Abstract
Over the past few decades, evidence has come to light that there is a rapid subcortical shortcut that transmits visual information to the amygdala, effectively bypassing the visual cortex. This pathway purportedly runs from the superior colliculus to the amygdala via the pulvinar, and thus presents a methodological challenge to study noninvasively in the human brain. Here, we present our recent work where we reliably reconstructed the white matter structure and directional flow of neural signal along this pathway in over 600 healthy young adults. Critically, we found structure-function relationships for the pulvinar-amygdala connection, where people with greater fibre density had stronger functional neural coupling and were also better at recognising fearful facial expressions. These results tie together recent anatomical evidence from other visual primates with very recent optogenetic research on rodents demonstrating a functional role of this pathway in producing fear responses. Here, we discuss how this pathway might operate alongside other thalamo-cortical circuits (such as pulvinar to middle temporal area) and how its structure and function may change according to the sensory input it receives. This newly established circuit might play a potentially important role in autism and/or anxiety disorders.
Keywords: Amygdala, pulvinar, superior colliculus, fear, connectivity
COMMENT ON: McFadyen J, Mattingley JB and Garrido MI. An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. eLife. 2019;8:e40766. PubMed PMID: 30648533; PubMed Central PMCID: PMC6335057 https://www.ncbi.nlm.nih.gov/pubmed/30648533
It is crucial to our survival that we can rapidly predict, detect, and respond to potential threats in our environment. As highly visual creatures, we have developed sophisticated neural networks for processing visual information about the world around us. Both the visual system and the amygdala are arguably the most thoroughly studied components of the brain and yet there is considerable controversy over how these two systems interact to rapidly respond to signs of threat. Specifically, there has been extensive debate over whether the amygdala receives visual input from a short, fast, subcortical pathway from the superior colliculus to the pulvinar.1 It might seem strange that the existence of this so-called ‘subcortical route to the amygdala’ could be so elusive, so much that it divides the neuroscientific community. In fact, the human subcortex is extremely difficult to study due to the problems associated with measuring very deep but also very fast neural activity, posing problems to both fMRI and M/EEG methods. Because of this (and the complete absence of postmortem human studies on this pathway), evidence for the subcortical route to the amygdala has had to be pieced together from an array of different studies using different approaches, each prone to its own set of methodological and interpretative limitations. Thus, there has been a great deal of discussion from opposing viewpoints (see review by Tamietto and de Gelder2 but also the review by Pessoa and Adolphs1).
In recent years, there have been significant advances in human neuroimaging methods that have brought us closer to resolving whether this pathway exists and, if so, what it might be used for. First, the computational methods applied to diffusion magnetic resonance imaging (MRI) images have significantly advanced in how accurately they can reconstruct the underlying white matter structure.3 Second, there now exist multiple consortiums around the world that collect high-quality human neuroimaging data from thousands of people, obtaining structural images as well as functional measures from an array of cognitive tasks.4 In our study,5 we exploited these two recent advancements and combined them with a long-standing network modelling method (i.e. dynamic causal modelling)6 to robustly investigate the evidence for a subcortical route to the amygdala in the human brain.
The results from our study provided strong evidence for the presence of an afferent subcortical pathway to the amygdala. Using data from the Human Connectome Project, we successfully reconstructed this pathway in over 600 young healthy adults using sophisticated local and global tractography methods. We also observed strong evidence that neural activity (as indicated by BOLD signal) flowed in a forward direction along this pathway (i.e. from the superior colliculus to the amygdala via the pulvinar; see Figure 1) while participants viewed angry and fearful faces. Taken together, these two findings provide convergent evidence for the presence of an afferent subcortical route to the amygdala in the human brain recruited for face processing.
Figure 1.
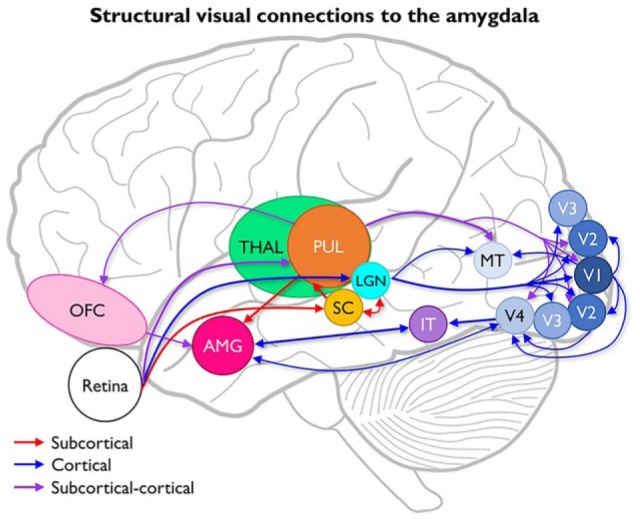
Anatomical diagram of subcortical and cortical visual afferents to the amygdala.
Subcortical-only pathways are indicated by red arrows, cortical visual pathways are indicated by blue arrows, and alternative subcortical-cortical pathways are indicated by purple arrows.
Abbreviations: AMG, amygdala; IT, inferior temporal cortex; LGN, lateral geniculate nucleus; MT, middle temporal area (V5); OFC, orbital frontal cortex; SC, superior colliculus; THAL, thalamus; V1-V4, visual areas 1 to 4.
We then investigated whether people with greater fibre density along this pathway might also have stronger forward-flowing neural connectivity during face viewing or perhaps be better at recognising different facial expressions. We discovered that fibre density along only the second half of the subcortical route significantly covaried with effective connectivity (i.e. people with greater fibre density also had stronger pulvinar-amygdala functional coupling) as well as better recognition of fearful (but not angry or sad) expressions. These significant intermodal relationships support the validity of the tractography results and also demonstrate the involvement of the pulvinar-amygdala connection in fearful face processing.
The results from our study resolve several key issues in the debate over the existence and function of the subcortical route to the amygdala. They also, however, raise new questions about how the subcortical route to the amygdala might contribute to individual differences in affective processing, as well as affective processing disorders like anxiety and autism. Let us frame this finding in the context of previous animal research and then postulate how these results might change our understanding of how emotion processing engages with hierarchical visual systems.
Comparing Evidence for the Subcortical Route to the Amygdala Across Species
The amygdala is well suited for comparison across species, as its evolution has been well-conserved, and it is essential for producing fear responses, which are critical to the survival of any animal.7 Hence, we might expect subcortical pathways to the amygdala to also be conserved across species. Indeed, a subcortical pathway was first identified from the inferior colliculus to the amygdala via the medial geniculate nucleus in the rodent brain for transmitting auditory signals,8 particularly those in the frequency range of warning calls from other rodents.9 While this rapid subcortical auditory pathway is also present in the human brain,10 research has focused almost exclusively on whether an analogous pathway exists in humans for vision. The primary reason for this was the discovery of blindsight (discussed in more detail below) but also because, unlike rodents, we are highly visual creatures. The majority (55%) of the human cortex is dedicated to visual processing while rodents have relatively poor vision.11 As such, their cortical and subcortical areas for visual processing less clearly resemble those of a human brain. Despite this, a number of recent studies have used tracing and optogenetics to uncover a visual subcortical pathway to the amygdala that transmits threat signals (for mice, this is looming stimuli) and directly triggers fearful behaviour.12,13 Pathways have been identified from the mouse superior colliculus (which is architecturally similar but functionally dissimilar to the primate brain), to the lateral posterior nucleus of the thalamus (considered analogous to the human pulvinar12,14,15 or the parabigeminal nucleus13 and on towards the amygdala). These studies provide compelling evidence for a subcortical visual path to the amygdala, even in a species whose primary sensory modality is touch via its whiskers. It is, however, essential to consider the dramatic evolutionary reorganisation seen in the primate brain to accommodate vision, as this may render the subcortical route absent or perhaps even redundant (our results, of course, suggest the opposite).
There is dramatic neuroplastic reorganisation in the early developmental years of the primate brain. Tectopulvinar pathways actually develop earlier than geniculostriate pathways but are pruned as we get older, leaving the geniculostriate pathways as the dominant transmission of visual information.16 Hence, the structure or functional relevance of the subcortical route to the amygdala is highly likely to be susceptible to change. Nonetheless, there is evidence that this pathway is intact in adult primates. Most recently, one study successfully traced axonal projections in the macaque brain. They discovered axons connecting the superior colliculus to the pulvinar, the terminals of which overlapped with axons connecting the pulvinar to the amygdala.17 Previous work has also identified these axonal projections in the tree shrew.18 Unlike the optogenetic innervation seen in the aforementioned rodent research, these neuroanatomical investigations into the primate brain do not relate the presence of the subcortical route to a particular functional role. This is where studies on humans have made the biggest impact, originating with the discovery of affective blindsight.
The Malleable Functional Role of the Pulvinar-Amygdala Connection
Blindsight describes the remarkable ability of people with a destroyed primary visual cortex (V1) to respond to visual stimuli that they cannot consciously perceive.19 When presented with affective stimuli, these cortically blind patients show significantly greater signal in the superior colliculus, pulvinar, and amygdala than healthy controls.20 This suggests that visual information, particularly fearful faces, is re-routed away from the damaged visual cortex and transmitted along the alternative, shorter subcortical pathway through the superior colliculus and pulvinar instead. One case study on a cortically blind patient has even shown that there is greater fibre density (as measured by fractional anisotropy) along the subcortical route in the damaged hemisphere than the intact hemisphere or the brains of healthy controls.21 This suggests that neural plasticity may have altered the strength of the subcortical route as a result of the patient’s reliance on unconscious visual processing.
An extraordinary case of a 7-year-old boy demonstrates the functional power of neuroplasticity for vision. At 2 weeks old, the boy sustained severe damage to both occipital lobes due to a disorder of fatty acid oxidation. Despite this, as well as significant damage to the eyes themselves (his left eye was completely blind), he reports near-normal conscious vision and can play video games, watch movies, and play soccer. He also performs perfectly on orientation, shape, colour, and face discrimination tasks. Instead of finding changes to the affective subcortical route to the amygdala, diffusion tractography instead revealed a significant increase in structural connections from the lateral geniculate nucleus and pulvinar to V5/MT.22 This thalamo-cortical circuit has been implicated in blindsight before, as well as in the healthy brain.2 It presents as a strong contender to the colliculo-pulvinar path to the amygdala we investigated in our study (see Figure 1). Crucially, we observed anatomical evidence for the full colliculo-pulvinar pathway but only found the latter half from the pulvinar to the amygdala to be functionally relevant. This supports the notion that perhaps there is significant cortical input to the pulvinar’s transmission to the amygdala, making it not quite so ‘subcortical’ after all. Future research is needed to directly compare the contributions of each circuit to producing fear responses in the amygdala. Importantly, this illustrates that visual affective processing is anything but serial. There are many different types of subcortical, cortical, and subcortical-cortical networks all operating in parallel; the specific combination might depend on particular tasks or perhaps is modulated by clinical disorders.
Regardless of whether the pulvinar receives input subcortically or cortically before communicating with the amygdala, the pulvinar-amygdala connection may play an important role in rapid relevance detection. We have previously shown this connection to innervate amygdala activity very quickly (~70 ms poststimulus onset).23 Other research has demonstrated the role of the pulvinar in relaying visual and higher-order cortical signals to orient attention, facilitate visual search, and recognise emotions.16 Hence, the pulvinar may gate or amplify information flow to the amygdala and cortex according to an assessment of relevance. Interestingly, people with high trait anxiety show heightened pulvinar-amygdala connectivity,24 while people with autism show reduced pulvinar-cortical connectivity,25 suggesting that modulation of this connection may play an important role in psychopathology.
Both autism and anxiety, which can be comorbid, can be characterised by atypical fear responses. People with autism tend to have impaired fear conditioning,26 atypical amygdala responses to stimuli like fearful faces,27 as well as unusual fears where, for example, a child with autism may be afraid of umbrellas but not afraid of deep bodies of water.28 Studies on autism have found that there is weaker structural connectivity along the subcortical route in humans and in an animal model of autism, coinciding with a reduction in typical fear responses.14 In another study, however, people with autism showed greater BOLD signal in the superior colliculus, pulvinar, and amygdala when fixating on the eyes of fearful faces.29 Taken together, these findings suggest that the subcortical route may be underused, due to reduced structural connectivity, but then also overused for processing certain visual input like fearful faces. Further research is needed to establish precisely how the subcortical route to the amygdala might be modulated in people higher on the autistic spectrum. Indeed, individual differences in anxiety may play a role in these autism-related findings and may be an interesting avenue to explore. There is already neuropharmacological research using dynamic causal modelling finding that the subcortical route to the amygdala significantly contributes to abnormal fear responses in phobia disorder.30 Therefore, this points towards a promising avenue of new research into how rapid affective processing subserved by the subcortical route to the amygdala might contribute to disordered fear responses.
Acknowledgments
The author would like to thank her PhD supervisors, Marta Garrido, and Jason Mattingley, for their work and support on this project. She also thanks the ARC Centre for Integrative Brain Function for funding this project and encouraging integrative neuroscience.
Footnotes
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by an Australian Postgraduate Award and the Australian Research Council Centre for Integrative Brain Function.
ORCID iD: Jessica McFadyen  https://orcid.org/0000-0003-1415-2286
https://orcid.org/0000-0003-1415-2286
References
- 1. Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamietto M, De Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2010;11:697–709. [DOI] [PubMed] [Google Scholar]
- 3. Tournier J-D. Diffusion MRI in the brain – theory and concepts. Prog Nucl Magn Reson Spectrosc. 2019;112–113:1–16. [DOI] [PubMed] [Google Scholar]
- 4. Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McFadyen J, Mattingley JB, Garrido MI. An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. eLife. 2019;8:e40766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. [DOI] [PubMed] [Google Scholar]
- 7. Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. Exp Brain Res. 1994;98:261–274. [DOI] [PubMed] [Google Scholar]
- 10. Garrido MI, Barnes GR, Sahani M, Dolan RJ. Functional evidence for a dual route to amygdala. Curr Biol. 2012;22:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex (New York, NY). 1991;1:1–47. [DOI] [PubMed] [Google Scholar]
- 12. Wei P, Liu N, Zhang Z, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6:6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shang C, Liu Z, Chen Z, et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–1477. [DOI] [PubMed] [Google Scholar]
- 14. Hu Y, Chen Z, Huang L, et al. A translational study on looming-evoked defensive response and the underlying subcortical pathway in autism. Sci Rep. 2017;7:14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou N, Masterson SP, Damron JK, Guido W, Bickford ME. The mouse pulvinar nucleus links the lateral extrastriate cortex, striatum, and amygdala. J Neurosci. 2018;38:347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bridge H, Leopold DA, Bourne JA. Adaptive pulvinar circuitry supports visual cognition. Trends Cogn Sci. 2016;20:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malkova L, Elorette C, Forcelli PA, Saunders RC. Co-localization of tectal inputs with amygdala-projecting neurons in the macaque pulvinar. Front Neural Circuits. 2018;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Day-Brown JD, Wei H, Chomsung RD, Petry HM, Bickford ME. Pulvinar projections to the striatum and amygdala in the tree shrew. Front Neuroanat. 2010;4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiskrantz L, Warrington EK, Sanders M, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. [DOI] [PubMed] [Google Scholar]
- 20. Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating ‘unseen’ fear. Proc Natl Acad Sci U S A. 1999;96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamietto M, Pullens P, de Gelder B, Weiskrantz L, Goebel R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Curr Biol. 2012;22:1449–1455. [DOI] [PubMed] [Google Scholar]
- 22. Mundinano I-C, Chen J, de Souza M, et al. More than blindsight: case report of a child with extraordinary visual capacity following perinatal bilateral occipital lobe injury [published online ahead of print November 13, 2017]. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 23. McFadyen J, Mermillod M, Mattingley JB, Halász V, Garrido MI. A rapid subcortical amygdala route for faces irrespective of spatial frequency and emotion. J Neurosci. 2017;37:3864–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hakamata Y, Sato E, Komi S, et al. The functional activity and effective connectivity of pulvinar are modulated by individual differences in threat-related attentional bias. Sci Rep. 2016;6:34777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green SA, Hernandez L, Bookheimer SY, Dapretto M. Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res. 2017;10:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. South M, Larson MJ, White SE, Dana J, Crowley MJ. Better fear conditioning is associated with reduced symptom severity in autism spectrum disorders. Autism Res. 2011;4:412–421. [DOI] [PubMed] [Google Scholar]
- 27. Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Dev Sci. 2004;7:340–359. [DOI] [PubMed] [Google Scholar]
- 28. Mayes SD, Calhoun SL, Aggarwal R, et al. Unusual fears in children with autism. Res Aut Spect Disord. 2013;7:151–158. [Google Scholar]
- 29. Hadjikhani N, Åsberg Johnels J, Zürcher NR, et al. Look me in the eyes: constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Sci Rep. 2017;7:3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakataki M, Soravia LM, Schwab S, et al. Glucocorticoid administration improves aberrant fear-processing networks in spider phobia. Neuropsychopharmacology. 2017;42:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]


