Abstract
Abstract. The main focus of this review is the role of mammary stem cells in normal breast development and carcinogenesis. We have developed a new in vitro culture system that permits, for the first time, the propagation of mammary stem and progenitor cells in an undifferentiated state, which should facilitate the elucidation of pathways that regulate normal mammary stem‐cell self‐renewal and differentiation. Furthermore, we propose a model in which transformation of stem cells, or early progenitor cells, results in carcinogenesis. A key event in this process is the deregulation of normal self‐renewal in these cells. Transformed mammary stem or progenitor cells undergo aberrant differentiation processes that result in generation of the phenotypic heterogeneity found in human and rodent breast cancers. This phenotypic diversity is driven by a small subset of mammary tumour stem cells. We will discuss the important implications of this mammary tumour stem‐cell model.
INTRODUCTION
The mammary gland in humans and other mammals is a dynamic organ that undergoes significant developmental changes during pregnancy, lactation, and involution. Research over the past several decades has helped to elucidate the pathways that regulate cellular growth, differentiation, and apoptosis (Hennighausen & Robinson 2001; Strange et al. 2001). These pathways involve stromal–epithelial interactions, and are modulated by circulating hormones, local growth factors, cellular/extracellular matrix interactions and by cell–cell interactions (Wiseman & Werb 2002). There is also increasing evidence that breast cancers arise from either inherited or acquired mutations that cause deregulation of these normal pathways in stem or early progenitor cells (Reya et al. 2001). Therefore, an understanding of the pathways that regulate normal mammary development is of fundamental importance in elucidating how these pathways are deregulated in mammary carcinogenesis. This understanding in turn may lead to more effective ways to diagnose, treat, and prevent breast cancer.
THE EXISTENCE OF STEM CELLS IN THE MATURE MAMMARY GLAND
Unlike other tissues and organs that are patterned during embryogenesis and preserve their architecture throughout adult life, the mammary gland is subjected to major changes in morphology during distinct developmental windows (Rudland et al. 1997). In humans, the mammary epithelium consists of a network of ducts that form before birth, by branching and invading the mammary fat pad. The ducts are formed by a basal layer of contractile, myoepithelial cells and a luminal layer of specialized epithelial cells. During puberty, ductal outgrowth rapidly increases under hormonal stimulation, resulting in side branching (Rudland et al. 1997; Hennighausen & Robinson 2001). The final differentiation stage is achieved in the mammary gland during pregnancy and lactation, when numerous lobulo‐acinar structures containing the milk‐secreting alveolar cells are formed through extensive proliferation, followed by terminal differentiation. Cessation of lactation following weaning is accompanied by massive apoptosis and tissue remodelling, and the gland reverts to a structure resembling that before pregnancy. Therefore, a compartment of cells with high proliferative potential and differentiation ability is needed in order to sustain numerous pregnancies, a description that fits the definition of stem cells or early progenitor cells.
Whereas mammary stem cells have not as yet been isolated and characterized, there is strong evidence for their existence in vivo. The existence of self‐renewing, multipotent mammary stem cells was first suggested decades ago by the work of Daniel et al. Their studies in mice and rats (Daniel et al. 1971; Kim et al. 2000) demonstrated that an entire mammary gland can be generated from serially transplanted random fragments of epithelium. Generally, senescence occurred after the fourth transplant, but in about 25% of cases, seven and eight serial transplantations were achieved. This indicates that mammary stem cells are dispersed throughout the entire gland and have a potent, although limited, self‐renewal capacity. More recently, Kordon and Smith, using mammary epithelium marked with mouse mammary tumour virus (MMTV) showed that clonal dominant populations were responsible for the generation of a new gland in recipient animals (Kordon & Smith 1998). Serial transplantation of the clonally derived outgrowth recapitulated the entire functional repertoire of the gland, demonstrating the existence of self‐renewing and multipotent mammary stem cells. While these studies provided indirect evidence for the existence of mammary stem cells, these cells have not yet been prospectively identified or isolated.
The isolation of mammary stem cells has been hindered by the lack of suitable systems that maintain these cells in culture in an undifferentiated state, and by the lack of defined stem‐cell markers. In an attempt to purify rodent stem cells, Welm and coworkers combined long‐term in vivo BrdU labelling with immunosorting and transplantation, and showed that cells that expressed stem‐cell antigen (SCA)‐1 were enriched in progenitor cells, able to regenerate the gland in vivo (Welm et al. 2002). In the same study, they showed that progenitor cells are also contained in a subpopulation of cells with increased ability to exclude the nuclear dye, Hoechst. This is a characteristic shared by a number of stem cells, including haematopoietic and neural stem cells, and is the result of increased expression of transporter proteins such as P‐glycoproteins or breast cancer resistance proteins (BCRP) (Zhou et al. 2001; Bunting 2002). It has been suggested that the functional significance of this increased transporter activity is that of protection of the long‐lived stem cells from damaging agents. Similarly, DNA repair pathways are active in a number of previously characterized stem cells, such as haematopoietic stem cells, neuronal stem cells, and embryonic stem cells. In addition to protecting cells from toxic insults, these transporters have been suggested to play a role in maintaining stem cells in an undifferentiated state. This has been shown in Dictyostelium, in which transporters are able to export differentiating‐inducing factor, maintaining the cells in an undifferentiated state (Good & Kuspa 2000).
An ultrastructural study performed by Smith and Chepko described a population of small light cells, without polarity, that are dispersed along the epithelial ducts of the rat mammary gland. They suggested that these cells might represent mammary stem cells (Smith & Chepko 2001). Based on these data, and the fact that human bi‐potent progenitor cells are contained in the luminal epithelial compartment, a number of candidate markers for mammary stem/progenitor cells were hypothesized: ESA+, Muc1−, alpha 6 integrin+, CD10+. Immunosorting using these markers, in combination with the exclusion of rhodamine dye (Stingl et al. 1998; Stingl et al. 2001) showed that bi‐potent luminal epithelial and myoepithelial progenitors are present in the in the ESA+ alpha 6 integrinhigh CD10+/low fraction of HMEC. Using these markers, a 80% increase in bipotent progenitors was obtained compared with 6% in nonsorted cells.
Other investigators have used an alternative approach, in which immortalized mammary cell lines are established from human or rodent tissues. For instance, Gudjonsson and coworkers (Gudjonsson et al. 2002), described an ESA+ MUC 1− cell‐line derived from human mammary cells, which was capable of generating ductal‐acinar structures in a basement‐membrane gel. Complete functional differentiation and synthesis of milk proteins was not shown for these cells. Although cell lines are useful for elucidating molecular pathways, the process of immortalization may introduce artefacts that significantly alter cellular properties and gene expression profiles.
As an alternative to the use of established cell lines, the cultivation of normal mammary progenitor cells has been limited by the lack of suitable systems that allow for the propagation of these cells in an undifferentiated state. When primary cultures of mammary epithelium from rodents or humans are cultured on solid substrata, they undergo limited replication and differentiation in a process that is regulated by hormonal factors, extracellular matrix and cell–cell interactions (Reynolds & Weiss 1996; Muschler et al. 1999; Romanov et al. 2001; Simian et al. 2001). A major advance in neural stem‐cell research was achieved when it was found that an undifferentiated multipotent population of neural cells can be grown in suspension as neurospheres (Reynolds & Weiss 1996; Weiss et al. 1996). Neurospheres were shown to contain between 4% and 20% stem cells, with the rest of the population representing progenitor cells in various stages of differentiation. This cultivation method was instrumental in a variety of experimental systems: stem‐cell enrichment assays (Uchida et al. 2000), comparative gene‐expression profiling (Geschwind et al. 2001), and in vitro models for the development of the nervous system (Caldwell et al. 2001). We have used a similar approach to develop a novel culture system for human mammary epithelial cells, which for the first time permits their propagation in an undifferentiated state.
MAMMOSPHERES, OBTAINED BY CULTURING HUMAN MAMMARY EPITHELIAL CELLS IN SUSPENSION, ARE ENRICHED IN STEM/PROGENITOR CELLS
It has been widely believed that normal epithelial cells are anchorage‐dependent and undergo a process of apoptosis, termed anoikis, when they are cultured in the absence of a substratum to which they can attach (Streuli & Gilmore 1999). Based on the model of neurospheres, we hypothesized that a small population of mammary cells with stem‐cell properties would be able to survive and proliferate in the absence of attachment to an exogenous substratum. We developed a culture system in which human mammary epithelial cells, isolated from reduction mammoplasties, are cultured on a nonadhesive substratum. Under these conditions, the vast majority of cells undergo anoikis. However, a small number of cells are able to survive and proliferate, and form multicellular spheroids (Fig. 1a). We have termed these spheroids ‘mammospheres’ by virtue of their resemblance to neurospheres cultured from primary neural cells. As is the case for neurospheres, we have demonstrated that mammospheres are highly enriched in undifferentiated cells, as demonstrated by the ability of single cells isolated from mammospheres to generate multilineage colonies when cultured in the presence of serum on a collagen substratum that promotes their differentiation (Fig. 1b). Primary mammospheres contain eight times more bilineage progenitor cells than freshly cultured human mammary cells. Secondary and later‐passaged mammospheres consist of virtually 100% bi‐potent progenitors. Furthermore, the majority of bipotent progenitors are able to generate colonies that contain all three lineages of the adult mammary gland, myeoepithelial, ductal epithelial, and alveolar epithelial cells (Dontu et al. 2003). We have also shown that mammospheres contain cells capable of clonally generating complex functional structures in reconstituted 3‐D culture systems in Matrigel (Fig. 1c)
Figure 1.
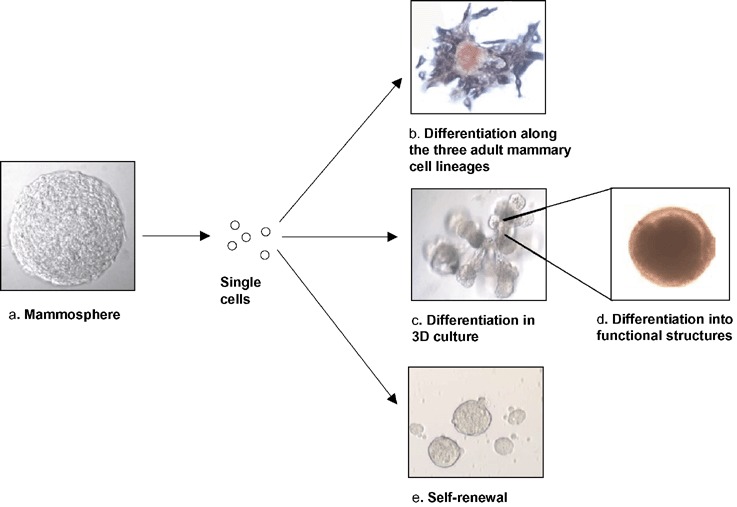
Mammosphere‐derived cells have stem‐cell characteristics. (a) Mammosphere structure (15 days’ growth). Mammosphere‐derived cells plated at clonogenic denstities can (b) generate mixed colonies, containing cells of all the three mammary lineage types (ductal epithelial, ESA, brown; myoepithelial, CD10, purple; alveolar, beta‐casein, red) or (c) generate complex structures in 3D Matrigel culture. (d) The structures clonally generated in vitro are functional, as demonstrated by beta‐casein secretion (immunostained red). Self‐renewal is demonstrated by serial passages of mammospheres.
Another important property of all stem cells is their ability to undergo self‐renewal. Self‐renewal of a population within mammospheres was demonstrated using an assay in which single cells from mammospheres were able to generate second‐ and later generation spheres (Fig. 1e). Furthermore, we demonstrated that the mammospheres derived form these passaged cells also have multipotent differentiation potential. These results resemble those reported for neurospheres (Scheffle et al. 1999), and are consistent with a model in which the mammosphere‐forming cell represents a mammary stem cell that undergoes limited self‐renewal, and then gives rise to mammary progenitors still capable of multilineage differentiation. Clonal experiments, in which spheres were grown from single cells and single mammospheres were passaged, suggest that one or two self‐renewal divisions are involved in the formation of a single mammosphere. This limited number of self‐renewal divisions is in agreement with the majority of studies involving adult stem cells, which indicate that expansion of the adult stem population does not occur ex vivo, presumably due to asymmetric cell kinetic divisions that result in a large number of progenitors and differentiated cells, and a small fixed number of stem cells. We are currently testing the stem‐cell properties of sphere‐forming cells in an NOD/SCID mouse model.
As mammospheres are able to maintain mammary stem and progenitor cells in a relatively undifferentiated state, this system can be used to study the pathways that regulate self‐renewal and differentiation. Indeed, we have preliminary data showing that activation of pathways such as Notch or LIF affects mammosphere formation and cellular differentiation.
TRANSCRIPTIONAL PROFILE OF HUMAN MAMMARY STEM/PROGENITOR CELLS
Another important application of the mammosphere cultivation system is that it has enabled us to obtain a transcriptional profile of mammary stem/progenitor cells, and thus identify a number of candidate markers that could be used for their purification. The gene expression profile of secondary mammospheres, consisting almost exclusively of multipotent cells, were compared with cells grown under differentiating conditions, using microarray analysis. The differences in observed expression profiles strongly supported the validity of this experimental system in reflecting the normal process of mammary development and differentiation (Dontu et al. 2003). Moreover, some of the genes upregulated in mammospheres have previously been found to be enriched in other stem cells. Two groups recently compared the gene expression profiles of adult stem cells with those of embryonic stem cells and proposed that the overlap represents a molecular signature for ‘stemness’ (Ivanova et al. 2002; Ramalho‐Santos et al. 2002). Despite limitations in gene expression comparison across different species and different experimental conditions, we detected a high degree of overlap between genes expressed in mammospheres and those expressed in haematopoietic, neuronal and embryonic stem cells (Table 1). The proposed attributes of stemness, such as active TGF‐beta signalling, growth hormone and thrombin receptor signalling, and upregulation of membrane transporters in the ABC family, were also identified in our study. In addition, deregulation of a number of the genes that we identified as being upregulated in mammospheres have previously been implicated in mammary tumorigenesis.
Table 1.
Examples of genes with overlapping expression patterns in mammospheres, neurospheres, haematopoietic stem cells and embryonic stem cells
| Genes upregulated in mammospheres, neurospheres, haematopoietic |
| stem cells, and embryonic stem cells |
| Integrin, beta 1 |
| Growth hormone receptor |
| Platelet‐derived growth factor receptor, beta polypeptide |
| p53 target zinc finger protein |
| Policystic kidney disease 2 |
| Genes upregulated in mammospheres and neurospheres |
| p59fyn (FYN) oncogene |
| ATP‐binding cassette, subfamily A, member 1 |
| Fibroblast growth factor receptor 1 |
| Tenascin C |
| Genes upregulated in mammospheres and haematopoietic stem cells |
| Notch 3 |
| Latent transforming growth factor beta binding protein 3 |
| Apolipoprotein E |
| GATA‐binding protein 2 |
| Genes upregulated in mammospheres and embryonic stem cells |
| Nidogen 1 and 2 |
| Glypican 4 |
| Insulin‐like growth factor binding protein 4 and 7 |
| WNT1 inducible signalling pathway protein 1 |
STEM CELLS AS PRIMARY TARGETS FOR TRANSFORMATION
A number of investigators have suggested that stem cells may represent important targets for transformational events (Reya et al. 2001; Tu et al. 2002). We propose a model in which mammary carcinogenesis is driven by tumour stem cells derived from mutated adult stem or progenitor cells. The central role of stem cells in normal mammary development and tumorigenesis is illustrated in Fig. 2. Carcinomas are believed to arise through a series of mutations that may occur over many years. Adult stem cells are slow‐dividing, long‐lived cells that by their very nature are exposed to damaging agents for long periods of time. Therefore, they may accumulate mutations that can result in their transformation. Evidence that long‐lived cells may be targets for transformation comes from data on breast‐cancer incidence following radiation exposure in atomic bomb victims at Hiroshima and Nagasaki. Women exposed to radiation during this period have experienced an increased incidence of breast cancer, often occurring 30 years after the time of exposure (Little & Boice 1999). Mutations found in these women's breast cancers are consistent with those known to be induced by radiation. Furthermore, women exposed to radiation during late adolescence had the highest susceptibility to breast‐cancer development. This is thought to be the period when the mammary gland has the highest number of stem cells (Smith & Chepko 2001).
Figure 2.
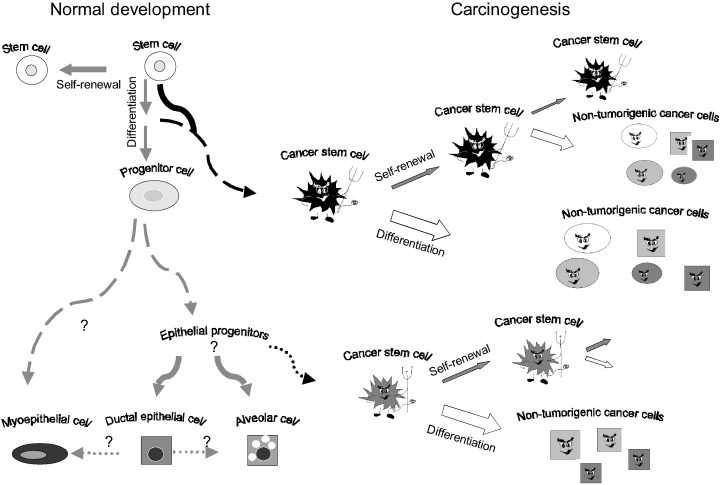
Role of stem cells in normal mammary development and carcinogenesis. Normal mammary stem cells are defined by their ability to undergo self‐renewal and to differentiate into the three cell lineages present in the mature gland. Mammary tumours arise from stem cells or progenitor cells through deregulation of normal self‐renewal. Tumour stem cells retain the ability to self‐renew as well as to differentiate. The tumorigenic stem‐cell population is maintained through self‐renewal. Aberrant differentiation of these cells generates nontumorigenic progeny, representing the bulk of the tumour.
The observation that normal stem cells and cancers share a number of important phenotypic properties provides further evidence for these cells playing a role in carcinogenesis. Important cellular properties shared by all normal stem cells and cancer cells are shown in Fig. 3 and include: (1) capacity for self‐renewal; (2) ability to differentiate; (3) active telomerase and antiapototic pathways; (4) increased membrane transporter activity; and (5) anchorage independence and ability to migrate.
Figure 3.
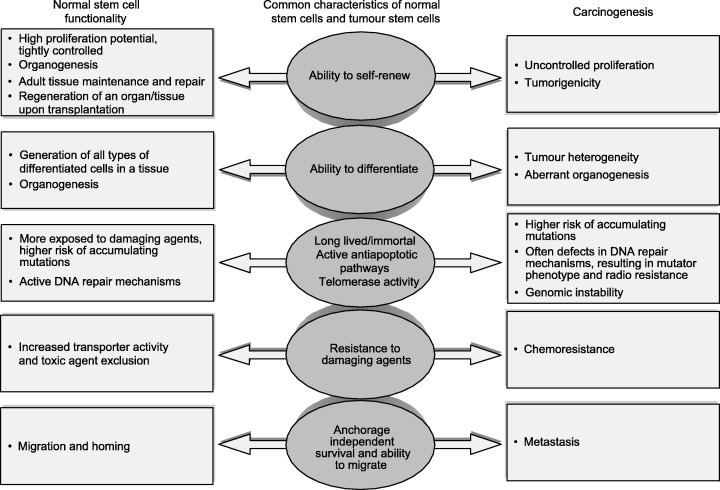
Similarities between normal stem cells and cancer cells and their impact on stem‐cell functionality and carcinogenesis.
In addition to cellular phenotypes shared by stem cells and tumour cells, there is evidence that deregulation of the pathways that are involved in stem‐cell functionality plays an important role in mammary carcinogenesis.
PATHWAYS INVOLVED IN SELF‐RENEWAL
The Notch transmembrane receptor proteins are part of a signalling pathway that is critical for the correct developmental fate of cells and various tissues. The Notch proteins, represented by four homologues in mammals (Notch 1 through 4), are expressed in a variety of stem or early progenitor cells (Gaiano & Fishell 2002). They interact with a number of surface‐bound or secreted ligands (Delta, Delta‐like, Jagged 1 and 2 in vertebrates) (Mumm & Kopan 2000). These interactions are in turn regulated by a number of modifiers that form the Fringe family (Lunatic, Manic and Radical Fringe) (Wu & Rao 1999). Activation of the Notch pathway results in changes of cell fate, such as proliferation of undifferentiated cells, blockage of differentiation, or differentiation along a particular lineage (Krause 2002). The vertebrate Notch 4 gene has been shown to be involved in normal mammary development (Uyttendaele et al. 1998; Soriano et al. 2000). In vitro, over‐expression of the constitutively active form of Notch 4 inhibits differentiation of normal breast epithelial cells (Uyttendaele et al. 1998). In vivo, transgenic mice expressing a constitutively active form of Notch 4 in the mammary gland fail to develop secretory lobules during gestation, and subsequently develop mammary tumours (Soriano et al. 2000). This supports a role for Notch involvement in normal breast development, and suggests that alterations in Notch 4 signalling might play a significant role in breast‐cancer development. Indeed, we have detected expression of Notch family members in mammospheres, and have evidence that Notch ligands affect the self‐renewal and differentiation of normal mammary epithelial cells.
Another pathway that regulates cell fate determination in a number of tissues, including the mammary gland, is Wnt. Molecules involved in the Wnt pathway have well‐established pro‐oncogenic roles (Wnt, β‐catenin) or tumour‐suppressor activities (APC, TCF‐1, axin) (Polakis 2000). Recently, a number of studies were published providing evidence for a direct role of Wnt signalling in self‐renewal of haematopoietic, epidermal and gut stem cells (Reya et al. 2001; Brittan & Wright 2002). In addition, retroviral transduction of activated β‐catenin results in increased epidermal stem‐cell self‐renewal and decreased differentiation. A direct correlation between cancer and dysfunction of this pathway in stem cells was established by experiments in transgenic mice that showed that activation of the Wnt signalling pathway in epidermal stem cells leads to epithelial cancers (Reya et al. 2001). We have identified differential expression of molecules in the Wnt pathways in mammospheres, compared with differentiated cells, which suggests the possible involvement of the Wnt pathway members in the regulation of normal mammary stem‐cell function. Furthermore, overexpressing Wnt in the mouse mammary gland using the MMTV promoter increased mammary tumour formation (Schroeder et al. 2002).
Leukaemia inhibitory factor (LIF) and PTEN are two other examples of proteins involved in stem‐cell self‐renewal, and normal development, which have also been shown to be involved in tumorigenesis. LIF stimulates self‐renewal of neural adult stem cells, as shown by the experiments using transgenic LIF deficient homo‐ and hetero‐zygous mice (Shimazaki et al. 2001). LIF has been shown to maintain embryonic cells in an undifferentiated state. Recently, transgenic animals expressing LIF in the mammary gland were shown to develop mammary tumours (Dhingra et al. 1998). Furthermore, LIF overexpression has been described in human mammary carcinomas. PTEN is a lipid phosphatase that is able to dephosphorylate active AKT, which plays a role in modulating cellular apoptotic pathways. PTEN has been shown to regulate self‐renewal of neural stem cells in vivo, as well as formation of neurospheres in vitro (Groszer et al. 2001). Mutations of the PTEN gene have been demonstrated in several human malignancies, including human mammary carcinomas (Petrocelli & Slingerland 2001).
PATHWAYS INVOLVED IN DIFFERENTIATION
LMO4 belongs to a family of LIM‐only transcriptional regulators, the first two members of which are oncoproteins in acute T‐cell leukaemia (Dawid et al. 1998). Initially LMO4 was described as a tumour autoantigen in breast cancer, and is over‐expressed in 60% of primary breast carcinomas (Visvader et al. 2001). Recently, the function of LMO4 in the normal gland was explored and, as suggested by an increased lobulo‐alveolar expression during pregnancy, overexpression of the gene inhibited differentiation of mammary cells in vitro (Visvader et al. 2001; Sum et al. 2002). Interestingly, LMO4 was found to be upregulated in all the stem‐cell types analysed in both Ramalho‐Santos’ and Ivanova's studies. We also found that it was upregulated in mammospheres compared with differentiated mammary cells.
One of the genes enriched in differentiated mammary cells compared with the mammosphere‐derived cells is WISP3, also termed LIBC (lost in inflammatory breast cancer), because it was identified in a differential display study as being downregulated in inflammatory breast cancer, an extremely aggressive variety of breast cancer that has a poor prognosis. Overexpression of WISP3 in a mammary cancer cell line reverses the malignant phenotype, inducing growth arrest and differentiation (Kleer et al. 2002).
PATHWAYS INVOLVED IN HOMING AND MIGRATION
It has been recently shown that migration of stem cells during the homing process is regulated by specific chemokines and their receptors (Peled et al. 1999). For example, CXCR4 is a receptor expressed by haematopoietic stem cells that interacts with the cytokines CXCL12/SCDF, secreted by the bone‐marrow stromal cells. Engraftment experiments in SCID mice have implicated this interaction in the repopulation of the bone marrow with haematopoietic cells (Peled et al. 1999). Interestingly CXCR4, which we found to be upregulated by a factor of four in mammospheres, is overexpressed in metastatic breast cancer (Müller et al. 2001) and neuroblastoma (Geminder et al. 2001). This observation has prompted speculation about the stem‐cell origin of metastasis, in a model in which tumorigenic stem cells not only are the origin of the primary tumour, but also are responsible for metastasis resulting from tumour cell homing and growth at metastatic sites (Tu et al. 2002).
PROGENITOR CELLS AS TARGETS FOR TRANSFORMATION
In addition to stem cells, early or late progenitors could also serve as targets for transforming events (Fig. 2). If this were the case, then these cells would need to acquire mutations that enabled them to undergo self‐renewal, a stem‐cell property. It is possible that different phenotypes of mammary carcinomas such as oestrogen receptor positivity and negativity may result from transformation of different cell types. One might speculate that oestrogen receptior‐negative tumours result from transformation of the most primitive mammary stem cells, which are oestrogen receptor‐negative. It is also possible that transformation of oestrogen receptor‐negative stem cells could result in a heterogeneous tumour composed of further oestrogen receptor‐negative stem cells, as well as more differentiated oestrogen receptor‐positive cells that are produced by differentiation of these cells. In contrast, transformation of later progenitors that express oestrogen receptors may result in oestrogen receptor‐positive tumours. This model suggests that phenotypic diversity of tumours in different patients may result from transformation of different ‘tumour’ stem/progenitor cell populations. The therapeutic implications of this model are very important, as tumours arising from transformation of different stem/progenitor cells may display different phenotypes, clinical behaviours and responses to therapy. This is consistent with recently published data that associated metastatic behaviour and prognosis with specific gene‐expression profiles (van De Vijver et al. 2002; van’t Veer et al. 2002). This suggests that the characteristics of tumours are dependent on the intrinsic biology of the target cells and not solely on the mutations that occur during cancer progression. The different phenotypes displayed by genotypically distinct subsets of tumours could potentially result from the transformation of cells in various stages of differentiation. Interestingly, a number of the markers associated with bad prognosis are upregulated in mammosphere‐derived cells and in stem cells from other tissues. WISP1, TGFB3, IGFBP4, and LMO are examples of such markers (Ramalho‐Santos et al. 2002; van’t Veer et al. 2002).
MAMMARY TUMOUR STEM CELLS
The studies cited above provide evidence that pathways involved in normal stem‐cell development may also be involved in tumorigenesis. This suggests that cancers in epithelial organs, including the mammary gland, may result from deregulation of normal stem‐cell functions, such as self‐renewal, which are normally tightly regulated. If this were the case, then these tumorigenic cells would maintain many of the properties of normal stem cells. However, mutations found in these cells could interfere with normal processes of cellular differentiation. Such a model could account for phenotypic heterogeneity within individual tumours, since tumours would be composed of tumour stem cells as well as other more differentiated progeny generated through aberrant differentiation (Fig. 2). Such a stem‐cell model of tumorigenesis would have important implications for the understanding of mammary tumour biology, as well as for the development of new therapeutic approaches for breast cancer.
In order to test the hypothesis that a tumour stem‐cell population drives carcinogenesis, we developed a sensitive NOD/SCID mouse model in which small numbers of human mammary tumour cells, obtained from primary tumours or metastases, could be grown in vivo (Al‐Hajj et al. submitted). As has been described by other investigators, we found that individual tumours contained highly heterogeneous populations, as defined by cell‐surface markers. We used flow cytometry to separate individual cells based on the expression of these heterogeneously expressed markers, and identified a combination of cell‐surface markers that defined a subset of tumour cells with highly tumorigenic capacities. The tumorigenic subset, representing a minority of the total cellular population within a tumour, was defined by the same markers in the majority of tumours examined CD44+ CD4− (lineage negative) (Al‐Hajj et al. ). As few as 200 of these cells consistently formed tumours in NOD/SCID mice. In contrast, the bulk of the tumour contained cells with different cell‐surface phenotypes, which failed to form tumours even when tens of thousands of cells were injected.
In order to determine whether this experimental system merely selected for a highly tumorigenic subset of cells, we analysed the phenotype of tumours produced in NOD/SCID mice by the prospectively isolated tumorigenic cells. The results showed that the small population of tumorigenic cells was able to regenerate the entire phenotypic heterogeneity found in the initial tumour. These findings support a stem‐cell model of carcinogenesis, in which a small population of tumorigenic cells with definable phenotype is able to give rise to more tumorigenic cells, as well as the bulk tumour population, without tumorigenic properties. The tumorigenic subset of cells, like their normal counterparts, are able to undergo both self‐renewal and differentiation.
THERAPEUTIC IMPLICATIONS OF THE TUMOUR STEM‐CELL MODEL
The stem‐cell model of mammary tumorigenesis has important clinical implications for the diagnosis and treatment of breast cancer. Current therapies for breast cancer are largely derived from animal models and clinical studies in which the endpoint is tumour regression. In many cases, this occurs through the induction of apoptosis in tumour cells. There is evidence that normal stem cells are more resistant to apoptosis than more differentiated cells, due to a number of resistance mechanisms, such as higher expression of genes of the Bcl‐2 family and transporters such as BCRP and MDR (multidrug resistance) (Bunting 2002; Tiberio et al. 2002). Similarly, tumorigenic stem cells may be inherently more resistant to our current therapies than are the other, more differentiated cells that comprise the bulk of the tumour. The stem‐cell model provides an explanation for tumour recurrence following therapy. A resistant tumorigenic subset of cells may drive recurrence by regenerating the bulk of the tumour after therapy. This may explain why current therapies, while able to induce tumour regression, may not always have a dramatic effect on patient longevity. Similarly, hormone therapies, such as tamoxifen may be ineffective in those oestrogen receptor‐positive tumours that are derived from oestrogen receptor‐negative stem cells, as the more differentiated cells killed by tamoxifen would be regenerated from oestrogen receptor‐negative stem cells through differentiation.
According to this proposed model, the tumorigenic population may also be the origin of clinically relevant metastatic cells. This is consistent with the observation that metastases are composed of a heterogeneous cell population, but does not exclude the contribution of genomic instability to tumour heterogeneity and metastasis.
Prospective isolation of the tumorigenic stem‐cell populations should facilitate the identification of specific pathways important for their growth and survival, thus permitting the development of new therapeutic strategies designed to target this important population. These strategies may ultimately result in more effective interventions for the treatment of breast cancer and other solid tumours.
CONCLUSION
We have developed a new system to culture normal human mammary stem cells in an undifferentiated state. This system should facilitate the elucidation of pathways that control self‐renewal and differentiation of these cells. Furthermore, we suggest that breast tumours may be derived from transformation of normal stem or progenitor cells by deregulation of normal self‐renewal. We propose a model in which carcinogenesis is driven by a tumour stem‐cell component. This model, summarized in Fig. 2, provides one explanation for tumour cell phenotypic heterogeneity, recurrence post‐therapy, and metastasis, and has profound implications for mammary tumour biology, as well as for the development of novel therapeutic strategies for breast cancer.
ACKNOWLEDGEMENTS
We would like to thank Dr Sean Morrison for many helpful discussions. This work was supported by NIH grant CCA66233.
REFERENCES
- Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 3983 [Erratum in: Proc. Natl. Acad. Sci. USA 2003; 100: 6890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittan M, Wright NA (2002) Gastrointestinal stem cells. J. Pathol. 197, 492. [DOI] [PubMed] [Google Scholar]
- Bunting KD (2002) ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20, 11. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN (2001) Growth factors regulate the survival and fate of cells derived from human neurospheres. Nature Biotech. 19, 475. [DOI] [PubMed] [Google Scholar]
- Daniel C, Young L, Medina D, Deome K (1971) The influence of mammogenic hormones on serially transplanted mouse mammary gland. Exp. Gerontol. 6, 95. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Breen JJ, Toyama R (1998) LIM domains: multiple roles as adapters and functional modifiers in protein interaction. Trends Genet 14, 156. [DOI] [PubMed] [Google Scholar]
- Dhingra K, Sahin A, Emami K, Hortobagyi GN, Estrov Z (1998) Expression of leukemia inhibitory factor and its receptor in breast cancer: a potential autocrine and paracrine growth regulatory mechanism, Breast Cancer Res. Treat. 48, 165. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Fishell G (2002) The role of notch in promoting glial and neural stem cell fates. Ann. Rev. Neurosci. 25, 471. [DOI] [PubMed] [Google Scholar]
- Geminder H, Sagi‐Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, Ben‐Baruch A (2001) A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell‐derived factor‐1, in the development of bone marrow metastases in neuroblastoma. J. Immunol. 167, 4747. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Ou J, Easterday MC, Dougherty JD, Jackson RL, Chen Z, Antoine H, Terskikh A, Weissman IL, Nelson SF, Kornblum HI (2001) A genetic analysis of neural progenitor differentiation. Neuron. 29, 325. [DOI] [PubMed] [Google Scholar]
- Good JR, Kuspa A (2000) Evidence that a cell‐type‐specific efflux pump regulates cell differentiation in Dictyostelium . Dev. Biol. 220, 53. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture‐Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H (2001) Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo . Science 294, 2186. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Rønnov‐Jessen L, Bissell MJ, Petersen OW (2002) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Gen. Dev. 16, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW (2001) Signaling pathways in mammary gland development. Dev. Cell. 1, 467. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR (2002) A stem cell molecular signature. Science 298, 601. [DOI] [PubMed] [Google Scholar]
- Kim ND, Oberley TD, Yasukawa‐Barnes J, Clifton KH (2000) Stem cell characteristics of transplanted rat mammary clonogens. Exp. Cell Res. 260, 146. [DOI] [PubMed] [Google Scholar]
- Kleer CG, Zhang Y, Pan Q, Van Golen KL, Wu Z‐F, Livant D, Merjaver SD (2002) WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene 21, 3172. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921. [DOI] [PubMed] [Google Scholar]
- Krause DS (2002) Regulation of hematopoietic stem cell fate. Oncogene 21, 3262. [DOI] [PubMed] [Google Scholar]
- Little M, Boice J (1999) Comparison of breast cancer incidence in the Massachusetts tuberculosis fluroscopy cohort and in the Japanese atomic bomb survivors. Rad. Res. 151, 218. [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev. Biol. 228, 151. [DOI] [PubMed] [Google Scholar]
- Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ (1999) Division of labor among the α6β4 integrin β1 integrins, and an E3 laminin receptor to signal morphogenesis and β‐casein expression in mammary epithelial cells. Mol. Biol. 10, 2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben‐Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T (1999) Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845. [DOI] [PubMed] [Google Scholar]
- Petrocelli T, Slingerland JM (2001) PTEN deficiency: a role in mammary carcinogenesis. Breast Cancer Res. 3, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Gen. Dev. 14, 1837. [PubMed] [Google Scholar]
- Ramalho‐Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA (2002) ‘Stemness’: transcriptional profiling of embryonic and adult stem cells. Science 298, 597. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1996) Clonal and population analyses demonstrate that an EGF‐responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175, 1. [DOI] [PubMed] [Google Scholar]
- Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tisty TD (2001) Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 409, 633. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Barraclough R, Fernig DG, Smith JA (1997) Mammary stem cells in normal development and cancer In: Potten CS. Stem Cells vol. 147 Academic Press, San Diego, CA. [Google Scholar]
- Scheffle B, Horn MIB, Laywell E, Coomes D, Kukekov V, Steindler D (1999) Marrow‐mindedness: a perspective on neuropoiesis. Trends Neurosci. 22, 348. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj B, Gendler SJ (2002) ErbB‐beta‐catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)‐Wnt‐1 and MMTV‐c‐Neu transgenic carcinomas. J. Biol. Chem. 277, 22692. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S (2001) The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci 21, 7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ (2001) The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128, 3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH, Chepko G (2001) Mammary epithelial stem cells. Micr. Res. Techn. 52, 190. [DOI] [PubMed] [Google Scholar]
- Soriano JV, Uyttendaele H, Kitajewski J, Montesano R (2000) Expression of an activated Notch4 (int‐3) oncoprotein disrupts morphogenesis and induces an invasive phenotype in mammary epithelial cells in vitro . Int. J. Cancer 86, 652. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63, 201. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandieh I, Emerman JT (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue, Breast Cancer Res. Treat. 67, 93. [DOI] [PubMed] [Google Scholar]
- Strange R, Metcalfe T, Thackray L, Dang M (2001) Apoptosis in normal and neoplastic mammary gland development. Micr. Res. Techn. 52, 171. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Gilmore AP (1999) Adhesion‐mediated signaling in the regulation of mammary epithelial cell survival. J. Mam. Gland Biol. Neopl. 4, 183. [DOI] [PubMed] [Google Scholar]
- Sum E, Peng B, Yu CX, Byrne J, Lindeman G, Visvader J (2002) The LIM domain protein LMO4 interacts with the cofactor CtIP and the tumor suppressor BRCA1 and inhibits BRCA1 activity. J. Biol. Chem. 277, 7849. [DOI] [PubMed] [Google Scholar]
- Tiberio R, Marconi A, Fila C, Fumelli C, Pignatti M, Krajewski S, Giannetti A, Reed JC, Pincelli C (2002) Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl‐2 dependent manner. FEBS Lett. 524, 139. [DOI] [PubMed] [Google Scholar]
- Tu S‐M, Lin S‐H, Logothetis CJ (2002) Stem‐cell origin of metastasis and heterogeneity in solid tumours, Lancet Oncol. 3, 508. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL (2000) Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA 97, 14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Soriano JV, Montesano R, Kitajewski J (1998) Notch4 and Wnt‐1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashion. Dev. Biol. 196, 204. [DOI] [PubMed] [Google Scholar]
- Van De Vijver MJ, He YD, Van’t Veer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, Van Der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene‐expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999. [DOI] [PubMed] [Google Scholar]
- Van’t Veer LJ, Dai H, Vijver MJVD, He YD, Hart AAM, Mao M, Peterse HL, Kooy KVD, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530. [DOI] [PubMed] [Google Scholar]
- Visvader J, Venter D, Hahm K, Santamaria M, Sum E, O'Reilly L, White D, Williams R, Armes J, Lindeman G (2001) The LIM domain gene LMO4 inhibits differentiation of mammary epithelial cells in vitro and is overexpressed in breast cancer. Proc. Natl Acad. Sci. USA 98, 14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead CM, Craig CG, Van Der Kooy D (1996) Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 19, 387. [DOI] [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA (2002) Sca‐1 (pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev. Biol. 245, 42. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb S (2002) Stromal effects on mammary gland development and breast cancer. Science 296, 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rao Y (1999) Fringe: defining borders by regulating the notch pathway. Curr. Opin. Neurobiol. 9, 537. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro A‐M, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nature Med. 7, 1028. [DOI] [PubMed] [Google Scholar]


