Abstract
A myocardial bridge (MB) is an anatomical abnormality of the coronary artery and is characterized by the systolic narrowing of the epicardial coronary artery caused by myocardial compression during systole. An MB is frequently observed on cardiac computed tomography or coronary angiography and generally appears to be harmless in the majority of patients. However, the presence of MB is reportedly associated with abnormalities of the cardiovascular system, including coronary artery diseases, arrhythmia, certain types of cardiomyopathy, and cardiac death, indicating that MB serves a pivotal role in the occurrence and/or development of such cardiovascular events. Recently, there has been an increasing interest in the coexistence of MB and coronary spasm in research due to opposing aspects regarding their treatments. For example, monotherapy using β-blockers, which are effective in patients with MB, may worsen symptoms in patients with coronary spasm. By contrast, nitroglycerin, which is an effective treatment option for coronary spasm, may worsen symptoms in patients with MB. This review focuses on the pathophysiology and diagnosis of MB and MB-related cardiovascular diseases, including coronary spasm, and on the treatment strategies for MB.
Keywords: myocardial bridge, exertional angina, rest angina, coronary spasm, vasospastic angina
Introduction
Coronary arteries are normally distributed over the epicardial surface of the heart; however, they occasionally run a segmental intramyocardial course. In such cases, this segment of the vessel is compressed during systole, a condition referred to as milking or a systolic myocardial bridge (MB).1–6 As coronary blood flow occurs primarily during diastole, this phenomenon appears to be harmless and does not significantly influence the myocardium in the majority of patients with MB.7 However, there have been cases for which MB has been reported as the cause of ischemic heart diseases, such as exertional angina, vasospastic angina, acute myocardial infarction, and sudden cardiac death.8–16 These findings have prompted significant research interest on the association between MB and coronary artery diseases, including not only coronary atherosclerosis but also coronary spasm.17–20 The present review focuses on the pathophysiology and diagnosis of MB and MB-related cardiovascular diseases, with particular emphasis on coronary spasm and treatment strategies for MB.
Pathophysiology and Prevalence of MB
The 2 main pathological findings of MB from autopsy and intravascular ultrasound (IVUS) are the absence of atherosclerosis at the intramural and distal segments of the MB, and the presence of developing atherosclerosis at the proximal segment of the MB.21–24 MB-related biomechanical forces may account for these findings.5,25 Systolic milking of the MB disturbs blood flow and may lead to the development of atherosclerosis at the entrance of the MB.26 Systolic compression of the coronary artery by an MB itself,27 in addition to the presence of atherosclerosis, may account for myocardial ischemia during exercise. In addition, autopsy findings have revealed that the systolic compression of an MB may lead to plaque rupture at atherosclerotic lesions proximal to the MB segments28 and that this possible mechanism may account for the occurrence of MB-related acute coronary syndrome (ACS). By contrast, several studies have reported abnormal vasoreactivity at the MB segments.17,18,29 Reportedly, turbulent flow may increase endothelial cell apoptosis or tumor necrosis factor-α–induced endothelial cell activation, whereas a steady laminar flow leads to the inhibition of endothelial cell apoptosis or tumor necrosis factor-α–induced endothelial cell activation.30 These factors may cause vascular dysfunction; therefore, turbulent shear stress secondary to the milking of MB segments may contribute to MB-induced vascular dysfunction. The abnormal vasoreactivity of the coronary artery at the MB segments may cause coronary spasm.17–20 A recent study using optical coherence tomography (OCT) revealed that the adventitial vasa vasorum (VV), which serves a significant role in the morphological and functional changes of the coronary vasculature, was clearly noted at the proximal and distal segments of the MB, whereas the adventitial VV was less frequently observed within the MB segments.31 As the increase in adventitial VV was associated with coronary spasm,32 these findings may also contribute to the occurrence of MB-related coronary spasm.
The prevalence of MB depends on the modalities of evaluation and the patients in question. In addition, it is established that the use of nitroglycerin (NTG) augments the degree of MB23,33 and that this may influence the prevalence of MB. The frequency of MB varies between 5.4% and 80% of autopsy cases,34–36 between 25% and 30.2% of cases assessed using cardiac computed tomography (CT),37–39 and between 0.5% and 16.0% of cases assessed using coronary angiography.1,3–5 According to the results of a recent meta-analysis of the prevalence of MB,40 the overall prevalence was 19%; autopsy studies revealed an overall prevalence of 42%, CT studies 22%, and coronary angiography 6%. The location of MB was primarily observed in the left anterior descending artery (LAD) in 67% to 98% of cases5,39–41 although MB was also detected in the left circumflex coronary artery and right coronary artery.
MB Diagnosis
There are several modalities used in the diagnosis of MB, including noninvasive and invasive types. The noninvasive modality for diagnosing MB is cardiac CT,37–39 which is reported to be superior to CAG in terms of detection sensitivity.40 Furthermore, cardiac CT can easily assess the length and thickness of MB over the coronary artery.42 Exercise echocardiography has recently been reported as an effective noninvasive modality for assessing MB; this method cannot assess or identify the presence of MB but can suggest the presence of MB by revealing the specific characteristic of focal buckling of the septum with apical sparing during the end-systolic and early-diastolic phases.43,44 By contrast, echocardiography, particularly, more advanced echocardiography, may be useful for the noninvasive assessment of the hemodynamic significance of MB.45
The invasive modality for diagnosing MB is CAG. A significant “milking effect” is present when there is ⩾70% reduction in minimal luminal diameter during systole and persistent ⩾35% reduction in minimal luminal diameter during mid-to-late diastole.3,5 As previously described, the use of NTG augments the severity of the compression caused by an MB.23,33 Therefore, NTG should be administered immediately prior to the CAG assessment of MB if hemodynamics are stable. Furthermore, as MB is primarily present at the midterm of the LAD,40 a straight cranial view may be desirable in the assessment of MB. There are several modalities for the evaluation of MB, in addition to CAG, which include IVUS,22,23,25,46,47 OCT,48,49 Doppler flow wire,22,50 and pressure wire.5,50,51 Using IVUS, the characteristic finding is a “half-moon” sign, an echolucent area present only between the MB segment and the epicardial tissue that persists throughout the cardiac cycle.22 OCT is also a useful modality for assessing the presence of MB.48,49 A previous study reported that the MB length was longer and the degree of MB was smaller when assessed using OCT compared with that when assessed using CAG.49 Such morphological assessments using IVUS and OCT can detect and assess the presence of atherosclerosis or intimal thickening proximal to the MB.5,47 By contrast, the Doppler flow wire and pressure wire methods can assess MB both physiologically and functionally.22,50,51 Using a Doppler flow wire, a characteristic velocity pattern termed the “fingertip” phenomenon is observed at the MB segments, exhibiting an abrupt early-diastolic acceleration, rapid middiastolic deceleration, and a mid-to-late diastolic plateau. Pharmacological stress intracoronary pressure using a pressure wire is useful when assessing the presence of myocardial ischemia or a significant MB, rather than the presence of the MB itself.5,50,51 In the physiological assessment of organic stenosis, the fractional flow reserve (FFR) has been widely used in the clinical setting using a pressure wire and intravenous adenosine. This method is also useful for the assessment of MB. A patient with MB and an FFR of <0.75 is likely to have MB-related myocardial ischemia.5 However, intravenous dobutamine may be more useful in the assessment of significant MB as it can increase the strength of myocardial contraction and subsequent compression of the coronary artery at MB segments.5,51 This method is of most value when an FFR with intravenous adenosine is considered nonsignificant. However, this method involving intravenous dobutamine stress is accompanied by complications and can be time-consuming. Recent reports highlight that FFR using hand-grip stress52 or instantaneous wave-free ratio (iFR)53 are useful in assessing myocardial ischemia in a patient with MB. In terms of the latter,53 it has been reported that iFR is more consistent with patients’ symptoms than FFR. Physiological assessment using iFR is desirable in the near future.
Influences of MB on the Cardiovascular System
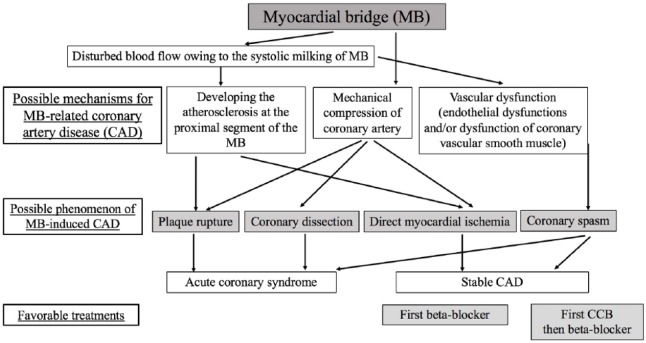
The influence of MB on the coronary vasculature includes the following effects (Figure 1): (1) myocardial ischemia due to the presence of mechanical compression of the coronary artery induced by systolic milking of MB or the coexistence of mechanical compression and atherosclerotic changes proximal to the MB segments;22 (2) development of ACS with thrombus formation, in part, due to plaque rupture induced by vascular compression at the atherosclerotic lesion proximal to the MB segments;24,28 and (3) coronary spasm (Figure 2).17–20 The presence of myocardial ischemia due to mechanical compression of an MB and atherosclerotic lesion proximal to the MB has been described in numerous studies.5,51,54,55 MB-related ACS may be due, at least in part, to plaque rupture induced by mechanical compression.24,28 However, spontaneous coronary dissection induced by MB56 or coronary spasm at the MB segments57,58 may also account for the development of ACS.59 The association between MB and coronary spasm has become a focus of increasing research interest (Table 1).17–20 Our previous study demonstrated that MB was one of the factors associated with the presence of coronary spasm.17 Kim et al18 also showed that the vasoconstrictive response was higher at the MB segments. In addition, Saito et al showed that a morphologically severe (longer) MB and a higher percentage of systolic compression were associated with MB-induced coronary spasm in the LAD.19 Nam et al also reported that the presence of MB is associated with the occurrence of coronary spasm and that patients with MB and coronary spasm have a higher rate of recurrent angina.20 Among these four studies,17–20 coronary spasm always occurred at the location of the MB, although another coronary spasm occurred at other locations and/or other vessels. Collectively, these data may provide evidence for an association between MB and coronary spasm. In addition, it has been reported that the length of MB is the important factor responsible for MB-related coronary spasm19,20 and that the presence of MB was associated with recurrent angina.20 It has previously been considered that coronary spasm occurs more frequently in Asians than in Caucasians;60 however, a recent study showed that the incidence of coronary spasm was similar between Asians and Caucasians.61–63 Furthermore, coronary spasm is the cause of not only rest angina but also exertional angina.64–68 Therefore, worldwide, increased attention on MB-related coronary spasm is required in patients with MB experiencing chest symptoms at rest and during exertion, as the treatments for MB and MB-related coronary spasm are different.
Figure 1.
Possible mechanisms of the development of the myocardial bridge responsible for coronary artery disease, and favorable treatments based on the causes.
CAD indicates coronary artery disease; CCB, calcium channel blocker.
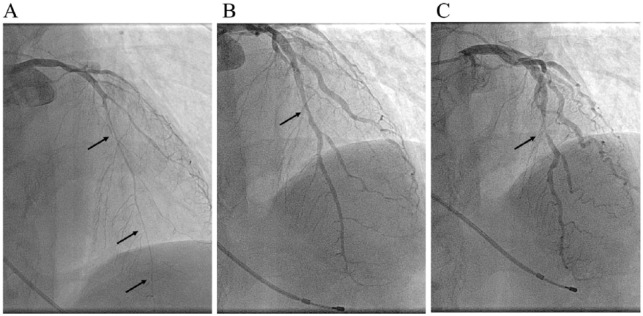
Figure 2.
Coronary angiograms in a representative patient with myocardial bridge (MB) and coronary spasm. The patient experienced chest symptoms during exercise in the early morning only. Coronary angiography revealed a severe vasospasm provoked by intracoronary infusions of acetylcholine (indicated by arrows) (A) and the presence of MB at spastic segment (B: diastole, C: systole), which is indicated by arrows.
MB indicates myocardial bridge.
Table 1.
Studies showing the relationship between MB and coronary spasm.
| No. | Authors | Reference No. | Year | Main findings |
| 1 | Teragawa et al | 16 | 2003 | In 114 patients who had any chest symptoms, MB was one of predictors of presence of coronary spasm, on logistic regression analysis. In addition, the MB segment in response to acetylcholine was more constrictive, even in patients without coronary spasm. |
| 2 | Kim et al | 17 | 2008 | In 128 patients with typical angiographic MB, coronary vasoconstriction (>50%) to acetylcholine was observed more often than control patients (89.1% vs 35.1%), despite the absence of plaque at the MB segments on intravascular ultrasonographic study. |
| 3 | Saito et al | 18 | 2017 | In 392 patients who underwent spasm provocation test, LAD spasm was provoked more frequently in patients with MB. Multivariate regression analysis demonstrated that the length of MB and percent systolic compression of MB were significant predictors for provoked LAD spasm. |
| 4 | Nam et al | 19 | 2018 | In 812 patients with MB, coronary spasm was provoked in 59.1% of patients. The length of MB, percent systolic compression of MB, and reference diameter were associated with coronary spasm. Patients with MB and coronary spasm experienced recurrent angina more frequently during the 5-year follow-up. |
Abbreviations: LAD, left anterior descending coronary artery; MB, myocardial bridge.
MB is reportedly associated with several types of cardiomyopathy69–73 or arrhythmia.74–76 MB-induced focal myocardial ischemia may account for the prognosis of hypertrophic cardiomyopathy69 and the development and prognosis of takotsubo cardiomyopathy.71–73 In terms of the association between MB and arrhythmia, exercise-induced QT dispersion has been shown to be increased in patients with MB, which suggests that MB-induced focal myocardial ischemia leads to arrhythmia.74–76
Treatment for Symptomatic MB
Pharmacological treatment is recommended as first-line therapy for patients with symptomatic MB. Nonpharmacological invasive treatments for symptomatic MB include percutaneous coronary intervention (PCI),5,77–79 coronary artery bypass grafting (CABG), and/or surgical unroofing.5,80–83 However, the results of these nonpharmacological treatments, with the exception of surgical unroofing,82,83 have not been satisfactory.5,77–79,81 In terms of surgical unroofing, which is either performed with or without CABG, the procedure may be promising in symptomatic patients with MB who are refractory to medical therapies,82,83 and further information on surgical unroofing is required. When administering these medications, attention to patient symptoms at rest and during exercise is required. The cause of symptoms during exercise is considered to be the mechanical compression of the coronary artery of MB, with the administration of β-blockers recommended as first-line therapy. However, the administration of β-blockers alone may worsen chest symptoms in patients with symptomatic MB and coronary spasm. Prior to the administration of β-blockers, it is important that the possibility of worsening chest symptoms be explained to patients (Figure 1).14,68 Therefore, the administration of pharmacological treatments requires strict control. If patients under optimal medical therapy experience life-restricting symptoms, then the consideration of nonpharmacological treatments, including PCI, CABG, and surgical unroofing, is required.
Conclusions
MB is an anatomical abnormality of the coronary artery, which runs on a segmental intramyocardial course. This abnormality is frequently observed, and the majority of patients with MB may be asymptomatic. However, this abnormality undoubtedly causes myocardial ischemia owing to the coexistence of mechanical compression of the MB and atherosclerotic changes proximal to the MB, which is likely to be the cause of coronary spasm in certain patients. Caution is required when taking both pathologies into account in determining the optimal medical therapy for symptomatic MB.
Footnotes
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: HT, CO, TU equally contributed to the conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
ORCID iD: Hiroki Teragawa  https://orcid.org/0000-0002-0183-2541
https://orcid.org/0000-0002-0183-2541
References
- 1. Mohlenkamp S, Hort W, Ge J, et al. Update on myocardial bridging. Circulation. 2002;106:2616–2622. [DOI] [PubMed] [Google Scholar]
- 2. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. [DOI] [PubMed] [Google Scholar]
- 3. Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–359. [DOI] [PubMed] [Google Scholar]
- 4. Alegria JR, Herrmann J, Holmes DR, Jr, et al. Myocardial bridging. Eur Heart J. 2005;26:1159–1168. [DOI] [PubMed] [Google Scholar]
- 5. Corban MT, Hung OY, Eshtehardi P, et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee MS, Chen CH. Myocardial bridging: a up-to-date review. J Invasive Cardiol. 2015;27:521–528. [PMC free article] [PubMed] [Google Scholar]
- 7. Hayashi T, Ishikawa K. Myocardial bridge: harmless or harmful. Intern Med. 2004;43:1097–1098. [DOI] [PubMed] [Google Scholar]
- 8. Morales AR, Romanelli R, Boucek RJ. The mural left anterior descending coronary artery, strenuous exercise and sudden death. Circulation. 1980;62:230–237. [DOI] [PubMed] [Google Scholar]
- 9. Munakata K, Sato N, Sasaki Y, et al. Two cases of variant form angina pectoris associated with myocardial bridge—a possible relationship among coronary vasospasm, atherosclerosis and myocardial bridge. Jpn Circ J. 1992;56:1248–1252. [DOI] [PubMed] [Google Scholar]
- 10. Ciampricotti R, el Gamal M. Vasospastic coronary occlusion associated with a myocardial bridge. Cathet Cardiovasc Diagn. 1988;14:118–120. [DOI] [PubMed] [Google Scholar]
- 11. Kodama K, Morioka N, Hara Y, Shigematsu Y, Hamada M, Hiwada K. Coronary vasospasm at the site of myocardial bridge—report of two cases. Angiology. 1998;49:659–663. [DOI] [PubMed] [Google Scholar]
- 12. Kurisu S, Inoue I, Kawagoe T, et al. Acute myocardial infarction associated with myocardial bridging in a young adult. Intern Med. 2004;43:1157–1161. [DOI] [PubMed] [Google Scholar]
- 13. Hong L, Liu J, Luo S, Li J. Relation of myocardial bridge to myocardial infarction: a meta-analysis. Chin Med J (Engl). 2014;127:945–950. [PubMed] [Google Scholar]
- 14. Teragawa H, Fujii Y, Ueda T, Murata D, Nomura S. Case of angina pectoris at rest and during effort due to coronary spasm and myocardial bridging. World J Cardiol. 2015;7:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerit L, Duygu H. Myocardial bridging and sudden death. Int J Cardiol. 2017;229:11. [DOI] [PubMed] [Google Scholar]
- 16. Hostiuc S, Rusu MC, Hostiuc M, Negoi RI, Negoi I. Cardiovascular consequences of myocardial bridging: a meta-analysis and meta-regression. Sci Rep. 2017;7:14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teragawa H, Fukuda Y, Matsuda K, et al. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol. 2003;26:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JW, Seo HS, Na JO, et al. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart. 2008;94:765–769. [DOI] [PubMed] [Google Scholar]
- 19. Saito Y, Kitahara H, Shoji T, et al. Relation between severity of myocardial bridge and vasospasm. Int J Cardiol. 2017;248:34–38. [DOI] [PubMed] [Google Scholar]
- 20. Nam P, Choi BG, Choi SY, et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. 2018;270:8–12. [DOI] [PubMed] [Google Scholar]
- 21. Ishii T, Asuwa N, Masuda S, Ishikawa Y, Kiguchi H, Shimada K. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: an ultrastructural study. Mod Pathol. 1991;4:424–431. [PubMed] [Google Scholar]
- 22. Ge J, Jeremias A, Rupp A, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. [DOI] [PubMed] [Google Scholar]
- 23. Hongo Y, Tada H, Ito K, Yasumura Y, Miyatake K, Yamagishi M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: study by quantitative coronary angiography and intravascular ultrasound. Am Heart J. 1999;138:345–350. [DOI] [PubMed] [Google Scholar]
- 24. Ishikawa Y, Akasaka Y, Suzuki K, et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–383. [DOI] [PubMed] [Google Scholar]
- 25. Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995;73:462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. [DOI] [PubMed] [Google Scholar]
- 27. Rouleau JR, Roy L, Dumesnil JG, Dagenais GR. Coronary vasodilator reserve impairment distal to systolic coronary artery compression in dogs. Cardiovasc Res. 1983;17:96–105. [DOI] [PubMed] [Google Scholar]
- 28. Ishikawa Y, Akasaka Y, Akishima-Fukasawa Y, et al. Histopathologic profiles of coronary atherosclerosis by myocardial bridge underlying myocardial infarction. Atherosclerosis. 2013;226:118–123. [DOI] [PubMed] [Google Scholar]
- 29. Shiode N, Kato M, Teragawa H, et al. Vasomotility and nitric oxide bioactivity of the bridging segments of the left anterior descending coronary artery. Am J Cardiol. 1998;81:341–343. [DOI] [PubMed] [Google Scholar]
- 30. Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001;947:93–109, discussion 109–111. [DOI] [PubMed] [Google Scholar]
- 31. Nishimiya K, Matsumoto Y, Wang H, et al. Absence of adventitial vasa vasorum formation at the coronary segment with myocardial bridge—An optical coherence tomography study. Int J Cardiol. 2018;250:275–277. [DOI] [PubMed] [Google Scholar]
- 32. Ohyama K, Matsumoto Y, Takanami K, et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71:414–425. [DOI] [PubMed] [Google Scholar]
- 33. Qian JY, Zhang F, Dong M, et al. Prevalence and characteristics of myocardial bridging in coronary angiogram—data from consecutive 5525 patients. Chin Med J (Engl). 2009;122:632–635. [PubMed] [Google Scholar]
- 34. Burnsides C, Edwards JC, Lansing AI, Swarm RL. Arteriosclerosis in the intramural and extramural portions of coronary arteries in the human heart. Circulation. 1956;13:235–241. [DOI] [PubMed] [Google Scholar]
- 35. Polacek P, Kralove H. Relation of myocardial bridges and loops on the coronary arteries to coronary occlusions. Am Heart J. 1961;61:44–52. [DOI] [PubMed] [Google Scholar]
- 36. Angelini P, Trivellato M, Donis J, et al. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26:75–88. [DOI] [PubMed] [Google Scholar]
- 37. La Grutta L, Runza G, Lo Re G, et al. Prevalence of myocardial bridging and correlation with coronary atherosclerosis studied with 64-slice CT coronary angiography. Radiol Med. 2009;114:1024–1036. [DOI] [PubMed] [Google Scholar]
- 38. Jeong YH, Kang MK, Park SR, et al. A head-to-head comparison between 64-slice multidetector computed tomographic and conventional coronary angiographies in measurement of myocardial bridge. Int J Cardiol. 2010;143:243–248. [DOI] [PubMed] [Google Scholar]
- 39. Liu G, Qu Y, Chen X, et al. Measurements of myocardial bridges on computed tomography predict presence of clinical symptoms and outcomes of adverse heart events: a retrospective study in a large population from China. Acta Radiol. 2017;58:1068–1076. [DOI] [PubMed] [Google Scholar]
- 40. Hostiuc S, Negoi I, Rusu MC, Hostiuc M. Myocardial bridging: a meta-analysis of prevalence. J Forensic Sci. 2018;63:1176–1185. [DOI] [PubMed] [Google Scholar]
- 41. Loukas M, Curry B, Bowers M, et al. The relationship of myocardial bridges to coronary artery dominance in the adult human heart. J Anat. 2006;209:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forsdahl SH, Rogers IS, Schnittger I, et al. Myocardial bridges on coronary computed tomography angiography- correlation with intravascular ultrasound and fractional flow reserve. Circ J. 2017;81:1894–1900. [DOI] [PubMed] [Google Scholar]
- 43. Lin S, Tremmel JA, Yamada R, et al. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Siciliano M, Migliore F, Piovesana P. Stress echocardiography pattern: a promising noninvasive test for detection of myocardial bridging with haemodynamic relevance. J Cardiovasc Med (Hagerstown). 2016;17:e208–e209. [DOI] [PubMed] [Google Scholar]
- 45. Kobayashi Y, Tremmel JA, Kobayashi Y, et al. Exercise strain echocardiography in patients with a hemodynamically significant myocardial bridge assessed by physiological study. J Am Heart Assoc. 2015;4:002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ge J, Erbel R, Rupprecht HJ, et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–1732. [DOI] [PubMed] [Google Scholar]
- 47. Yamada R, Tremmel JA, Tanaka S, et al. Functional versus anatomic assessment of myocardial bridging by intravascular ultrasound: impact of arterial compression on proximal atherosclerotic plaque. J Am Heart Assoc. 2016;5:e001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bose D, Philipp S. Images in clinical medicine. High-resolution imaging of myocardial bridging. N Engl J Med. 2008;358:392. [DOI] [PubMed] [Google Scholar]
- 49. Cao HM, Jiang JF, Deng B, Xu JH, Xu WJ. Evaluation of myocardial bridges with optical coherence tomography. J Int Med Res. 2010;38:681–685. [DOI] [PubMed] [Google Scholar]
- 50. Gould KL, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging. 2015;8:705–709. [DOI] [PubMed] [Google Scholar]
- 51. Escaned J, Cortes J, Flores A, et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–233. [DOI] [PubMed] [Google Scholar]
- 52. Ryan N, Escaned J. Myocardial bridge as a cause of persistent post percutaneous coronary intervention angina identified with exercise intracoronary physiology. Eur Heart J. 2017;38:1001. [DOI] [PubMed] [Google Scholar]
- 53. Tarantini G, Barioli A, Nai Fovino L, et al. Unmasking myocardial bridge-related ischemia by intracoronary functional evaluation. Circ Cardiovasc Interv. 2018;11:e006247. [DOI] [PubMed] [Google Scholar]
- 54. Mouratidis B, Lomas FE, McGill D. Thallium-201 myocardial SPECT in myocardial bridging. J Nucl Med. 1995;36:1031–1033. [PubMed] [Google Scholar]
- 55. Lee YS, Moon DH, Shin JW, Park SW, Park SJ, Lee HK. Dipyridamole TI-201 SPECT imaging in patients with myocardial bridging. Clin Nucl Med. 1999;24:759–764. [DOI] [PubMed] [Google Scholar]
- 56. Ge JB, Huang ZY, Liu XB, Qian JY. Spontaneous coronary dissection associated with myocardial bridge causing acute myocardial infarction. Chin Med J (Engl). 2008;121:2450–2453. [PubMed] [Google Scholar]
- 57. Sakuma M, Kamishirado H, Inoue T, et al. Acute myocardial infarction associated with myocardial bridge and coronary artery vasospasm. Int J Clin Pract. 2002;56:721–722. [PubMed] [Google Scholar]
- 58. Fujibayashi D, Morino Y, Ikari Y. A case of acute myocardial infarction due to coronary spasm in the myocardial bridge. J Invasive Cardiol. 2008;20:E217–E219. [PubMed] [Google Scholar]
- 59. Sheikh AR, Sidharta S, Worthley MI, Yeend R, Di Fiore DP, Beltrame JF. The importance of evaluating patients with MINOCA (myocardial infarction with non-obstructive coronary arteries). Int J Cardiol. 2015;199:386–388. [DOI] [PubMed] [Google Scholar]
- 60. Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–1452. [DOI] [PubMed] [Google Scholar]
- 61. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 62. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 63. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 64. Waters DD, Szlachcic J, Bourassa MG, Scholl JM, Theroux P. Exercise testing in patients with variant angina: results, correlation with clinical and angiographic features and prognostic significance. Circulation. 1982;65:265–274. [DOI] [PubMed] [Google Scholar]
- 65. Sakata K, Hoshino T, Yoshida H, Shugino H, Miura F, Takada A. Characteristics of vasospastic angina with exercised-induced ischemia—analysis of parameters of hemostasis and fibrinolysis. Jpn Circ J. 1996;60:277–284. [DOI] [PubMed] [Google Scholar]
- 66. Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760–765. [DOI] [PubMed] [Google Scholar]
- 67. Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm—clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. [DOI] [PubMed] [Google Scholar]
- 68. Teragawa H, Fujii Y, Oshita C, et al. What factors contribute to chest symptoms during exercise in patients with vasospastic angina? Angiol. 2017;5:202. [Google Scholar]
- 69. Yetman AT, Hamilton RM, Benson LN, McCrindle BW. Long-term outcome and prognostic determinants in children with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1998;32:1943–1950. [DOI] [PubMed] [Google Scholar]
- 70. Mohiddin SA, Begley D, Shih J, et al. Myocardial bridging does not predict sudden death in children with hypertrophic cardiomyopathy but is associated with more severe cardiac disease. J Am Coll Cardiol. 2000;36:2270–2278. [DOI] [PubMed] [Google Scholar]
- 71. Boktor M, Mansi IA, Troxclair S, Modi K. Association of myocardial bridge and Takotsubo cardiomyopathy: a case report and literature review. South Med J. 2009;102:957–960. [DOI] [PubMed] [Google Scholar]
- 72. Ando G, Trio O, de Gregorio C. Coronary spasm and myocardial bridging: an elusive pathophysiological mechanism leading to apical ballooning syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:501–504. [DOI] [PubMed] [Google Scholar]
- 73. Kato K, Kitahara H, Saito Y, et al. Impact of myocardial bridging on in-hospital outcome in patients with takotsubo syndrome. J Cardiol. 2017;70:615–619. [DOI] [PubMed] [Google Scholar]
- 74. Barutcu I, Sezgin AT, Gullu H, Topal E, Acikgoz N, Ozdemir R. Exercise-induced changes in QT interval duration and dispersion in patients with isolated myocardial bridging. Int J Cardiol. 2004;94:177–180. [DOI] [PubMed] [Google Scholar]
- 75. Aksan G, Nar G, Inci S, et al. Exercise-induced repolarization changes in patients with isolated myocardial bridging. Med Sci Monit. 2015;21:2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nishikii-Tachibana M, Pargaonkar VS, Schnittger I, et al. Myocardial bridging is associated with exercise-induced ventricular arrhythmia and increases in QT dispersion. Ann Noninvasive Electrocardiol. 2018;23:e12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ernst A, Bulum J, Separovic Hanzevacki J, Lovric Bencic M, Strozzi M. Five-year angiographic and clinical follow-up of patients with drug-eluting stent implantation for symptomatic myocardial bridging in absence of coronary atherosclerotic disease. J Invasive Cardiol. 2013;25:586–592. [PubMed] [Google Scholar]
- 78. Tandar A, Whisenant BK, Michaels AD. Stent fracture following stenting of a myocardial bridge: report of two cases. Catheter Cardiovasc Interv. 2008;71:191–196. [DOI] [PubMed] [Google Scholar]
- 79. Tsujita K, Maehara A, Mintz GS, et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol. 2009;103:1344–1348. [DOI] [PubMed] [Google Scholar]
- 80. Sun X, Chen H, Xia L, Zhao D, Ding W, Wang C. Coronary artery bypass grafting for myocardial bridges of the left anterior descending artery. J Card Surg. 2012;27:405–407. [DOI] [PubMed] [Google Scholar]
- 81. Bockeria LA, Sukhanov SG, Orekhova EN, Shatakhyan MP, Korotayev DA, Sternik L. Results of coronary artery bypass grafting in myocardial bridging of left anterior descending artery. J Card Surg. 2013;28:218–221. [DOI] [PubMed] [Google Scholar]
- 82. Boyd JH, Pargaonkar VS, Scoville DH, et al. Surgical Unroofing of Hemodynamically Significant Left Anterior Descending Myocardial Bridges. Ann Thorac Surg. 2017;103:1443–1450. [DOI] [PubMed] [Google Scholar]
- 83. Maeda K, Schnittger I, Murphy DJ, et al. Surgical unroofing of hemodynamically significant myocardial bridges in a pediatric population. J Thorac Cardiovasc Surg. 2018;156:1618–1626. [DOI] [PubMed] [Google Scholar]