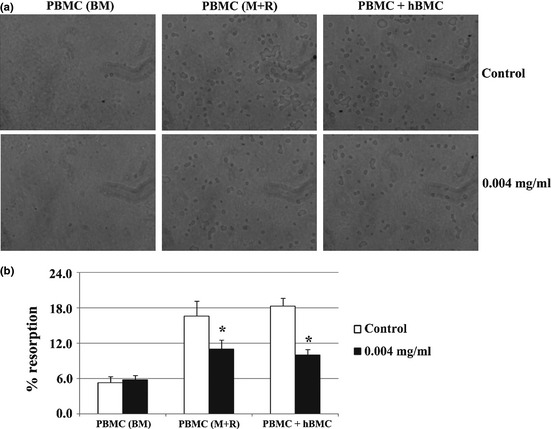
Calcium phosphate resorbing activity of PBMC cultures. Cells cultures, maintained in base medium (BM, a) or supplemented with M‐CSF and RANKL (M+R, b), and co‐cultures of PBMC + hBMC cells (c), were performed in absence (control) or presence of 0.004 mg/ml Equisetum arvense extract. a – Representative images of calcium phosphate layers after cell removal. b – Quantification of resorbed areas expressed as percentage of total area. *Significantly different from control cultures.
Intracellular signalling pathways
Involvement of MEK and NFκB pathways and PGE2 production of the inhibitory effect of E. arvense, was addressed in cultures exposed to 0.004 mg/ml extract, and assessed for TRAP activity (Fig. 6). For each of the three different cell cultures tested, cell responses were compared with that obtained in absence of any signalling pathway inhibitor.
Figure 6.
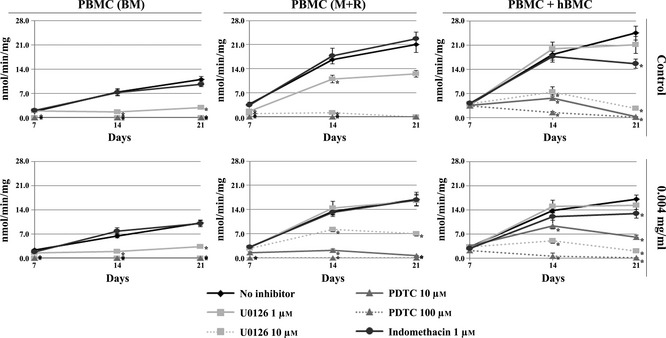
Involvement of MEKand NFκB pathways and PGE2 production on cellular response to E. arvense extract. TRAP activity of PBMC cultures maintained in base medium (BM) or supplemented with M‐CSF and RANKL (M+R) and co‐cultures of PBMC + hBMC cells, maintained in absence (control) or presence of 0.004 mg/ml Equisetum arvense extract. Cell cultures were treated with 1 or 10 μm U0126, 10 or 100 μm PDTC and 1 μm indomethacin. *Significantly different from control cultures.
Control PBMC cultures maintained in BM or in presence of M‐CSF and RANKL were very sensitive to presence of U0126, an inhibitor of the MEK signalling pathway; at 1 μm, significant reduction in TRAP activity was noted, and at 10 μm, inhibition was total. Moreover, full inhibition was also observed in presence of PDTC, an inhibitor of the NFκB pathway (at both tested concentrations). Indomethacin did not elicit significant changes in TRAP activity. Globally, in PBMC cultures performed in BM, presence of extract did not change this behaviour. However, in PBMC supplemented with M‐CSF and RANKL, some important changes were noted. Addition of U0126, at 1 μm, did not affect TRAP activity, and, at 10 μm, only partial inhibition was achieved; also, presence of PDTC at 10 μm, did not completely abolish TRAP activity during the first 14 days of culture (although values were very low), unlike as was observed at 100 μm. Again, presence of indomethacin did not affect the cell response.
Co‐cultures of PBMC + hBMC were not affected by presence of 1 μm U0126, but addition of 10 μm sharply reduced TRAP activity. Treatment with PDTC elicited concentration‐dependent reduction in TRAP activity on day 14 and complete inhibition by day 21. Indomethacin caused partial inhibition of enzyme activity at day 21. Comparatively, following exposure to E. arvense extract, presence of U0126 at 1 and 10 μm resulted in similar behaviour. However, PDTC, at 10 μm, inhibited TRAP activity only partially, but, at 100 μm, total inhibition was observed. Indomethacin caused low levels of inhibition, specially at day 21, although slightly lower than that noted in control co‐cultures.
Discussion
Traditional medicines rely mainly on use of herbal extracts to treat a wide range of disorders, including those that affect bone tissue. Indeed, there are numerous plants employed for treatment of osteoporosis and arthropathies, among others 3, 7, 8, 9, 10, 32. Although use of phytomedicines might considered with care, there are cases in which use of such substances demonstrate potential use for application, this being supported both by in vitro and in vivo studies 10, 32, 33, 34, 35, 36. Here, effects of an E. arvense hydromethanolic extract on human osteoclastogenesis were assessed. Three osteoclastogenic models were tested, namely, non‐stimulated (base medium, absence of any exogenous osteoclastogenic stimuli) and stimulated (presence of recombinant M‐CSF and RANKL) osteoclast precursor cells, and co‐cultures of osteoclastic and osteoblastic cells. As there is no published information regarding concentrations of E. arvense extract components used in the traditional medicines, this study has been conducted using a wide range of concentrations, to obtain global perspective of effects of E. arvense on human osteoclasts.
Results showed that E. arvense extract did not significantly affect spontaneous osteoclastogenesis; occurring in base medium, neither signalling pathway was involved. However, when osteoclast precursors were committed towards osteoclastogenesis (either by supplementation with M‐CSF and RANKL or by presence of osteoblastic cells), clear and significant dose‐dependent inhibition on osteoclastogenesis was observed. It is important to mention that total protein content (which is, with some reservations, related to cell density) of cell cultures did not significantly change by presence of the extract, except for the two higher tested concentrations (data not shown). This suggests that the observed inhibition of osteoclastogenesis might mainly be due to specific effects of the processes related to osteoclast differentiation, rather than being a non‐specific toxic effect on cell density.
The present study has demonstrated that E. arvense can negatively modulate osteoclastogenesis. This is in line with some applications suggested for this plant in traditional medicine, particularly as treatment for bone disorders characterized by a hyperactivation of osteoclastic cells (such as osteoporosis and osteoarthritis) 3, 7, 8, 9, 10. This ability might be related to several characteristics of the plant, with regard to its composition. It is known that in life E. arvense accumulates high quantities of silica 11, 12, a characteristic that is highly variable among different plant species 37. No other plant reveals such high concentrations of this element, which can reach values as higher as 25% dry weight 12. Despite the many beneficial properties of silica for the plants, [specially in the relief of stress 11], this element is thought to mainly be responsible for the medicinal properties attributed to it 7, 8, particularly, for treatment of bone disorders 7, 8, 9. In this context, it is important to mention that silica is found in bone tissue, where it is known to have an important role in maintenance of normal physicochemical properties of this tissue 38. Due to that, several studies have demonstrated that silica has high potential for application in numerous biomaterial‐based bone regeneration strategies 39, 40, 41.
In this context, a relationship between silica content of E. arvense and observed osteoclastogenic response, was aimed to be established. Although some effects of silica on osteoclastogenic behaviour of cell cultures were observed (data not shown), clearly there were also further factors of the extract contributed to the process. Detailed characterization of components of E. arvense extract that could modulate osteoclast differentiation and function are underway.
In addition to its high silica content, it is known that E. arvense also contains numerous phenolic compounds. In a previous study, it has been observed that one hydroalcoholic extract of the plant contained tannins 1, saponins 1, flavonoids [particularly isoquercetin, kaempferol glycosides, flavones glycosides and apigenin 1, 4, 42] and steroids [especially β‐sitosterol, campesterol and isofucosterol 1, 43]. These are natural antioxidant molecules well known for their anti‐inflammatory effects [see 1, 44 and refs. cited therein]. In addition, some of them have been linked to positive effects on bone metabolism and preservation of a long‐term bone health 45, 46, 47, 48, 49. For example, in an in vitro study conducted with RAW264.7 cells treated with furosin (tannin), it was observed that osteoclast differentiation and function were compromised in the presence of this molecule 45. A similar inhibitory effect has been observed in osteoclast cultures performed in presence of the steroidal saponin diosgenin 47. Moreover, E. arvense is also rich in further molecules that can affect bone cells, such as vitamins C, E, K and some of the B group 3.
Characterization of intracellular processes involved in the observed osteoclastogenic responses revealed some important aspects, and represents, to our knowledge, the only information regarding the mechanism of action of E. arvense for bone diseases. In PBMC cultures supplemented with M‐CSF and RANKL, presence of the extract seemed to downregulate the MEK signalling pathway. Indeed, in this condition, inhibition of TRAP activity achieved by treatment with U0126, was significantly lower than in the control. Strong dependence on the NFκB signalling pathway, and absence of involvement of PGE2 production, on the observed cell response remained identical to control.
Osteoclastogenesis observed in co‐cultures of PBMC + hBMC treated with E. arvense extract appeared to be slightly more dependent on the MEK pathway, while NFκB pathway, although very important for the process, seemed to be downregulated in that condition. PGE2 production was also involved in osteoclast development in the co‐cultures, but its relative importance appears to be slightly lower in presence of the extract. In this context, it is noteworthy to highlight that, in a previous report 50, it was observed that the flavonoid portion of an E. arvense hydroalcoholic extract was mainly composed of isoquercitin, an important antioxidant 1 that is known to inhibit both synthesis and release of PG‐like substances 51. This property may be the main reason behind less pronounced inhibitory effects of indomethacin on co‐cultures supplemented with the extract.
Taken together, intracellular mechanisms involved in osteoclastogenic response in the presence of E. arvense extract appeared to involve, in all tested conditions, one or more of the tested signalling pathways. However, those, per se, cannot be considered to be sole mechanisms involved in the cell response, as osteoclastogenesis is a complex process that requires a network of multiple intracellular pathways 18, 25. Moreover, although the overall degree of osteoclast differentiation and activation was identical in both PBMC supplemented with M‐CSF and RANKL or co‐cultured with osteoblastic cells, differences observed in intracellular processes suggest that E. arvense can affect osteoclastogenesis in different ways. As osteoblasts are key players in the osteoclastogenic process 15, 16, it is tempting to speculate that the plant extract can not only act directly on osteoclast precursor cells, but also indirectly, via the osteoblastic cells, probably through modulation of expression of different molecules that can affect osteoclastogenesis. M‐CSF and RANKL production were not significantly affected by the extract, which suggests that other mediators, in addition to these, may be involved in the process. In both situations (PBMC treated with M‐CSF and RANKL and PBMC + hBMC co‐cultures), an inhibitory effect on osteoclastogenesis was achieved. Recently, we have shown that the same hydromethanolic extract of E. arvense revealed osteogenic properties when used to supplement human bone marrow cell cultures, performed in standard cell culture plates or over hydroxyapatite disks 19. Thus, combining those observations with the ones obtained in the present work, it is tempting to suggest that E. arvense reveals an interesting and potentially applicable profile, when considering bone regeneration/repair strategies.
Conclusions
The present work showed that the E. arvense hydromethanolic tested extract could effectively reduce human osteoclast development and function, both in osteoclast precursor cell cultures and in co‐cultures of osteoclastic and osteoblastic cells. Although the present results might mainly be attributed to some individual molecules, it seems more likely that the complex composition of the extract can act as a whole in the process, with possible antagonistic, additive and/or synergistic relationships among its components. To take advantage of the use of E. arvense derivatives as potential medicines for bone disorders, particularly those related to a high osteoclastic activity, or as a useful tool for bone regeneration strategies, a detailed understanding of the effects of the plant on bone cells must be performed. The present data shed some new light on this subject.
Acknowledgements
Financial support was provided by Faculdade de Medicina Dentária, Universidade do Porto, Portugal. CLSM observation was performed at Advanced Light Microscopy, IBMC, University of Porto (IBMC.INEB) under the responsibility of Dr Paula Sampaio.
References
- 1. Do Monte FH, dos Santos JG Jr, Russi M, Lanziotti VM, Leal LK, Cunha GM (2004) Antinociceptive and anti‐inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 49, 239–243. [DOI] [PubMed] [Google Scholar]
- 2. Dos Santos JG Jr, Blanco MM, Do Monte FH, Russi M, Lanziotti VM, Leal LK et al (2005) Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense . Fitoterapia 76, 508–513. [DOI] [PubMed] [Google Scholar]
- 3. Stajner D, Popovic BM, Canadanovic‐Brunet J, Anackov G (2009) Exploring Equisetum arvense L., Equisetum ramosissimum L. and Equisetum telmateia L. as sources of natural antioxidants. Phytother. Res. 23, 546–550. [DOI] [PubMed] [Google Scholar]
- 4. Milovanovic V, Radulovic N, Todorovic Z, Stankovic M, Stojanovic G (2007) Antioxidant, antimicrobial and genotoxicity screening of hydro‐alcoholic extracts of five serbian Equisetum species. Plant Foods Hum. Nutr. 62, 113–119. [DOI] [PubMed] [Google Scholar]
- 5. Oh H, Kim DH, Cho JH, Kim YC (2004) Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense . J. Ethnopharmacol. 95, 421–424. [DOI] [PubMed] [Google Scholar]
- 6. Radulovic N, Stojanovic G, Palic R (2006) Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 20, 85–88. [DOI] [PubMed] [Google Scholar]
- 7. Duke J, Bogenschutz J, du Cellier J, Duke P (2002) Handbook of Medicinal Herbs, 2nd edn, pp. 391–392. Boca Raton, FL: CRC Press. [Google Scholar]
- 8. Van Wyk B, Wink M (2004) Medicinal Plants of the World, pp. 136 Portland, OR: Timber Press. [Google Scholar]
- 9. Wichtl M (2004) Herbal Drugs and Phytopharmaceuticals, 3rd edn, pp. 195–199. Boca Raton, FL: CRC Press. [Google Scholar]
- 10. Putnam SE, Scutt AM, Bicknell K, Priestley CM, Williamson EM (2007) Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. 21, 99–112. [DOI] [PubMed] [Google Scholar]
- 11. Currie HA, Perry CC (2009) Chemical evidence for intrinsic ‘Si’ within Equisetum cell walls. Phytochemistry 70, 2089–2095. [DOI] [PubMed] [Google Scholar]
- 12. Holzhuter G, Narayanan K, Gerber T (2003) Structure of silica in Equisetum arvense . Anal. Bioanal. Chem. 376, 512–517. [DOI] [PubMed] [Google Scholar]
- 13. DiCarlo EF, Kahn LB (2011) Inflammatory diseases of the bones and joints. Semin. Diagn. Pathol. 28, 53–64. [DOI] [PubMed] [Google Scholar]
- 14. Zaidi M (2007) Skeletal remodeling in health and disease. Nat. Med. 13, 791–801. [DOI] [PubMed] [Google Scholar]
- 15. Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J. Clin. Pathol. 61, 577–587. [DOI] [PubMed] [Google Scholar]
- 16. Costa‐Rodrigues J, Teixeira CA, Sampaio P, Fernandes MH (2010) Characterisation of the osteoclastogenic potential of human osteoblastic and fibroblastic conditioned media. J. Cell. Biochem. 109, 205–216. [DOI] [PubMed] [Google Scholar]
- 17. Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 473, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423, 337–342. [DOI] [PubMed] [Google Scholar]
- 19. Bessa Pereira C, Gomes PS, Costa‐Rodrigues J, Almeida Palmas R, Vieira L, Ferraz MP et al (2012). Equisetum arvense hydromethanolic extracts in bone tissue regeneration: in vitro osteoblastic modulation and antibacterial activity. Cell Prolif. 45, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa‐Rodrigues J, Moniz KA, Teixeira MR, Fernandes MH (2012) Variability of the paracrine‐induced osteoclastogenesis by human breast cancer cell lines. J. Cell. Biochem. 113, 1069–1079. [DOI] [PubMed] [Google Scholar]
- 21. Costa‐Rodrigues J, Fernandes A, Fernandes MH (2011) Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14(+) and CD14(−) cell fractions. Cell Prolif. 44, 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa‐Rodrigues J, Fernandes A, Lopes MA, Fernandes MH (2012) Hydroxyapatite surface roughness: complex modulation of the osteoclastogenesis of human precursor cells. Acta Biomater. 8, 1137–1145. [DOI] [PubMed] [Google Scholar]
- 23. Costa‐Rodrigues J, Fernandes MH (2011) Paracrine‐mediated differentiation and activation of human haematopoietic osteoclast precursor cells by skin and gingival fibroblasts. Cell Prolif. 44, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72, 2448–2454. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Q, Shao J, Chen W, Li YP (2007) Osteoclast differentiation and gene regulation. Front. Biosci. 12, 2519–2529. [DOI] [PubMed] [Google Scholar]
- 26. Hotokezaka H, Sakai E, Ohara N, Hotokezaka Y, Gonzales C, Matsuo K et al (2007) Molecular analysis of RANKL‐independent cell fusion of osteoclast‐like cells induced by TNF‐alpha, lipopolysaccharide, or peptidoglycan. J. Cell. Biochem. 101, 122–134. [DOI] [PubMed] [Google Scholar]
- 27. Costa‐Rodrigues J, Fernandes A, Fernandes MH (2011) Reciprocal osteoblastic and osteoclastic modulation in co‐cultured MG63 osteosarcoma cells and human osteoclast precursors. J. Cell. Biochem. 112, 3704–3713. [DOI] [PubMed] [Google Scholar]
- 28. Costa‐Rodrigues J, Teixeira CA, Fernandes MH (2011) Paracrine‐mediated osteoclastogenesis by the osteosarcoma MG63 cell line: is RANKL/RANK signalling really important? Clin. Exp. Metastasis 28, 505–514. [DOI] [PubMed] [Google Scholar]
- 29. Hall TJ, Schaeublin M, Jeker H, Fuller K, Chambers TJ (1995) The role of reactive oxygen intermediates in osteoclastic bone resorption. Biochem. Biophys. Res. Commun. 207, 280–287. [DOI] [PubMed] [Google Scholar]
- 30. Kawashima M, Fujikawa Y, Itonaga I, Takita C, Tsumura H (2009) The effect of selective cyclooxygenase‐2 inhibitor on human osteoclast precursors to influence osteoclastogenesis in vitro. Mod. Rheumatol. 19, 192–198. [DOI] [PubMed] [Google Scholar]
- 31. Kellinsalmi M, Parikka V, Risteli J, Hentunen T, Leskela HV, Lehtonen S et al (2007) Inhibition of cyclooxygenase‐2 down‐regulates osteoclast and osteoblast differentiation and favours adipocyte formation in vitro. Eur. J. Pharmacol. 572, 102–110. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Leung PC, Che CT, Chow HK, Wu CF, Wong MS (2008) Improvement of bone properties and enhancement of mineralization by ethanol extract of Fructus Ligustri Lucidi. Br. J. Nutr. 99, 494–502. [DOI] [PubMed] [Google Scholar]
- 33. Jeong JC, Lee JW, Yoon CH, Lee YC, Chung KH, Kim MG et al (2005) Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3‐E1 cells. J. Ethnopharmacol. 96, 489–495. [DOI] [PubMed] [Google Scholar]
- 34. Dong GC, Chen HM, Yao CH (2008) A novel bone substitute composite composed of tricalcium phosphate, gelatin and drynaria fortunei herbal extract. J. Biomed. Mater. Res. A 84, 167–177. [DOI] [PubMed] [Google Scholar]
- 35. Yao CH, Tsai HM, Chen YS, Liu BS (2005) Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin, and Chinese medicine as a bone substitute. J. Biomed. Mater. Res. B Appl. Biomater. 75, 277–288. [DOI] [PubMed] [Google Scholar]
- 36. Sun JS, Dong GC, Lin CY, Sheu SY, Lin FH, Chen LT et al (2003) The effect of Gu‐Sui‐Bu (Drynaria fortunei J. Sm) immobilized modified calcium hydrogenphosphate on bone cell activities. Biomaterials 24, 873–882. [DOI] [PubMed] [Google Scholar]
- 37. Hodson MJ, White PJ, Mead A, Broadley MR (2005) Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96, 1027–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jugdaohsingh R (2007) Silicon and bone health. J. Nutr. Health Aging 11, 99–110. [PMC free article] [PubMed] [Google Scholar]
- 39. Pietak AM, Reid JW, Stott MJ, Sayer M (2007) Silicon substitution in the calcium phosphate bioceramics. Biomaterials 28, 4023–4032. [DOI] [PubMed] [Google Scholar]
- 40. Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E et al (2002) A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 13, 1199–1206. [DOI] [PubMed] [Google Scholar]
- 41. Gomes PS, Botelho C, Lopes MA, Santos JD, Fernandes MH (2010) Evaluation of human osteoblastic cell response to plasma‐sprayed silicon‐substituted hydroxyapatite coatings over titanium substrates. J. Biomed. Mater. Res. B Appl. Biomater. 94, 337–346. [DOI] [PubMed] [Google Scholar]
- 42. Graefe EU, Veit M (1999) Urinary metabolites of flavonoids and hydroxycinnamic acids in humans after application of a crude extract from Equisetum arvense . Phytomedicine 6, 239–246. [DOI] [PubMed] [Google Scholar]
- 43. D'Agostino M, Dini A, Pizza C, Senatore F, Aquino R (1984) Sterols from Equisetum arvense . Boll. Soc. Ital. Biol. Sper. 60, 2241–2245. [PubMed] [Google Scholar]
- 44. Cetojevic‐Simin DD, Canadanovic‐Brunet JM, Bogdanovic GM, Djilas SM, Cetkovic GS, Tumbas VT et al (2010) Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense L.) extracts. J. Med. Food 13, 452–459. [DOI] [PubMed] [Google Scholar]
- 45. Park EK, Kim MS, Lee SH, Kim KH, Park JY, Kim TH et al (2004) Furosin, an ellagitannin, suppresses RANKL‐induced osteoclast differentiation and function through inhibition of MAP kinase activation and actin ring formation. Biochem. Biophys. Res. Commun. 325, 1472–1480. [DOI] [PubMed] [Google Scholar]
- 46. Nian H, Qin LP, Chen WS, Zhang QY, Zheng HC, Wang Y (2006) Protective effect of steroidal saponins from rhizome of Anemarrhena asphodeloides on ovariectomy‐induced bone loss in rats. Acta Pharmacol. Sin. 27, 728–734. [DOI] [PubMed] [Google Scholar]
- 47. Shishodia S, Aggarwal BB (2006) Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF‐kappa B‐regulated gene expression. Oncogene 25, 1463–1473. [DOI] [PubMed] [Google Scholar]
- 48. Dew TP, Day AJ, Morgan MR (2007) Bone mineral density, polyphenols and caffeine: a reassessment. Nutr. Res. Rev. 20, 89–105. [DOI] [PubMed] [Google Scholar]
- 49. Bandyopadhyay S, Lion JM, Mentaverri R, Ricupero DA, Kamel S, Romero JR et al (2006) Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 72, 184–197. [DOI] [PubMed] [Google Scholar]
- 50. Broudiscou LP, Lassalas B (2000) Effects of Lavandula officinalis and Equisetum arvense dry extracts and isoquercitrin on the fermentation of diets varying in forage contents by rumen microorganisms in batch culture. Reprod. Nutr. Dev. 40, 431–440. [DOI] [PubMed] [Google Scholar]
- 51. Chanh PH, Ifansyah N, Chahine R, Mounayar‐Chalfoun A, Gleye J, Moulis C (1986) Comparative effects of total flavonoids extracted from Ribes nigrum leaves, rutin and isoquercitrin on biosynthesis and release of prostaglandins in the ex vivo rabbit heart. Prostaglandins Leukot. Med. 22, 295–300. [DOI] [PubMed] [Google Scholar]


