Short abstract
Effective pharmacological treatment options for chronic pain remain very limited, and continued reliance on opioid analgesics has contributed to an epidemic in the United States. On the other hand, nonpharmacologic neuromodulatory interventions provide a promising avenue for relief of chronic pain without the complications of dependence and addiction. An especially attractive neuromodulation strategy is to optimize endogenous pain regulatory circuits. The prefrontal cortex is known to provide top-down control of pain, and hence neuromodulation methods that selectively enhance the activities in this brain region during pain episodes have the potential to provide analgesia. In this study, we designed a low-frequency (2 Hz) electrical stimulation protocol to provide temporally and spatially specific enhancement of the prefrontal control of pain in rats. We showed that low-frequency electrical stimulation of the prelimbic region of the prefrontal cortex relieved both sensory and affective responses to acute pain in naive rats. Furthermore, we found that low-frequency electrical stimulation of the prefrontal cortex also attenuated mechanical allodynia in a rat model of chronic pain. Together, our findings demonstrated that low-frequency electrical stimulation of the prefrontal cortex represents a promising new method of neuromodulation to inhibit pain.
Keywords: Neuromodulation, low-frequency stimulation, prefrontal cortex, acute pain, chronic pain
Introduction
Chronic pain affects close to one in three or four adults.1 Despite progress in understanding basic mechanisms of chronic pain, few highly effective analgesics have been discovered in the last 50 years. This lack of translation has resulted in the overuse of opioids for chronic pain treatment, particularly in the United States in recent years, leading to an opioid epidemic.2 In this context, neuromodulation holds promise as a method of nonpharmacologic pain treatment with minimal risks for addiction or abuse. Electrical stimulation methods such as transcranial direct or alternating current stimulation and deep brain stimulation (DBS) have been developed for patients with neuropsychiatric diseases. In a limited number of studies, some of these brain stimulation methods have been shown to be effective in pain management.3–8 In addition, spinal cord stimulation (SCS), which is based in part on the gate control theory,9 has been in clinical use for the treatment of chronic neuropathic pain.10 However, current neuromodulation methods typically require continuous, relatively high-frequency electrical stimulations of peripheral and/or central neurons. The purpose of such methods is to take over the native property—most often inhibiting the function—of a group of neurons in a specific pain transmission pathway. Unfortunately, such methods are often associated with decreased efficacy over time as well as sensory and cognitive side effects.11–15 These issues have limited the application of neuromodulation therapies. Thus, more work is needed to improve the neuromodulation approach for analgesia.
In addition to taking over a neural circuit, neuromodulation can also be used to enhance the endogenous pain-inhibitory function of select group of neurons. The prefrontal cortex (PFC) exerts profound top-down regulation of pain processing16,17. Nonphysiologic high-frequency (>20 Hz) stimulation of the PFC has been shown to inhibit withdrawal reflexes and aversive responses to pain in animal models, further indicating this region as a critical area for endogenous pain regulation and possible target for neuromodulation.18–22 Specifically, previous studies have demonstrated that optogenetic activation of the prelimbic region of the PFC (PL-PFC) in rodents can relieve pain.20–22 Furthermore, studies have shown that the PFC projects to a number of subcortical brain regions, including the periaqueductal gray (PAG), nucleus accumbens (NAc), and amygdala to mediate pain-modulatory effects.20–26 A recent study found that chronic pain reduces the basal firing rates of PFC neurons, resulting in decreased cortical pain-regulatory outputs. Meanwhile, low-frequency (2 Hz) optogenetic stimulation of the PFC increased basal firing rates of neurons in this region, which in turn resulted in an increase in the neuronal response to nociceptive inputs. These results indicate a cortical gain control mechanism, where the gain of the nociceptive cortical control is regulated by intrinsic excitability of PFC neurons. Chronic pain reduces this gain, resulting in decreased cortical modulatory outputs. Conversely, low-frequency stimulation can increase the gain of function in the PFC, leading to enhanced endogenous descending regulation of pain.22 Such cortical gain mechanism, where the gain of cortical function can be modulated by small changes in basal firing properties, has also been proposed for other sensory and affective processes.27,28 However, optogenetic stimulation is not practical clinically. Thus, in order to demonstrate the usefulness of prefrontal stimulation as a novel targeted therapy, we need to show that electrical stimulation of this region, in the form of DBS, can relieve pain.
In this study, we designed a novel neuromodulation protocol to test the effect of low-frequency electrical stimulation of the PL-PFC in freely moving rats. We found that 2-Hz stimulations of this region increased the threshold of thermal pain on the Hargreaves’ test in naive rats. In addition, on a well-established conditioned place preference assay, when rats received noxious stimulations in both chambers, rats preferred the chamber associated with PFC electrical stimulation. This indicated that PFC stimulation also removed the aversive effect of pain. Finally, we showed that low-frequency stimulation of the PFC relieved mechanical allodynia associated with chronic inflammatory pain, in a complete Freund’s adjuvant (CFA) model. Together, these results demonstrate that low-frequency stimulation of the PFC can be a highly effective method in treating sensory and affective component of pain.
Material and methods
Animals
All procedures were approved by the New York University School of Medicine Institutional Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ensure minimal animal use and discomfort. Eight-week-old male Sprague-Dawley rats were purchased from Taconic Farms, Albany, NY, and kept at Mispro Biotech Services Facility in the Alexandria Center for Life Science, with controlled humidity, temperature, and 12-h (6:30 a.m. to 6:30 p.m.) light–dark cycle. Food and water were available ad libitum. Animals arrived to the animal facility at 250 to 300 g and were given on average 10 days to adjust to the new environment prior to the onset of experiments.
CFA injection
To induce chronic inflammatory pain, 0.1 ml of CFA (mycobacterium tuberculosis, Sigma-Aldrich) was suspended in an oil–saline (1:1) emulsion and injected subcutaneously into the plantar aspect of the hindpaw opposite to the paw that was stimulated by pin prick (PP). Control rats received equal volume of saline injection.
Intracranial electrode implant
Intracranial bipolar electrodes were constructed from twisting together two 35 µm diameter formvar insulated Stablohm 675 wires (California Fine Wire Company).29,30 On the stimulus end, one wire of the bipolar pair was cut 0.5 mm shorter than the other wire to provide a distance across which the applied current will traverse while also staying within the same cortical layer. The other end of the bipolar pair was stripped of the insulation and coupled to connector header (2163S-36-ND, Digi-Key). Rats were anesthetized with isoflurane (1.5%–2%). Depth of anesthesia was verified by tail pinch, which was performed throughout the surgery. The skull was exposed and a 0.7-mm-diameter hole was drilled above the target region. A 30G needle was used to pierce the dura before electrodes were slowly lowered bilaterally into the PL-PFC with the stereotaxic apparatus. Coordinates for PL-PFC bipolar electrodes were as follows: Anteroposterior (AP) +2.9 mm, Mediolateral (ML) ±1.5 mm, Dorsoventral (DV) −3.7 mm, angled at 13° toward the midline. The electrodes and connector header were secured to the skull screws with dental cement. Rats were given on average 10 days to recovery from the surgery prior to any further testing.
Animals were sacrificed after electrical lesions. Rats were anesthetized with isoflurane (>2%), and once deep anesthesia was confirmed by tail pinch, they were sacrificed. Following sacrifice, brain sections (20 mm) were collected using a Microm HM525 cryostat machine and analyzed for the electrode location with histology staining. Animals with improper electrode implantation were excluded from the further analysis.
Electrical stimulation protocols
All electrical stimulations were applied using Constant Current Stimulus Isolator (A365, World Precision Instruments). The stimulus sequence was trigger by Transistor-Transistor Logic (TTL) pulse generators (OPTG_4, Doric Lenses). Bilateral electrical stimulation was applied at 2 Hz with a pulse width of 5 ms. Current amplitudes of 5, 10, 20, and 40 uA were used during behavioral testing. All electrical stimulations were biphasic.
Hargreaves’ test (Plantar Test)
The Hargreaves’ test was performed to evaluate the response to acute thermal stimulation.20 A mobile radiant heat-emitting device with an aperture of 10 mm (37370-Plantar Test, Ugo Basile, Italy) was used to produce acute thermal stimulation of the plantar surface of the right hind paw. The latency to paw withdrawal was recorded automatically. Paw withdrawals due to locomotion or weight shifting were not counted, and the trials were repeated. Measurements were repeated five times at 5-min intervals.
Conditioned place preference assay
Conditioned place preference (CPP) experiments were conducted similar to what has been described previously.31 The movements of animals in each chamber were recorded by a camera and analyzed with the Any-maze software. The CPP protocol included preconditioning (baseline), conditioning, and testing phases (10 min during each phase). Animals spending more than 500 s or less than 100 s of the total time in either main chamber in the preconditioning phase were eliminated from further analysis. Immediately following the preconditioning phase, the rats underwent conditioning for 10 min. During conditioning, rats received peripheral noxious stimulation (PP) every 15 s in both chambers. One of the chambers was paired with electrical treatment; the other chamber was paired with no treatment. Peripheral stimulation, electrical stimulation, and chamber pairings were counterbalanced. During the test phase, the animals did not receive any stimulation and had free access to both compartments for a total of 10 min. Animal movements in each of the chambers were recorded, and the time spent in either of the treatment chambers was analyzed by the AnyMaze software. Decreased time spent in a chamber during the test phase as compared with the baseline indicates avoidance (aversion) for that chamber.
Mechanical allodynia test
A Dixon up-down method with von Frey (VF) filaments was used to measure mechanical allodynia. Rats were individually placed into plexiglass chambers over a mesh table and acclimated for 20 min before testing. Beginning with 2.55 g, VF filaments in a set with logarithmically incremental stiffness (0.45, 0.75, 1.20, 2.55, 4.40, 6.10, 10.50, and 15.10 g) were applied to the paws of rats. A traditional Dixon up-down method with VF filaments was used to measure 50% withdrawal threshold, as described previously.20,32,33 Electrical stimulation was applied throughout the time course of the allodynia test.
Statistical analysis
The results of behavioral experiments were given as mean ± SEM. A two-tailed paired Student’s t test was used to analyze the results from the Hargreaves’ test. For mechanical allodynia, a two-way analysis of variance (ANOVA) with repeated measures and post hoc multiple pairwise comparison Bonferroni tests was used. During the CPP test, a paired Student’s t test was used to compare the time spent in each treatment chamber before and after conditioning (i.e., baseline vs. test phase for each chamber). Decreased time spent in a chamber during the test phase as compared with the baseline indicates avoidance (aversion) for that chamber. A conditioned placed aversion (CPA) score was computed by subtracting the time spent in the more noxious chamber during the test phase from the time spent in that chamber at baseline. A two-tailed unpaired Student’s t test was used to compare differences in CPA scores under various testing conditions.
For all tests, a p value < 0.05 was considered statistically significant. All data were analyzed using the GraphPad Prism Version 7 software (GraphPad).
Results
A novel low-frequency neurostimulation strategy can relieve both sensory and affective components of acute pain
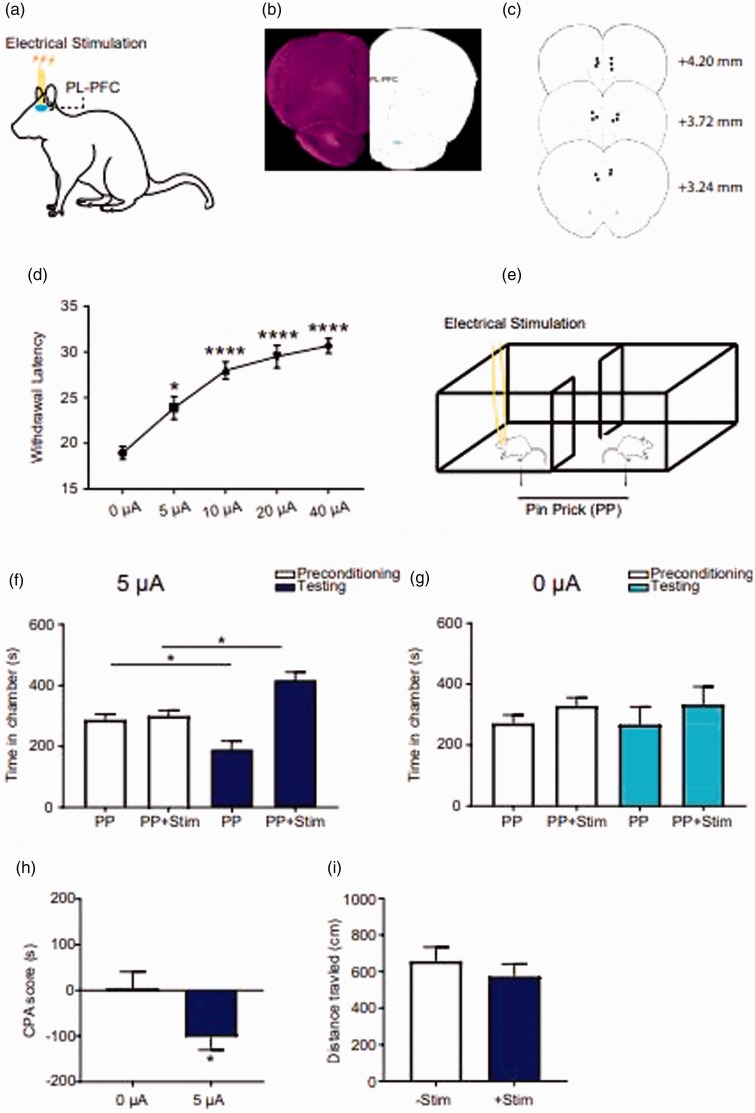
Based on the concept of cortical gain control for pain,22 where low-frequency stimulation of the cortex can improve its basal and stimulus-induced functions, we performed preclinical testing of low-frequency electrical stimulation of the PL-PFC in freely moving rats (Figure 1(a)). We chose 2-Hz stimulation for two reasons. First, this frequency is not much higher than basal cortical spontaneous firing rates, and so it falls within the physiological range. Second, 2 Hz frequency has been successfully used in a previous optogenetic study to provide pain relief.22 In rodents, the PL-PFC is positioned dorsal to the anterior cingulate cortex (ACC). Hence, to specifically target the PL-PFC, we inserted electrodes directly into this region (Figure 1(b) and (c)). In order to provide a low level of neuromodulation, rather than supraphysiological activation, we chose to stimulate this region at 2 Hz frequency, resembling the basal firing rates for the pyramidal neurons in this region.22 We found that across a range of current intensities, 2-Hz stimulation of the PFC was highly effective in reducing acute nociceptive withdrawals (Figure 1(d)). Next, we tested if low-frequency and low-intensity electrical stimulation could also reduce the aversive response to pain by testing with a two-chamber CPP assay.21,22,31 We paired 2-Hz stimulation of the PFC in one chamber with a peripheral pain stimulus, and the other chamber with that pain stimulus alone (Figure 1(e)). Rats preferred the chamber associated with electrical stimulation in the testing phase (Figure 1(f)). In contrast, rats demonstrated no preference when the same treatment chamber was paired with sham stimulation (Figure 1(g)). To further quantitate these results on the CPP assay, we calculated a CPA score by subtracting the amount of time rats stayed in the electrical stimulus-paired chamber during the test phase from the amount of time they stayed in the preconditioning phase. We found that low-frequency electrical stimulation of the PL-PFC reduced this CPA score compared with sham stimulation (Figure 1(h)). Importantly, 2-Hz stimulations of the PFC did not alter baseline locomotor behavior, demonstrating relative behavioral specificity for this neuromodulation approach (Figure 1(i)).
Figure 1.
Low-frequency electrical stimulation in the PFC relieved pain in naive rats. (a) Schematic of the electrical stimulation in the PL-PFC. (b) Histology showing the location of tetrodes in the PL-PFC. (c) Schematic showing the intracranial electrode sites. (d) Low-frequency (2 Hz) electrical stimulation of the PL-PFC increased the latency to paw withdrawal latency during Hargreaves’ test across a range of stimulation intensities, n = 8; p = 0.0156 (baseline vs. 5 µA), p < 0.0001 (baseline vs. 10, 20, or 40 µA), one-way ANOVA with repeated measures and Bonferroni posttests. (e) Schematic of conditioned place preference protocol. During the conditioning phase, one chamber was paired with the noxious mechanical stimulus—PP, coupled with simultaneous electrical stimulation of the PL-PFC, the other chamber was paired with PP only. (f) Rats displayed preference to the chamber associated with electrical stimulation in the testing phase, n = 7; p = 0.0124, paired t test. (g) Rats showed no preference to the chamber associated with sham electrical stimulation, n = 5; p = 0.9095, paired t test. (h) 2-Hz electrical stimulation in the PFC provided relief of aversive response to acute pain, as demonstrated by the decreasing CPA score. n = 5–7; p = 0.0435, unpaired t test. (i) Electrical stimulation of the PL-PFC did not alter the locomotion. n = 5; p = 0.4455, unpaired t test. CPA: conditioned placed aversion; PP: pin prick.
Low-frequency neurostimulation of the PFC relieves persistent inflammatory pain
To further investigate the impact of this low-frequency neuromodulatory strategy on chronic pain, we used a traditional inflammatory pain (CFA) model.34,35 CFA was administrated subcutaneously into one hind paw of the rat to induce persistent inflammatory pain (Figure 2(a)). We chose a low current intensity (20 µA) that has been shown to be well tolerated according to the previous literature.36 At this intensity, when administered concurrently with behavior tests, 2-Hz stimulation provided effective relief of mechanical allodynia in CFA-treated rats (Figure 2(b) and (c)). These results indicate that low-frequency and low-intensity stimulation of the PFC can relieve symptoms of chronic pain as well.
Figure 2.
Low-frequency electrical stimulation in the PFC relieved pain in a rodent chronic pain model. (a) Rats developed mechanical allodynia after CFA injection in their hind paws, n = 5; p < 0.0001, two-way ANOVA with repeated measures and Bonferroni posttests. (b) Schematic of electrical stimulation of the PL-PFC in CFA-treated rats. (c) Low-frequency electrical stimulation of the PL-PFC relieved mechanical allodynia in CFA-treated rats, n = 7; p = 0.0045 (CFA vs. CFA + Stim), one-way ANOVA with repeated measures and Bonferroni posttests. CFA: complete Freund’s adjuvant; PFC: prefrontal cortex; PL-PFC: prelimbic region of the PFC.
Discussion
Multimodal analgesia is critical to combat the dual public health crises of undertreatment of pain and the opioid epidemic. A key component of a multimodal strategy is the integration of nonpharmacological neuromodulation therapies. Current neuromodulation for pain has been limited to SCS in the United States. The frequency of SCS is typically 40–60 Hz.10 Newer advances include the use of very high-frequency stimulation (10,000 Hz) and burst stimulation.37–41 Numerous studies have confirmed the analgesic efficacy for SCS.42,43 However, three conditions limit its overall use. First, due to anatomical considerations, SCS is primarily used for lower extremity neuropathic pain. Second, the SCS is implanted in the epidural space. With constant movement of the spine, there is a high risk for lead displacement, with rates reported to be as high as 30% in some studies.44 Finally, there has been noted loss of efficacy with SCS over time, likely due to desensitization secondary to constant and chronic stimulations.
Over the last 70 years, electrical brain neuromodulation has been studied as a treatment modality for refractory pain conditions. The overall goal in these studies has been to reduce neural activities in pain-producing or processing regions with high-frequency stimulations in a protocol similar to DBS utilized for Parkinson’s disease.45 Select targets include the ACC, motor cortex, sensory thalamus, intralaminar parafascicular complex (CMP), PAG, and NAc.46 A number of studies targeting these areas have shown a varying degree of success in pain relief, but no method has provided consistent efficacy. Depending on the brain region being stimulated, side effects include nausea, dizziness, loss of appetite, nystagmus, vertigo, and nausea.47
In our study, we have taken a different approach. Rather than inhibiting brain and spinal regions that may play a role in pain transmission, we have opted to enhance the function of endogenous cortical control. We have chosen the PFC as a target, as a large number of studies have shown the efficacy of PL-PFC for pain regulation in rodents.18–22,48–52 Our most recent study indicates that low-frequency optogenetic stimulation of the PFC can enhance the endogenous function of this region, as evidenced by increased basal spontaneous firing rates and increased firing rates in response to a noxious stimulus.22 While optogenetic stimulation is impractical as a treatment modality, our results here with low-frequency electrical stimulations of the PFC generated similar behavioral outcomes in naive rats and rats with persistent pain, demonstrating the promise for this therapeutic approach based on the cortical gain control mechanism. Importantly, the PL-PFC in rodents corresponds to the dorsolateral PFC (DL-PFC) in primates. Thus, our success in this study suggests the possibility that the DL-PFC may form a therapeutic target for low-frequency and low-intensity neuromodulation. While in this feasibility study we chose 2-Hz stimulation to mimic basal spontaneous firing rates of the PFC neurons,22 future studies are needed to test a wide range of stimulation frequencies. Furthermore, in this study, we aimed to achieve the qualitative pain relief with electrical PFC stimulations using stimulation parameters that are established and safe. Thus, we used low-intensity stimulation, and we did not observe any obvious side effects such as seizure or altered locomotion. At these low intensities, however, we only achieved partial relief of mechanical allodynia. Thus, future studies are needed to optimize the stimulation parameters for achieving pain relief without unwanted side effects.
In this study, we stimulated PFC bilaterally. However, in an earlier study, we showed that ipsilateral or contralateral PFC stimulation had similar effects as bilateral stimulation.21 It is possible that what matters for pain regulation is the total number of neurons that are activated in the PFC rather than laterality. Future studies are needed, however, to further characterize the laterality of electrical stimulation in the PFC for delivery analgesia.
We found that PFC stimulation relieved both nociceptive reflexive withdrawals and pain aversion. However, it is possible that the two behavioral effects are related. Thus, descending inhibition may have reduced ascending nociceptive inputs, resulting in less aversion. Alternatively, cortico–subcortical or cortico–cortical interactions may have reduced pain aversion independently. Future studies are needed to differentiate between these two possibilities.
In conclusion, we have shown that low-frequency electrical stimulation of the PFC can achieve considerable analgesic efficacy. Future studies should focus on examinations of additional cortical and subcortical targets for such neuromodulation approach and the application of this approach in clinical settings.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81771101), National Institute of General Medical Sciences (GM115384), and China Scholarship Council (201606370208).
References
- 1.de Souza JB. Prevalence of chronic pain, treatments, perception, and interference on life activities: Brazilian population-based survey. Pain Res Manag 2017; 2017: 4643830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan AT. Opioid abuse in chronic pain – misconceptions and mitigation strategies. N Engl J Med 2016; 374: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 3.Katayama Y, Tsubokawa T, Yamamoto T. Chronic motor cortex stimulation for central deafferentation pain: experience with bulbar pain secondary to Wallenberg syndrome. Stereotact Funct Neurosurg 1994; 62: 295–299. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg 1998; 89: 585–591. [DOI] [PubMed] [Google Scholar]
- 5.Herregodts P, Stadnik T, De Ridder F, D’Haens J. Cortical stimulation for central neuropathic pain: 3-D surface MRI for easy determination of the motor cortex. Acta Neurochir Suppl 1995; 64: 132–135. [DOI] [PubMed] [Google Scholar]
- 6.Ebel H, Rust D, Tronnier V, BöKer D, Kunze S. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir 1996; 138: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen JP. [Treatment of central and neuropathic facial pain by chronic stimulation of the motor cortex: value of neuronavigation guidance systems for the localization of the motor cortex]. Neurochirurgie 2000; 46: 483–491. [PubMed] [Google Scholar]
- 8.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguiere F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 1999; 83: 259–273. [DOI] [PubMed] [Google Scholar]
- 9.Nathan PW. The gate-control theory of pain. A critical review. Brain 1976; 99: 123–158. [DOI] [PubMed] [Google Scholar]
- 10.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014; 17: 515–550; discussion 550. [DOI] [PubMed] [Google Scholar]
- 11.Deer TR, Krames E, Mekhail N, Pope J, Leong M, Stanton-Hicks M, Golovac S, Kapural L, Alo K, Anderson J, Foreman RD, Caraway D, Narouze S, Linderoth B, Buvanendran A, Feler C, Poree L, Lynch P, McJunkin T, Swing T, Staats P, Liem L, Williams K. The Appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation. 2014; 17: 599–615. [DOI] [PubMed] [Google Scholar]
- 12.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl 1991; 52: 137–139. [DOI] [PubMed] [Google Scholar]
- 13.Lewin W, Whitty CW. Effects of anterior cingulate stimulation in conscious human subjects. J Neurophysiol 1960; 23: 445–447. [DOI] [PubMed] [Google Scholar]
- 14.Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol 2017; 13: 548–554. [DOI] [PubMed] [Google Scholar]
- 15.De Ridder D, Perera S, Vanneste S. State of the art: novel applications for cortical stimulation. Neuromodulation 2017; 20: 206–214. [DOI] [PubMed] [Google Scholar]
- 16.Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol 2019; 56: 1137–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci 2010; 33: 173–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res 1985; 339: 281–284. [DOI] [PubMed] [Google Scholar]
- 19.Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat Commun 2015; 6: 7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 2015; 35: 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez E, Lin HH, Zhou H, Dale J, Liu K, Wang J. Corticostriatal regulation of acute pain. Front Cell Neurosci 2017; 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale J, Zhou H, Zhang Q, Martinez E, Hu S, Liu K, Urien L, Chen Z, Wang J. Scaling up cortical control inhibits pain. Cell Rep 2018; 23: 1301–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji G, Neugebauer V. CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur J Neurosci 2014; 39: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiritoshi T, Neugebauer V. Pathway-specific alterations of cortico-amygdala transmission in an arthritis pain model. ACS Chem Neurosci 2018; 9: 2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Martinez E, Lin HH, Yang R, Dale JA, Liu K, Huang D, Wang J. Inhibition of the prefrontal projection to the nucleus accumbens enhances pain sensitivity and affect. Front Cell Neurosci 2018; 12: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheriyan J, Sheets PL. Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. J Neurosci 2018; 38: 4829–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzsaki G, Mizuseki K. The log-dynamic brain: how skewed distributions affect network operations. Nat Rev Neurosci 2014; 15: 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stark E, Roux L, Eichler R, Buzsaki G. Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proc Natl Acad Sci USA 2015; 112: 10521–10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geddes LA, Roeder R. Criteria for the selection of materials for implanted electrodes. Ann Biomed Eng 2003; 31: 879–890. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Ke Y, Chan DCW, Qian Z-M, Yung KKL, Ko H, Arbuthnott GW, Yung W-H. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012; 76: 1030–1041. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Manders T, Tong AP, Yang R, Garg A, Martinez E, Zhou H, Dale J, Goyal A, Urien L, Yang G. Chronic pain induces generalized enhancement of aversion. Elife 2017; 6: e25302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 33.Bourquin AF, Süveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 2006; 122: 14 e11–14. [DOI] [PubMed] [Google Scholar]
- 34.Le AM, Lee M, Su C, Zou A, Wang J. AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology 2014; 121: 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su C. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol Brain 2015; 8: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffrey M, Lang M, Gane J, Wu C, Burnham W, Zhang L. A reliable method for intracranial electrode implantation and chronic electrical stimulation in the mouse brain. BMC Neurosci 2013; 14: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liem L, Russo M, Huygen FJPM, Van Buyten J-P, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015; 18: 41–48; discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 38.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013; 80: 642–649.e641. [DOI] [PubMed] [Google Scholar]
- 39.De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery 2010; 66: 986–990. [DOI] [PubMed] [Google Scholar]
- 40.Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 2013; 16: 59–65; discussion 65–56. [DOI] [PubMed] [Google Scholar]
- 41.Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation 2013; 16: 370–375. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract 2014; 14: 489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, Grami V, Justiz R, Nouri KH, Hayek SM, Vallejo R, Christo PJ. Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician 2016; 19: E33–E54. [PubMed] [Google Scholar]
- 44.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Thomson S, Raso L, Burton A, DeAndres J, Buchser E, Buvanendran A, Liem L, Kumar K, Rizvi S, Feler C, Abejon D, Anderson J, Eldabe S, Kim P, Leong M, Hayek S, McDowell G, Poree L, Brooks ES, McJunkin T, Lynch P, Kapural L, Foreman RD, Caraway D, Alo K, Narouze S, Levy RM, North R. The appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation 2014; 17: 571–598. [DOI] [PubMed] [Google Scholar]
- 45.Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery 1987; 21: 885–893. [DOI] [PubMed] [Google Scholar]
- 46.van Kuyck K, Gabriëls L, Cosyns P, Arckens L, Sturm V, Rasmussen S, Nuttin B. Behavioural and physiological effects of electrical stimulation in the nucleus accumbens: a review. Acta Neurochir Suppl 2007; 97: 375–391. [DOI] [PubMed] [Google Scholar]
- 47.Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg 1977; 47: 178–183. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep 2015; 12: 752–759. [DOI] [PubMed] [Google Scholar]
- 49.Kiritoshi T, Ji G, Neugebauer V. Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci 2016; 36: 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly CJ, Huang M, Meltzer H, Martina M. Reduced glutamatergic currents and dendritic branching of layer 5 pyramidal cells contribute to medial prefrontal cortex deactivation in a rat model of neuropathic pain. Front Cell Neurosci 2016; 10: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radzicki D, Pollema-Mays SL, Sanz-Clemente A, Martina M. Loss of M1 receptor dependent cholinergic excitation contributes to mPFC deactivation in neuropathic pain. J Neurosci 2017; 37: 2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol 2011; 106: 2642–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]