Summary
Aim
Nerve growth factor (NGF) regulates neuronal survival and differentiation by activating extracellular signal‐regulated‐kinases (ERK) 1/2 and phosphoinositide‐3‐kinase (PI3K)/Akt pathways in two distinct processes: latency process and neurite extension process. This study was designed to investigate whether botanical drug C‐glucosylated isoflavone puerarin coordinates with NGF to regulate neuritogenesis via activating ERK1/2 and PI3K/Akt in neurite extension process.
Methods
We investigated the neuroprotective and neurotrophic activities of puerarin in MPTP‐lesioned mice and dopaminergic PC12 cells. The effects of puerarin on ERK1/2, Akt, Nrf2, and HO‐1 were assessed by Western blotting. The neurite outgrowth was assayed by neurite outgrowth staining kit.
Results
Puerarin protected dopaminergic cells and ameliorated the behavioral impairments in MPTP‐lesioned mice. Puerarin potentiated the effect of NGF on neuritogenesis in PC12 cells by >10‐fold. Mechanistic studies revealed: (1) puerarin rapidly activated ERK1/2 and Akt, leading to the activation of Nrf2/heme oxygenase‐1 (HO‐1) pathways; (2) ERK1/2, PI3K/Akt, and HO‐1 inhibitors attenuated the neuritogenic activity of puerarin. Notably, puerarin enhanced NGF‐induced neuritogenesis in a timing‐dependent manner.
Conclusion
Puerarin effectively coordinated with NGF to stimulate neuritogenesis via activating ERK1/2 and PI3K/Akt pathways in neurite extension process. These results demonstrated a general mechanism supporting the therapeutic application of puerarin‐related compounds in neurodegenerative diseases.
Keywords: ERK1/2, Neuritogenesis, Nrf2/heme oxygenase‐1, PI3K/Akt, Puerarin
Introduction
Parkinson's disease (PD) is the second common neurodegenerative disease hallmarked by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) 1, 2. The pathogenesis of PD has been attributed to several factors including oxidative stress, inflammation, mitochondrial disruption, protein aggregation, excitotoxicity, and autophagic–lysosomal alterations 3, 4. Oxidative stress disrupts the functions of neurotrophins including nerve growth factor (NGF) and brain‐derived neurotrophic factor (BDNF) and their tyrosine kinase receptors (Trks), thereby suppressing the survival and differentiation of neuronal cells 5, 6. Consistently, the levels of BDNF and NGF were markedly decreased in the nigrostriatal regions and ventricular and lumbar cerebrospinal fluid of patients with PD 7, 8. The reduction of neurotrophins was also observed in PD models induced by 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) and 6‐hydroxydopamine (6‐OHDA) 7. These results highlight the pivotal roles of neurotrophins in neuronal functions, survival, and regeneration 9, 10. Genetic and direct introduction of various neurotrophins improved the parkinsonian symptoms in several animal PD models and reassured the growth of neurons in cell culture 11, 12. For example, NGF stimulates neuronal growth and differentiation and consequently delays neurodegeneration in PD 13, 14. The neuroprotective and neuritogenic activities of NGF are mainly mediated by the extracellular signal‐regulated kinase (ERK) and phosphatidylinositol 3‐kinase (PI3K)/Akt pathways upon binding to its high affinity receptor TrkA 15, 16, 17. Continuous NGF stimulation is critical to sustain the activation of ERK and subsequent induction of various transcriptional factors such as c‐fos. Such stimulation induces a panel of NGF target genes to support neuronal differentiation 18, 19, 20. A recent study revealed that NGF induced the neuritogenesis in dopaminergic cells via two distinct processes, namely, the early ERK‐driven and transcription‐dependent latency process, and the later ERK‐ and PI3K/Akt‐driven and transcription‐independent neurite extension process 21.
Many natural products are highly active neuroprotective and neuritogenic agents for promoting neuronal differentiation and maturation or potentiating the actions of NGF with low cost and few side effects 22, 23, 24. Interestingly, various flavonoids can potentiate the effect of NGF on neuritogenesis and exhibit the antiparkinsonian activities 25, 26, 27. Puerarin (Figure 1) is a C‐glucosylated isoflavone derived from herbal medicine Radix Puerariae (the dried root of Pueraria lobata (Wild.) Ohwi). Puerarin is well known for a range of pharmacological activities such as vasodilatory, anticonvulsive, retinoprotective, cardioprotective, and neuroprotective activities 28, 29, 30. Recent studies have demonstrated that puerarin activates MEK/ERK and PI3K/Akt pathways in many cell types including neuronal cells 31, 32.
Figure 1.
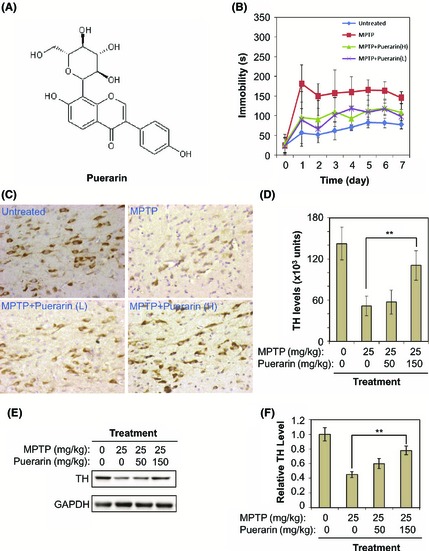
In vivo neuroprotective and neurotrophic activities of puerarin against MPTP neurotoxicity in a murine model of PD. (A) Chemical structure of puerarin. The structure was generated by ChemSketch software (http://www.acdlabs.com). (B) Amelioration of MPTP‐induced behavioral impairments. A total of 24 mice were divided into four groups (n = 6): Untreated, no treatment; MPTP, 25 mg/kg MPTP (i.p.) per day; MPTP+Puerarin (L), 50 mg/kg of puerarin (i.p.) and 25 mg/kg MPTP (i.p.) per day; MPTP+Puerarin (H), 150 mg/kg of puerarin (i.p.) and 25 mg/kg MPTP (i.p.) per day. The immobility of mice in forced swim test was recorded as an indicator of behavioral performance. (C) Reduction of MPTP neurotoxicity on tyrosine hydroxylase positive (TH +) dopaminergic neurons. The animals were treated as described in Panel B. TH protein was immunostained with specific antibody and visualized as dark brown stains produced by the HRP conjugate of secondary antibody. (D) Data analysis of TH immunostaining. Following detection described in Panel C, TH expression levels were determined by reading the optical density of stains. The mean values of the optical intensity for each group of animals (n = 6) were analyzed by one‐way ANOVA (**P < 0.01, Puerarin + MPTP group vs MPTP group). (E) Recovery of MPTP‐induced loss of TH expression. The animals were treated as described in Panel B. TH protein was determined by Western blotting with specific antibody. (F) Data analysis of TH expression. The optical density of TH bands in Panel C was determined by software Quantity One (Bio‐Rad). The mean values of the optical intensity for each group of animals (n = 6) were analyzed by Student's t‐test (**P < 0.01, Puerarin + MPTP group vs MPTP group).
In this study, we tested the hypothesis that puerarin may coordinate with NGF to induce neuritogenesis by activating ERK and PI3K/Akt pathways in a timing‐dependent manner. We investigated the effect of puerarin on the survival of the dopaminergic neurons in the SNpc and the deficits in motor performance in MPTP‐induced parkinsonian model in mice. We carefully examined the timing dependence of the activation of ERK1/2 and PI3K/Akt pathways in the effect of puerarin on NGF‐induced neuritogenesis through three different protocols.
Materials and Methods
Materials
Antibodies against ERK1/2, phospho‐ERK1/2, Akt, phospho‐Akt, β3‐tubulin, microtubule‐associated protein 2 (MAP2), glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), and Alexa Fluor 594‐conjugated goat anti‐rabbit IgG antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Heme oxygenase 1 (HO‐1) antibody was purchased from Stressgene (Ann Arbor, MI, USA). Antityrosine hydroxylase (TH) antibody was purchased from BOSTER (Wuhan, China). Anti‐Nrf2, antilamin B, and anti‐rabbit horseradish peroxidase (HRP)‐conjugated IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti‐rabbit HRP‐conjugated IgG secondary antibody was purchased from Sigma‐Aldrich (St. Louis, MO, USA). Immunohistochemical DAB Detection Kit (Streptavidin‐Biotin) was purchased from MAIXIN Biotechnology (Fuzhou, China). Sn(IV) protoporphyrin IX dichloride (SnPP) was obtained from Frontier Scientific (Logan, UT, USA). Puerarin, NGF‐2.5S, collagen I, and other biochemicals were purchased from Sigma‐Aldrich unless indicated otherwise.
Animals and Drug Intervention
Adult male C57BL/6 mice (8 weeks, 22–25 g) were supplied by the Laboratory Animal Center of Fujian University of Traditional Chinese Medicine (Fuzhou, Fujian, China) and housed in a temperature‐ and humidity‐controlled environment on a 12 h light–dark cycle, and fed with a standard laboratory mice chow and free‐drinking water. All animal experimentation procedures were conducted in compliance with the guidelines of the Animal Ethics Committee of Fujian University of Traditional Chinese Medicine on the Care and Use of Animals for Laboratory Research. Prior to the drug intervention in mice, 1.5 g puerarin was dissolved in 100 mL of 50% 1, 3‐propanediol saline solution (v/v) to give the final concentration of 15 mg/mL. The solution was sterilized by passing through a 0.22‐μm filter. Mice were randomly divided into four experimental groups (n = 6): control group; MPTP group; MPTP + puerarin (L) group, and MPTP + puerarin (H) group. Mice were administered with MPTP (25 mg/kg/day in 100 μL saline) through intraperitoneal (i.p.) injection every afternoon for seven consecutive days, whereas control animals received seven injections of the same volume of saline containing 50% 1,3‐propanediol. Puerarin (50 mg/kg/day or 150 mg/kg/day in 100 μL saline containing 50% 1,3‐propanediol) or the same volume of saline containing 50% 1,3‐propanediol was administered via i.p. injection every morning over 7 days before MPTP injection. The mice were sacrificed at 24 h after the last MPTP injection.
Behavioral Assessment in Forced Swim Test
The effects of drugs on animal behavior were assessed in a blind fashion by experienced examiners who were not involved in the design of the experiments. The mice were forced to swim in an acrylic plastic cylinder filled with water as described 33. Briefly, mice were placed individually into plastic cylinders (height, 25 cm; diameter, 10 cm) filled with 10 cm of water, conditioned at 21 ± 2°C and left there for 5 min. A mouse was classified to be immobile when it floated in an upright position and only made small movements to keep its head above water. The duration of immobility was recorded during the 5‐min testing period.
Immunohistochemical Staining
Immunohistochemical examination of TH expression in mice midbrain was investigated as previously described 28, 34. Briefly, the paraffin sections of mouse midbrain were deparaffinized and hydrated in Millipore water. The antigenic sites were exposed by 20 min of incubation in 10 mM citrate buffer (pH 6.0) at 90°C. Endogenous peroxidase activity was quenched with 3.0% hydrogen peroxide solution. The sections were sequentially blocked in 5% BSA in PBST (PBS with Tween‐20) for 30 min and incubated with rabbit anti‐TH (1:100) overnight at 4°C. The bound antibodies were tracked with biotinylated goat anti‐rabbit secondary antibody for 30 min and horseradish peroxidase‐labeled streptavidin for 30 min. The bound horseradish peroxidase was assayed with 3,3‐N‐diaminobenzidine tetrahydrochloride (DAB). The sections were restained with hematoxylin. The numbers of positive cells from each animal were counted within three nonoverlapping areas (total number of about 300 cells) at 400‐fold magnifications on an Olympus microscope (Olympus Corp., Tokyo, Japan).
Cell Culture and Drug Treatments
Rat pheochromocytoma cell line PC12 was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat‐inactivated horse serum (HS), 5% heat‐inactivated fetal bovine serum (FBS), and 1% penicillin/streptomycin on collagen I‐coated dishes at 37°C under a humidified atmosphere of 5% CO2 and 95% air. For drug treatments, the cells were seeded at the density of 2 × 104 cells/mL in 6‐well plate for 24 h, incubated in differentiation medium (DMEM + 1% HS + 1% FBS) for 24 h and then treated with puerarin and NGF, alone or in combination.
Assay of Neurite Outgrowth
Neurite outgrowth was measured by Neurite Outgrowth Staining Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Briefly, PC12 cells were seeded at 4 × 104 cells per well in a collagen I‐coated 6‐well plate for 24 h, cultured in differentiation medium for another 24 h, and then treated with puerarin and NGF, alone or in combination. The cells were incubated with cell membrane stain and cell viability indicator for 20 min at 37°C, washed twice with PBS, and subsequently incubated with background suppression dye. The images were acquired by a Zeiss fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The cells were scored at different times for the proportion of neurite‐bearing cells relative to the cell monolayer per view or alternatively by measuring the average neurite length in the indicated cell cultures. Triplicate wells were scored, and counts represent means ± SD.
Immunofluorescence Staining of Neuronal Biomarker MAP2
PC12 cells were seeded at 4 × 104 per well onto collagen I‐coated 35‐mm confocal dishes for 24 h and treated with NGF and puerarin, alone or in combination, in differentiation medium for 72 h. The cells were fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized with 0.5% Triton X‐100 for 30 min, and blocked in 5% normal goat serum in PBS for 2 h at room temperature. The cells were then incubated with anti‐MAP2 antibody in 1% BSA at 4°C overnight. The bound antibodies were detected by Alexa Fluor 594‐conjugated goat anti‐rabbit IgG secondary antibody. At the end of detection, the cell nuclei were indicated by staining with 4′‐6‐diamidino‐2‐phenylindole (DAPI) for 15 min. The immunofluorescence images were acquired on Zeiss fluorescence microscopy (Carl Zeiss).
Inhibition of Neurotrophic Signaling Pathways
PC12 cells were pretreated with MEK inhibitors PD98059 (20 μM) and U0126 (2 μM), HO‐1 inhibitor SnPP (20 μM) for 1 h, and treated with puerarin and NGF, alone or a combination for 72 h. Neurite outgrowth was studied as described above.
Western Blot Analysis
At the end of drug treatment, mouse brain tissues and PC12 cells were lysed in ice‐cold RIPA buffer (20 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP‐40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta‐glycerophosphate, 1 mM Na3VO4, and 1 μg/mL leupeptin). The cellular proteins were recovered by centrifugation at 14000 rpm for 15 min. The protein concentrations were determined with protein assay dye reagent from Bio‐Rad (Hercules, CA, USA). The brain tissue and cellular proteins (30 μg) were resolved by gel electrophoresis on 10% SDS‐polyacrylamide gels and subsequently transferred onto PVDF membranes. Following blocking overnight with TBS‐T (Tris‐buffered saline with 0.2% Tween‐20) buffer containing 5% nonfat milk powder or BSA at 4°C, the blots were probed with specific primary antibodies, detected with HRP‐conjugated secondary antibody, and finally visualized by Amersham™ ECL™ Select Western blotting detection reagent from GE Healthcare Biosciences (Uppsala, Sweden).
Statistical Analysis
The results were presented as means ± SD and analyzed by one‐way ANOVA test with SPSS 13.0 software (IBM, New York, NY, USA). A P‐value of <0.05 was considered statistically significant.
Results
Puerarin Protected Dopaminergic Neurons Against MPTP Neurotoxicity in Mice
To investigate the in vivo neuroprotective and neurotrophic activities of puerarin, we examined whether puerarin could ameliorate the impairments of motor functions in MPTP‐lesioned mice. As shown in Figure 1B, compared with untreated controls, MPTP‐treated mice became less active in swim test. Interestingly, puerarin at the doses of 50 and 150 mg/kg/day decreased the immobility time of mice in swim test. We further examined the effect of puerarin on dopaminergic neurons in the substantia nigra. TH levels in dopaminergic neurons were detected by immunohistochemical staining. As shown in Figure 1C,D, MPTP treatment caused dramatic loss of TH expression in mice. Puerarin at the doses of 50 and 150 mg/kg/day preserved TH expression in MPTP‐treated mice. Western blot analysis of TH expression in mouse brains further supported that puerarin (150 mg/kg) significantly rescued MPTP‐induced loss of TH expression (Figure 1E,F).
Puerarin Enhanced NGF‐Induced Neuritogenesis
To explore the effect of puerarin on the neurotrophic activity of NGF, we treated PC12 cells with NGF (2 ng/mL) alone or in combination with puerarin at the concentrations of 10, 25, and 50 μM. After 72 h of treatment, the neurite‐bearing cells were stained and enumerated under a fluorescence microscope. As shown in Figure 2A,B, puerarin induced the formation and growth of neurites in a concentration‐dependent manner and profoundly increased the length of neurites in the presence of NGF (2 ng/mL). Importantly, the combination of NGF (2 ng/mL) and puerarin (50 μM) achieved better stimulation of neurite outgrowth than NGF (20 ng/mL) alone. These results indicated that puerarin could enhance the activity of NGF on neuritogenesis by >10‐fold. The expression of neuronal biomarkers MAP2 and β3‐tubulin in puerarin‐treated cells was determined by immunocytochemical staining or Western blotting. As shown in Figure 2C, puerarin (50 μM) marginally induced MAP2 expression, but effectively enhanced the effect of NGF on MAP2 induction. Furthermore, Western blot analysis revealed that β3‐tubulin expression was also increased by puerarin (10 μM), NGF (2 ng/mL), or the combination of both reagents (Figure 2D). These results suggested that puerarin could effectively stimulate neuritogenesis even when NGF level was largely declined.
Figure 2.

Potentiation of NGF‐induced neuritogenesis by puerarin. (A) Representative images of PC12 cells with or without neurites. PC12 cells were treated with puerarin alone or in combination with NGF (2 ng/mL). The cells were stained by neurite outgrowth staining kit and examined under a Zeiss fluorescence microscope. Scale bar, 100 μm. (B) Puerarin potentiates NGF‐induced neuritogenesis in a concentration‐dependent manner. The neurite‐bearing cells were enumerated with NIH Image J. Untreated PC12 cells were used as control. The values represent the means ± SD (n = 3). **P < 0.01; *** P < 0.001 (sample vs NGF 2 ng/mL). (C) Immunofluorescence staining of neuronal biomarker MAP2. The cells were treated with puerarin and NGF for 72 h as follows: A, control; B, NGF (2 ng/mL); C, puerarin (50 μM); D, NGF (2 ng/mL) and puerarin (50 μM). Following staining with anti‐MAP2 and DAPI, the cells were examined under a Zeiss fluorescence microscope. (D) Western blot analysis of neuronal biomarker β3‐tubulin in PC12 cells. The cells were treated with vehicle control, NGF (2 ng/mL), puerarin (10 μM) or combination of NGF (2 ng/mL) and puerarin (10 μM) for 72 h. β3‐tubulin expression was analyzed by Western blotting using specific antibody, whereas GAPDH was analyzed as the control of protein loading.
Puerarin Potentiated NGF‐Induced Neuritogenesis via Activating ERK1/2, PI3K/Akt, and Nrf2/HO‐1 Pathways
To investigate the mechanisms underlying the neurotrophic activity of puerarin, we initially examined the effect of puerarin on ERK and Akt. PC12 cells were treated with puerarin (50 μM) for 0, 0.25, 0.5, 1, 3, or 6 h. The protein lysates were subsequently analyzed by Western blotting. As shown in Figure 3A, puerarin rapidly induced the phosphorylation of ERK1/2 and Akt. Interestingly, the phosphorylation of ERK1/2 and Akt was detected with a peak at 15 min and sustained for approximately 3 h. We also verified the in vivo effects of puerarin on ERK1/2 and Akt pathways. Following drug treatment for consecutive 7 days, mouse brains were recovered and analyzed by Western blotting. As shown in Figure 3B, puerarin also activated ERK1/2 in MPTP‐lesioned mice in a dose‐dependent manner, whereas puerarin induced somewhat phosphorylation of Akt. Secondly, we investigated the effect of puerarin on Nrf2 nuclear translocation and subsequent HO‐1 induction. After 6 h of treatment with or without puerarin (50 μM), the nuclear and cytosolic fractions were prepared for Western blot analysis of Nrf2, whereas the cells exposed to drugs for 24 h were used for Western blot analysis of HO‐1. Protein lamin B was detected as nuclear biomarker whereas GAPDH was examined as cytosolic biomarker. As shown in Figure 3C, puerarin effectively increased the level of nuclear Nrf2 and subsequent HO‐1 induction. To further explore whether puerarin induced Nrf2 translocation and HO‐1 expression via activating ERK1/2 and Akt, PC12 cells were cotreated with puerarin and MEK inhibitors (e.g., PD98059 and U0126) or PI3K inhibitor LY294002 (Figure 3C). Surprisingly, U0126 somewhat enhanced puerarin‐induced increase in the level of nuclear Nrf2 and HO‐1 expression, whereas PD98059 and LY294002 showed strong inhibitory effect against the actions of puerarin on Nrf2/HO‐1 pathway. Thirdly, we explored whether puerarin potentiated NGF‐induced neurite outgrowth via activating ERK1/2, Akt, and HO‐1 pathways. PC12 cells were cotreated with puerarin, NGF and MEK inhibitors (e.g., PD98059 and U0126), PI3K inhibitor LY294002 or HO‐1 inhibitor SnPP. As shown in Figure 3D,E, the effect of puerarin and NGF on neurite outgrowth was effectively blocked by MEK inhibitor PD98059, PI3K inhibitor LY294002, and HO‐1 inhibitor SnPP although MEK inhibitor U0126 showed relatively less inhibitory potency. The results confirmed that puerarin potentiated NGF‐induced neuritogenesis via ERK1/2‐ and PI3K/Akt‐dependent induction of HO‐1 expression.
Figure 3.
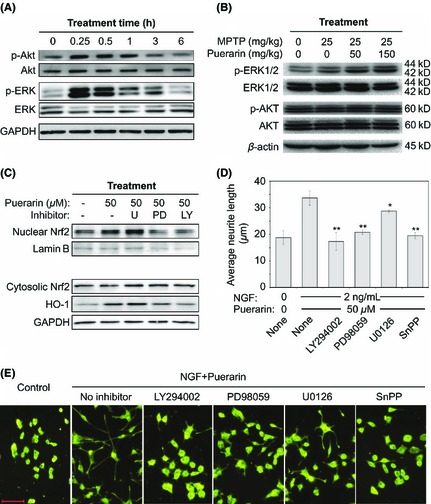
Potential mechanisms underlying the effect of puerarin on NGF‐induced neuritogenesis. (A) Puerarin rapidly activates kinases ERK1/2 and Akt in PC12 cells. Following treatment with puerarin 50 μM for the indicated times, phosphorylation of ERK1/2 and Akt was analyzed by Western blotting using specific antibodies. (B) In vivo effects of puerarin on ERK1/2 and Akt pathways. Following drug treatment for 7 days as described in Figure 1, phosphorylation of ERK1/2 and Akt in mouse brains was analyzed by Western blotting using specific antibodies. (C) Effects of puerarin on the nuclear translocation of Nrf2 and subsequent induction of HO‐1. Following drug treatment as indicated, nuclear proteins and cytosolic proteins were isolated and analyzed for the levels of Nrf2 and HO‐1 by Western blotting using specific antibodies. Protein kinase inhibitors were as follows: U, U0126 for MEK; PD, PD98059 for MEK; LY, LY294002 for PI3K. (D) Regulation of puerarin‐enhanced neuritogenesis by kinase inhibitors and HO‐1 inhibitor. Following drug treatment as indicated, the cells were stained by neurite outgrowth staining kit. The average neurite lengths were examined under a Zeiss fluorescence microscope and analyzed by NIH Image J. Untreated PC12 cells were used as control. The values represent the means ± SD (n = 3). *P < 0.05; **P < 0.01 (sample vs NGF+Puerarin). (E) Representative images of PC12 cells with or without neurites. Scale bar, 100 μm.
Puerarin Potentiated NGF‐Induced Neurite Extension in a Timing‐Dependent Manner
To clarify whether the activation of ERK1/2 and PI3K/Akt pathways by puerarin was sufficient to drive neurite extension, we treated PC12 cells by three different protocols: (1) transient stimulation with NGF and puerarin; (2) simultaneous addition of NGF and puerarin; and (3) sequential addition of two reagents with a time gap of 12 h (Figure 4A). As shown in Figure 4B,C, transient stimulation with NGF and puerarin provided enough signals to initiate the neurite extension process, but failed to sustain the continuous extension of neurites. It was not surprising that NGF at higher concentration induced better neurite extension. When NGF concentration was reduced to 2 ng/mL, little neurite outgrowth was actually promoted although the cells survived. No matter whether added simultaneously or sequentially, however, puerarin could effectively coordinate with NGF to drive neurite extension. The average neurite lengths were not significantly different in the cells treated with NGF and puerarin simultaneously or sequentially.
Figure 4.

Timing dependence of the coordination between puerarin and NGF in neuritogenesis. (A) Experimental protocols for timing‐dependent induction of neuritogenesis by NGF and puerarin. For transient stimulation, PC12 cells were incubated with NGF and puerarin for 1 h and differentiation medium for another 23 h; and for simultaneous stimulation, PC12 cells were incubated with NGF and puerarin for 24 h; for sequential stimulation, PC12 cells were sequentially incubated with NGF for 1 h, differentiation medium alone for 11 h, and then puerarin for 12 h. (B) Quantification of neuritogenesis regulated by NGF and puerarin. Following drug treatment as outlined in Panel A, the neurites were stained and measured as described in “Materials and Methods”. Untreated PC12 cells were used as control. The values represent the means ± SD (n = 3). (C) Representative images of PC12 cells following the treatments with NGF and puerarin. Following staining with neurite outgrowth assay kit, the images were acquired on a Zeiss fluorescence microscopy. Scale bar, 100 μm.
Structure‐Activity Relationship in the Potentiation of NGF‐Induced Neurite Outgrowth
To compare the neurotrophic effects of puerarin and its structural analogs (e.g., daidzein and genistein), we treated PC12 cells with NGF in combination with puerarin, daidzein, or genistein in a similar fashion. As shown in Figure 5, puerarin, daidzein, and genistein similarly potentiated NGF‐induced neurite outgrowth.
Figure 5.
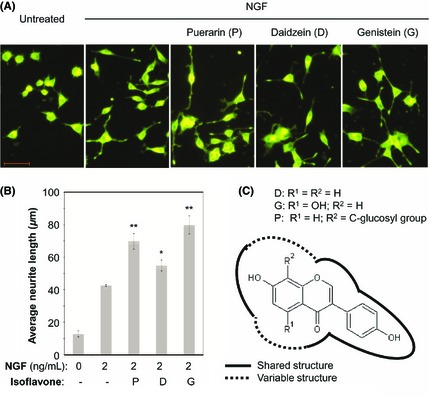
Structure‐activity relationship in NGF‐induced neuritogenesis. (A) Representative images of neuritogenesis induced by NGF and puerarin analogs. At the end of drug treatment with NGF and puerarin analogs, the neurites were stained and examined under a Zeiss fluorescence microscopy. Scale bar, 100 μm. (B) Quantification of neuritogenesis regulated by NGF and puerarin analogs. The average neurite lengths were determined by NIH Image J software. The values represent the means ± SD (n = 3). **P < 0.01 (NGF + isoflavone vs NGF alone). (C) Chemical structures of the active isoflavones. The backbone structure was created by ChemSketch software (R1 = H, OH; R2 = H, C‐glucosyl group). The solid lines indicate the shared structural properties, whereas the dashed lines indicate the variable structural properties.
Discussion
In the present study, we first validated that continuous puerarin treatment could protect TH‐expressing neurons in the midbrain substantia nigra region and improve motor performance in MPTP‐lesioned mice (Figure 1). Secondly, using dopaminergic PC12 cells as in vitro model, we found that puerarin potentiated NGF‐induced neuritogenesis by more than 10‐fold, possibly via activating ERK1/2 and PI3K/Akt pathways (Figures 2 and 3). According to a recent report 21, the actions of NGF on neuritogenesis can be divided into two different processes, namely, ERK‐driven and transcription‐dependent latency process and ERK‐ and PI3K/Akt‐driven and transcription‐independent neurite extension process. Thus, the aim of the present study was to investigate whether botanical drug puerarin could coordinate with NGF to induce neuritogenesis via activating ERK1/2 and PI3K/Akt pathways in the neurite extension process.
Neurotrophins including NGF regulate neuronal survival, growth and differentiation through multiple mechanisms, especially, Raf/MEK/ERK and PI3K/Akt pathways 17. Sustained activation of ERK1/2 is essential to support the actions of NGF on neurite extension 19, 35, 36. The functional neurites are generated through a complex process involving three stages: (1) initiation of neurite formation; (2) elongation of neurites to meet the appropriate targets; and (3) synapse formation and functional maturation of the newly formed neuronal networks 37. Many structural and signaling proteins are involved in modulating such a complex differentiation process 38. It is now known that the ERK1/2 pathway governs two dispensable processes: transcription‐involving latency process and neurite extension process 21. In the transcription‐involving latency process, neurotrophic factors activate ERK1/2 pathway and modulate the transcription of various genes for the initiation and elongation of neurites. On the other hand, in the neurite extension process, the activation of ERK1/2 and PI3K/Akt pathways triggers the extension of neurites, whereas gene transcription is no longer required for neurite outgrowth. Encouragingly, many small molecules are known to induce the activation of ERK1/2 and PI3K/Akt pathways 39, 40. Thus, it is of therapeutic interest to use these synthetic/natural small molecule drugs to mimic the actions of NGF on ERK1/2 and PI3K/Akt pathways and neurite extension.
Moreover, the activation of ERK1/2 and PI3K/Akt pathways often induces the nuclear translocation of transcriptional factor Nrf2 and subsequent HO‐1 expression 41, 42, 43. Upon the stimulation by electrophilic phase II inducers and oxidative stimuli, Nrf2 migrates into the nucleus, binds to antioxidant response element (ARE) in the promoter, and mediates the expression of various antioxidant enzymes including HO‐1 44, 45, 46. HO‐1 is a typical stress responsive enzyme that catalyzes the metabolism of heme into bilirubin, free iron, and carbon monoxide (CO), exhibiting antioxidant and cytoprotective activities. Previous studies suggest that Nrf2/HO‐1 pathway plays a key role to stimulate neurite outgrowth 41, 47. It is also known that the activation of Nrf2 pathway triggers cross‐talking between Nrf2 pathway and TrkA signaling pathway via inducing adaptor protein p62/ZIP 48. The activation of Nrf2/HO‐1 and Nrf2/p62/ZIP pathways could enhance NGF/TrkA‐mediated neuronal differentiation. Collectively, a variety of synthetic and natural small molecules may potentiate the neurotrophic activity of NGF through the activation of ERK1/2, PI3K/Akt, and Nrf2 pathways.
The present study not only revealed that puerarin rapidly activated ERK1/2 and PI3K/Akt, leading to the activation of Nrf2/HO‐1 pathway, but also demonstrated that puerarin potentiated NGF‐induced neuritogenesis in ERK1/2‐, PI3K/Akt‐, and Nrf2/HO‐1‐dependent manner. MEK inhibitor PD98059 and PI3K inhibitor LY294002 attenuated the actions of puerarin on the Nrf2/HO‐1 pathway. These results support the role of ERK and PI3K pathways in the regulation of HO‐1 expression 49. Moreover, MEK, PI3K, and HO‐1 inhibitors U0126, PD98059, LY294002, and SnPP diminished the effect of puerarin on NGF‐induced neurite outgrowth. The results also suggest that cross talks between ERK1/2, PI3K/Akt, and Nrf2/HO‐1 pathways may mediate the coordination between puerarin and NGF in the induction of neurite outgrowth. The present study verified that puerarin regulated the differentiation of PC12 cells into neuron by immunostaining and Western blot analysis of common neuronal biomarkers (e.g., MAP2 and β3‐tubulin) 50. Highly consistent with several previous studies 26, 41, 51, puerarin increased the expression of neuronal biomarkers MAP2 and β3‐tubulin. By transient, simultaneous and sequential stimulations of PC12 cells with NGF and puerarin, we for the first time observed that puerarin (50 μM) effectively coordinated with NGF (2 ng/mL) in driving neurite outgrowth in three stimulation experiments (Figure 4). It is critical to maintain NGF at a sufficiently high level (e.g., 20 ng/mL) to effectively stimulate the extension of neurites. NGF at lower concentrations (e.g., 2 ng/mL) could not drive the extension of neurites. In the presence of NGF (2 ng/mL), however, puerarin (50 μM), added either simultaneously or sequentially, could drive neurite outgrowth to a comparable extent that was achieved by NGF at higher concentrations. These results consolidated the pharmacological potential of puerarin to maintain neuronal differentiation when NGF level is low.
Natural products are increasingly recognized for the promotion of neuronal survival and neurogenesis against neuronal injury and chronic neurodegeneration 51, 52. Isoflavones including daidzein and genistein are commonly classified as phytoestrogens with the potential to enhance NGF‐induced neurite outgrowth, although the underlying mechanisms remain elusive 53, 54, 55. After assaying puerarin, daidzein, genistein, calycosin, onion, and naringin in parallel, we found that puerarin, daidzein, and genistein could similarly potentiate NGF‐induced neurite outgrowth (Figure 5). In terms of the chemical structures of these isoflavones (Figure 5C), daidzein represents the backbone structure for all three testing compounds, whereas puerarin bears a unique C‐glucosyl moiety and genistein is modified by one additional 5‐hydroxyl group. These isoflavones shared the common structural characteristics (solid lines) and differ in two minor variable substitutions (dashed lines). Presumably, these analogs may activate ERK1/2 and PI3K/Akt to the similar extent via similar or different mechanisms. These results shed light on structural optimization to potentiate the neurotrophic activity of NGF with better in vivo potency and efficacy.
In conclusion, the present study for the first time demonstrated that pharmacological activation of ERK1/2 and PI3K/Akt pathways could effectively drive the neurite extension process following NGF‐initiated latency process (Figure 6). The results of this study may suggest a general mechanism underlying the neurotrophic application of puerarin and related small molecule compounds in the therapy of PD.
Figure 6.

Schematic illustration of the coordination between puerarin and NGF to regulate neuritogenesis. NGF induces Signal‐1 to prime the cells to the ready‐to‐go differentiation state, whereas puerarin effectively promotes the activation of protein kinases ERK1/2 and Akt, leading to nuclear translocation of Nrf2 as Signal‐2. Ultimately, the cross talks between Signal‐1 and Signal‐2 drive the neurite outgrowth.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This project was supported by General Research Fund (GRF) (HKU 775812M) from the Research Grants Council of Hong Kong and the Seed Fund for Basic Research Programme, the University of Hong Kong (to J. R.).
References
- 1. Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron 2003;39:889–909. [DOI] [PubMed] [Google Scholar]
- 2. Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science 2003;302:819–822. [DOI] [PubMed] [Google Scholar]
- 3. Navarro A, Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson's disease. J Bioenerg Biomembr 2009;41:517–521. [DOI] [PubMed] [Google Scholar]
- 4. Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J. Mitochondrial dysfunction: A potential link between neuroinflammation and neurodegeneration? Mitochondrion 2010;10:411–418. [DOI] [PubMed] [Google Scholar]
- 5. Jonnala RR, Buccafusco JJ. Inhibition of nerve growth factor signaling by peroxynitrite. J Neurosci Res 2001;63:27–34. [DOI] [PubMed] [Google Scholar]
- 6. Bruno MA, Cuello AC. Cortical peroxynitration of nerve growth factor in aged and cognitively impaired rats. Neurobiol Aging 2012;33:1927–1937. [DOI] [PubMed] [Google Scholar]
- 7. Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl 2000;60:277–290. [DOI] [PubMed] [Google Scholar]
- 8. Ubhi K, Rockenstein E, Mante M, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial‐derived neurotrophic factors. J Neurosci Res 2010;30:6236–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci 2003;4:299–309. [DOI] [PubMed] [Google Scholar]
- 10. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001;24:1217–1281. [DOI] [PubMed] [Google Scholar]
- 11. Aron L, Klein R. Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 2011;34:88–100. [DOI] [PubMed] [Google Scholar]
- 12. Geral C, Angelova A, Lesieur S. From molecular to nanotechnology strategies for delivery of neurotrophins: Emphasis on brain‐derived neurotrophic factor (BDNF). Pharmaceutics 2013;5:127–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: From the early discoveries to the potential clinical use. J Transl Med 2012;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olson L, Backman L, Ebendal T, et al. Role of growth factors in degeneration and regeneration in the central nervous system; clinical experiences with NGF in Parkinson's and Alzheimer's diseases. J Neurol 1994;242(1 Suppl 1):S12–S15. [DOI] [PubMed] [Google Scholar]
- 15. Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron 1992;9:1067–1079. [DOI] [PubMed] [Google Scholar]
- 16. Kaplan DR, Martin‐Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto‐oncogene product induced by NGF. Nature 1991;350:158–160. [DOI] [PubMed] [Google Scholar]
- 17. Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002;296:1648–1649. [DOI] [PubMed] [Google Scholar]
- 18. Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 1994;77:841–852. [DOI] [PubMed] [Google Scholar]
- 19. Pellegrino MJ, Stork PJ. Sustained activation of extracellular signal‐regulated kinase by nerve growth factor regulates c‐fos protein stabilization and transactivation in PC12 cells. J Neurochem 2006;99:1480–1493. [DOI] [PubMed] [Google Scholar]
- 20. Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal‐regulated kinase activation. Cell 1995;80:179–185. [DOI] [PubMed] [Google Scholar]
- 21. Chung J, Kubota H, Ozaki Y, Uda S, Kuroda S. Timing‐dependent actions of NGF required for cell differentiation. PLoS One 2010;5:e9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson's disease. CNS Neurol Disord Drug Targets 2007;6:273–281. [DOI] [PubMed] [Google Scholar]
- 23. Heurteaux C, Widmann C, Moha ou Maati H, et al. NeuroAiD: Properties for neuroprotection and neurorepair. Cerebrovasc Dis 2013;35(Suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- 24. More SV, Kumar H, Kang SM, Song SY, Lee K, Choi DK. Advances in neuroprotective ingredients of medicinal herbs by using cellular and animal models of Parkinson's disease. Evid Based Complement Alternat Med 2013;2013:957875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song JX, Sze SC, Ng TB, et al. Anti‐Parkinsonian drug discovery from herbal medicines: What have we got from neurotoxic models? J Ethnopharmacol 2012;139:698–711. [DOI] [PubMed] [Google Scholar]
- 26. Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr 2010;104(Suppl 3):S40–S47. [DOI] [PubMed] [Google Scholar]
- 27. Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK‐dependent induction of Nrf2‐driven HO‐1 expression. J Agric Food Chem 2010;58:4477–4486. [DOI] [PubMed] [Google Scholar]
- 28. Li R, Liang T, Xu L, Zheng N, Zhang K, Duan X. Puerarin attenuates neuronal degeneration in the substantia nigra of 6‐OHDA‐lesioned rats through regulating BDNF expression and activating the Nrf2/ARE signaling pathway. Brain Res 2013;1523:1–9. [DOI] [PubMed] [Google Scholar]
- 29. Zhou YX, Zhang H, Peng C. Puerarin: A review of pharmacological effects. Phytother Res 2014;28:961–975. [DOI] [PubMed] [Google Scholar]
- 30. Zhu G, Wang X, Wu S, Li X, Li Q. Neuroprotective effects of puerarin on 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine induced Parkinson's disease model in mice. Phytother Res 2014;28:179–186. [DOI] [PubMed] [Google Scholar]
- 31. Zhu G, Wang X, Wu S, Li Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+‐induced human neuroblastoma SH‐SY5Y cell death. Neurochem Int 2012;60:400–408. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Wang WL, Xie WL, et al. Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG‐63 cells via ER‐dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 2013;20:787–796. [DOI] [PubMed] [Google Scholar]
- 33. Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977;229:327–336. [PubMed] [Google Scholar]
- 34. Sathiya S, Ranju V, Kalaivani P, et al. Telmisartan attenuates MPTP induced dopaminergic degeneration and motor dysfunction through regulation of alpha‐synuclein and neurotrophic factors (BDNF and GDNF) expression in C57BL/6J mice. Neuropharmacology 2013;73:98–110. [DOI] [PubMed] [Google Scholar]
- 35. Kao S, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem 2001;276:18169–18177. [DOI] [PubMed] [Google Scholar]
- 36. Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol 2003;13:391–398. [DOI] [PubMed] [Google Scholar]
- 37. da Silva JS, Dotti CG. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci 2002;3:694–704. [DOI] [PubMed] [Google Scholar]
- 38. Laketa V, Simpson JC, Bechtel S, Wiemann S, Pepperkok R. High‐content microscopy identifies new neurite outgrowth regulators. Mol Biol Cell 2007;18:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riese U, Ziegler E, Hamburger M. Militarinone A induces differentiation in PC12 cells via MAP and Akt kinase signal transduction pathways. FEBS Lett 2004;577:455–459. [DOI] [PubMed] [Google Scholar]
- 40. El Omri A, Han J, Kawada K, Ben Abdrabbah M, Isoda H. Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res 2012;1437:16–25. [DOI] [PubMed] [Google Scholar]
- 41. Gundimeda U, McNeill TH, Schiffman JE, Hinton DR, Gopalakrishna R. Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: Possible role of reactive oxygen species. J Neurosci Res 2010;88:3644–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qi H, Han Y, Rong J. Potential roles of PI3K/Akt and Nrf2‐Keap1 pathways in regulating hormesis of Z‐ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology 2012;62:1659–1670. [DOI] [PubMed] [Google Scholar]
- 43. Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6‐hydroxydopamine‐induced oxidative stress by increasing heme oxygenase‐1 expression through an estrogen receptor‐related PI3K/Akt/Nrf2‐dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol 2010;242:18–28. [DOI] [PubMed] [Google Scholar]
- 44. Qi H, Siu SO, Chen Y, et al. Senkyunolides reduce hydrogen peroxide‐induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase‐1. Chem Biol Interact 2010;183:380–389. [DOI] [PubMed] [Google Scholar]
- 45. Ryter SW, Morse D, Choi AM. Carbon monoxide: To boldly go where NO has gone before. Sci STKE 2004;2004:RE6. [DOI] [PubMed] [Google Scholar]
- 46. Satoh T, Okamoto SI, Cui J, et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci USA 2006;103:768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hsu YY, Tseng YT, Lo YC. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2‐related neurite outgrowth. Toxicol Appl Pharmacol 2013;272:787–796. [DOI] [PubMed] [Google Scholar]
- 48. Kosaka K, Mimura J, Itoh K, et al. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12 h cells. J Biochem 2010;147:73–81. [DOI] [PubMed] [Google Scholar]
- 49. Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase‐1 induction: Implications for chemoprevention and chemoprotection. Antioxid Redox Signal 2005;7:1688–1703. [DOI] [PubMed] [Google Scholar]
- 50. Cui W, Cui GZ, Li W, et al. Bis(12)‐hupyridone, a novel multifunctional dimer, promotes neuronal differentiation more potently than its monomeric natural analog huperzine A possibly through alpha7 nAChR. Brain Res 2011;1401:10–17. [DOI] [PubMed] [Google Scholar]
- 51. Oberbauer E, Urmann C, Steffenhagen C, et al. Chroman‐like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J Nutr Biochem 2013;24:1953–1962. [DOI] [PubMed] [Google Scholar]
- 52. More SV, Koppula S, Kim IS, Kumar H, Kim BW, Choi DK. The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 2012;17:6728–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao L, Chen Q, Diaz Brinton R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp Biol Med (Maywood) 2002;227:509–519. [DOI] [PubMed] [Google Scholar]
- 54. Yang SH, Liao CC, Chen Y, Syu JP, Jeng CJ, Wang SM. Daidzein induces neuritogenesis in DRG neuronal cultures. J Biomed Sci 2012;19:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakajima K, Niisato N, Marunaka Y. Genistein enhances the NGF‐induced neurite outgrowth. Biomed Res 2011;32:351–356. [DOI] [PubMed] [Google Scholar]


