Abstract
Abstract. Objectives: Mesotheliomas occur in occult serous cavities after chronic exposure of mesothelial cells to asbestos fibres. Molecular events that contribute to the development of this cancer are therefore not readily accessible for study. We have used in vitro culture systems to study and compare induced and spontaneous transformation events in primary mouse mesothelial cells. Materials and methods: Mouse mesothelial cells were cultivated until small populations of proliferating cells emerged from senescing cultures. Spontaneously transformed cultures of cells were characterized and compared to malignantly transformed cells. Results: Human mesothelial cells had a finite lifespan of 10–15 population doublings when cultured in vitro; mouse mesothelial cells typically exhibit this same pattern. Here, we show that mouse mesothelial cells can be cultured for extended periods and that these cells can transform spontaneously. Lines of spontaneously transformed cells generated in this study are immortal and growth factor‐independent. They display the salient characteristic features of transformation, including increased proliferation rate, lack of contact inhibition, aneuploidy and ability to grow in anchorage‐independent conditions. A subset of these cell lines developed into tumours in syngeneic mice. Comparative gene expression analysis demonstrated that spontaneously transformed cell lines were more closely related to neoplastic cells than to primary cells. Conclusion: These findings have implications for interpretation of in vitro transformation studies, demonstrating broad similarity between spontaneous and induced genetic changes.
INTRODUCTION
Mesothelial cells form a monolayer on the surface of serosal cavities and the internal organs. These cells secrete proteoglycans and surfactant‐like molecules, thus providing a lubricated surface for the movement of organs against each other within their cavities (Mutsaers 2004). Malignant transformation of mesothelial cells leads to the development of mesothelioma, a highly invasive, aggressive and incurable cancer. A principle causative agent of mesothelioma is asbestos, although the specific mechanisms leading to malignant transformation are poorly understood (Robinson & Lake 2005; Robinson et al. 2005). Typically, there is a long latency (~40 years) between initial exposure to asbestos and presentation with disease, and current therapies can at best extend life expectancy by a few months. This is in part due to late diagnosis of this disease when patients already have large, disseminated tumours that are difficult to completely resect. If the early stages of mesothelial cell transformation could be identified, this information might lead to early detection, better prognostication and provide new targets for therapy.
Cancer is a genetic disease and both endogenous and exogenous factors invoke changes that lead to malignant transformation. Specific genetic aberrations and particular mutagenic agents have been identified in the aetiology of many cancers, although the overall process is likely to be probabilistic in nature. There are at least six essential changes common to all human cell types that contribute to cancer progression (Hanahan & Weinberg 2000). These changes generally occur over a period of years to decades and typically confer a growth advantage to cells (Cahill et al. 1999). The hallmarks of cancer include the ability of cancer cells to: acquire unlimited proliferation (immortalization), synthesize and respond to endogenous mitogenic mediators, acquire insensitivity to exogenous growth inhibitory signals, evade cell death signals (apoptosis), acquire vascularization (angiogenesis), and migrate to and invade other tissues (metastasize).
Mesotheliomas occur in experimental animals subsequent to injection of asbestos into either the peritoneal cavity or pleural space. Rodents, like humans, develop mesotheliomas after a long latency and in the same way, stages and sites of transformation are difficult to determine (Davis et al. 1992). In contrast, a pure population of cultured cells can be monitored for phenotypic and genotypic changes after exposure to known mutagenic agents. However, it is well documented that many rodent cell types are capable of spontaneous immortalization in vitro (Davis & Kipling 2005). Immortalized cell lines emerge from replicative senescence, losing their dependence on growth factors and therefore acquiring the ability to grow in low serum conditions. These cells can also lose contact inhibition and pile up on top of each other to form foci, and may become able to form colonies in soft agarose; as such, they become anchorage‐independent. However, the ultimate test of malignant transformation is the ability to form tumours in vivo.
A key process that limits outgrowth of primary human mesothelial cells in vitro is the onset of senescence (‘crisis’). Erosion of telomeres with each round of replication causes crisis until they reach a critical length. This can lead to end‐to‐end fusion or degradation of chromosomes, subsequently triggering apoptosis (Sedivy 1998). Telomere‐induced senescence is as effective as apoptosis in reducing cancer incidence and is mediated by the tumour suppressor p53 (Xue et al. 2007). Telomere maintenance is facilitated by the reverse transcriptase enzyme telomerase, which is not expressed in most normal human cell types. Ability to restore telomeres is required for the immortalization of cells, an important step in the transformation process, and telomerase is activated in up to 90% of human tumours, including mesotheliomas. Transfection of mesothelial cells with the catalytic component of telomerase, hTERT, the proto‐oncogene Ras, or SV40 TAg/tAg oncogenes leads to cell immortalization (Ke et al. 1989; Reddel et al. 1989). Although such cell lines appear to bypass senescence, they are not necessarily neoplastic. This suggests that transformation of human mesothelial cells may be a multistage process and that more than one ‘hit’ is required to induce their malignant transformation.
In contrast, many primary rodent cell types constitutively express telomerase and do not undergo crisis. Rat mesothelial cells undergo spontaneous transformation in vitro (Funaki et al. 1991). Spontaneous transformation seems to be a phenomenon isolated to rodent cells and has been described before in cultures of embryonic stem cells, hepatocytes, keratinocytes, splenic macrophages and mammary epithelial cells (Suda et al. 1987; Kaighn et al. 1988; Ehmann et al. 1991; Huggett et al. 1991; Wilson et al. 1991; Kittrell et al. 1992); however, there are few reports of rat mesothelial cells undergoing spontaneous transformation in vitro (Funaki et al. 1991; Kravchenko et al. 1998) and the present study, to our knowledge, is the first demonstration of spontaneous transformation in mouse mesothelial cell cultures.
Here, we show that mouse mesothelial cells acquire features of immortalization and transformation after extended culture, and that these cells are more closely related to the malignant state than to primary mesothelial cells. The difficulty in distinguishing between spontaneous and induced transformation events is an important consideration for all in vitro studies.
MATERIALS AND METHODS
Cell culture
Primary mesothelial cells (MTM) were isolated from peritoneal tissue of C57Bl/6 mice and were cultured as described previously (Bot et al. 2003); tissue was recovered from three female mice to generate each cell line. By approximately five population doublings (passage 3), these cultures contained >95% mesothelial cells when analysed by transmission electron microscopy. Spontaneously transformed cell lines were generated by continuously culturing ‘senescing’ cells for up to 4 weeks. Emerging isolated populations of proliferating cells were pooled to generate representative spontaneously transformed high passage mesothelial cell lines (MTMhp) and were used in experiments at 75–100 population doublings. Mesothelioma cell lines were derived from mice that were injected with crocidolite asbestos as described previously (Davis et al. 1992).
Transmission electron microscopy
Cultured cells were pelleted in 11% bovine serum albumin in phosphate‐buffered saline, fixed in 2.5% glutaraldehyde and embedded in araldite. Thin (60–90 nm) sections were cut on an LKB III ultratome, they were stained with uranyl acetate and lead citrate and examined using a Philips 410 transmission electron microscope (Philips, Eindhoven, The Netherlands) at an accelerating voltage of 80 kV.
Proliferation assays
For each population of cells, viability and proliferation rate were determined by trypan blue exclusion. The proportion of cells in each culture that transited S phase within 24 h was determined using the BrdU Flow Kit (BD Pharmingen, San Diego, CA, USA).
Senescence associated‐β‐galactosidase staining
Cultures were stained for senescence using methods described previously (Dimri et al. 1995). Senescent (stained) and non‐senescent (unstained) cells were manually counted using light microscopy.
DNA content determination
Cells were assessed for propidium iodide (PI) incorporation using an excitation wavelength of 417 nm by flow cytometry. At least 10 000 cells were analysed. For a diploid profile, cells in the G0/G1 phase of the cell cycle (2 N) peaked at approximately 200 (arbitrary units) on the PI intensity scale and those in G2/M (4 N) peaked at approximately 400.
Soft agarose assay
Cells (2 × 104/mL) were re‐suspended in 0.35% low‐melt agarose (Roche, Mannheim, Germany) in 15% foetal calf serum (FCS) Dulbecco's modified Eagle's medium (DMEM). Single cell suspensions were added dropwise to pre‐coated wells containing 0.7% low‐melt agarose in 15% FCS DMEM. Cultures were incubated for 28 days when colonies were stained with crystal violet and then counted manually using a light microscope. MTM and mesothelioma cells were used as controls and colonies were counted if they consisted of five or more cells. Experiments were repeated at least twice using three biological replicate cell lines. We endeavoured to produce single cell suspensions by vigorous agitation of enzyme treated cultures. After plating into soft agarose, we found less than 1% doublets and colonies of five cells or more were never observed, after a 24‐h incubation period. There was no bias in the distribution of doublets between the cell lines. Therefore, the measurement of five cells was used arbitrarily as the lower limit to represent colony formation.
Tumour growth in mice
Cells were cultured until approximately 80% confluent and were injected as single cell suspensions (5 × 106 cells in saline), subcutaneously into the right flank of syngeneic (C57Bl/6) or athymic (nu/nu) mice. Mice were monitored weekly for tumour formation for up to 100 days. Mice with tumours that reached more than 100 mm2 in size were euthanized by cervical dislocation.
Microarray and analysis
Total RNA was extracted from cell cultures using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by purification with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Twenty micrograms of total RNA were labelled using an amino allyl direct labelling method, incorporating either a CyDye Cy3 or Cy5 fluorescent dye (GE‐Healthcare, Chalfont St. Giles, UK). Labelled cDNA was manually hybridized on to 22 K oligonucleotide microarrays (Compugen; manufactured by the LotteryWest Microarray Facility, Perth, Australia). Slides were subsequently washed and then scanned using a white light, CCD‐based ArrayWorX e Biochip Reader (Applied Precision Inc., Issaquah, WA, USA).
Signal intensity was quantified using spot finding software (Imagene, version 6.1; BioDiscovery Inc., El Seguendo, CA, USA) and any genes flagged as ‘absent’ were discarded from further data analysis. All subsequent analysis was performed using GeneSpring GX 7.3.1 Expression Analysis Software (Agilent Technologies, Palo Alto, CA, USA). LOWESS normalization was used to minimize bias in results due to unequal labelling of cDNA with fluorescent dyes. A data transformation step was also applied to change any normalized values below 0.1, to 0.1. The ratio of test versus reference intensity values for each gene was calculated for each experiment and log2 transformed. These expression values were then filtered on confidence across three biological replicate experiments, so that only genes with a P value less than 0.05 were included in subsequent analysis. Of this gene set, genes were ranked on mean fold change. For Gene Set Enrichment Analysis, GSEA v2.0 software was used (Subramanian et al. 2005). Genes were pre‐ranked based on mean fold change and were analysed against manually generated gene sets. These gene sets were based on an independent study, which determined differentially expressed genes in human mesothelioma patient specimens when compared to normal lung and pleural tissues (Gordon et al. 2005). Two gene sets were generated representing up‐ or down‐regulated genes from this study. The false discovery rate and P value of a normalized enrichment score were considered significant if less than 5% and 0.05, respectively.
Statistical analyses
Statistic analyses were calculated using GraphPad Prism software, version 4.0 (San Diego, CA, USA). Comparisons between cell lines at multiple time points were assessed using two‐way analysis of variance. Other comparisons were made using Student's t‐test. Differences were considered significant if the P value was less than 0.05.
RESULTS
Morphological changes associated with spontaneous transformation of mesothelial cells
MTM cultures displayed mixed morphology of epithelial and fibroblast‐like cells (Fig. 1a). Cells grew continuously in culture for six to eight passages (~9–13 population doublings), when the majority of cells displayed typical characteristics of senescence: cells became round and flattened, with the appearance of increased cytoplasmic volume. Senescent cells remained viable in culture for many weeks without division, eventually disintegrating. When cultures containing a high proportion of senescing cells were re‐cultured at a higher density, so that cells were maintained at 90–100% confluence, isolated populations of proliferating cells began to emerge. These small proliferating populations were observed 1–2 weeks after cells were replated and were subcultured to generate cell lines. Transmission electron microscopy (TEM) confirmed that spontaneously transformed cultures contained a pure population of mesothelial cells, as shown by the presence of microvilli at the cell surface (Fig. 1b).
Figure 1.
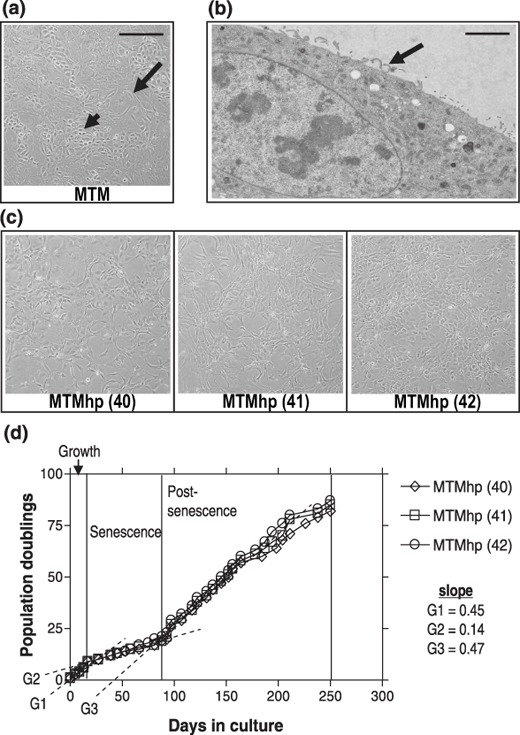
(a) Primary mesothelial cultures (MTM) contained cells with mixed epithelial (small arrow) and fibroblastic‐like morphologies (large arrow). (b) Representative transmission electron micrograph (TEM) of a spontaneously transformed mesothelial cell with characteristic surface microvilli (arrow). Scale bar = 2.5 µm. (c) Spontaneously transformed mesothelial cell lines MTMhp (40) and MTMhp (41) display fibroblastic morphology and MTMhp (42) cells have epithelioid morphology. (a) and (c) scale bar = 100 µm. (d) Population doubling profiles of cell lines. G1, G2 and G3 represent a gradient calculated by linear regression.
Three independently derived, spontaneously transformed mesothelial cell lines were established that displayed predominantly fibroblastic [MTMhp (40–41)] or epithelioid [MTMhp (42)] morphology (Fig. 1c). Although all cell lines were clearly mesothelial in origin, MTMhp (40) cells were less differentiated with no obvious condensation of intermediate filaments, few glycogen granules and no obvious junctional complexes. All cell lines were cultured for up to 90 population doublings over a 250‐day period, demonstrating three phases of growth: pre‐senescence, senescence and post‐senescence (Fig. 1d). Post‐senescent cells (G3) cultured for up to 70 population doublings grew faster compared to pre‐senescent cells (G1).
Reduced growth factor dependence of spontaneously transformed mesothelial cells
MTMhp cell lines grown in optimal serum conditions displayed a significantly higher rate of proliferation than MTM cells (P < 0.001; Fig. 2a). These results are consistent with the finding of a shorter doubling time of post‐senescent cells when compared to pre‐senescent cells (Fig. 1d). In contrast to MTM cells, MTMhp cell lines could proliferate in low serum conditions (0.5% FCS; P < 0.001; Fig. 2b), but more slowly than cells grown in optimal serum conditions.
Figure 2.
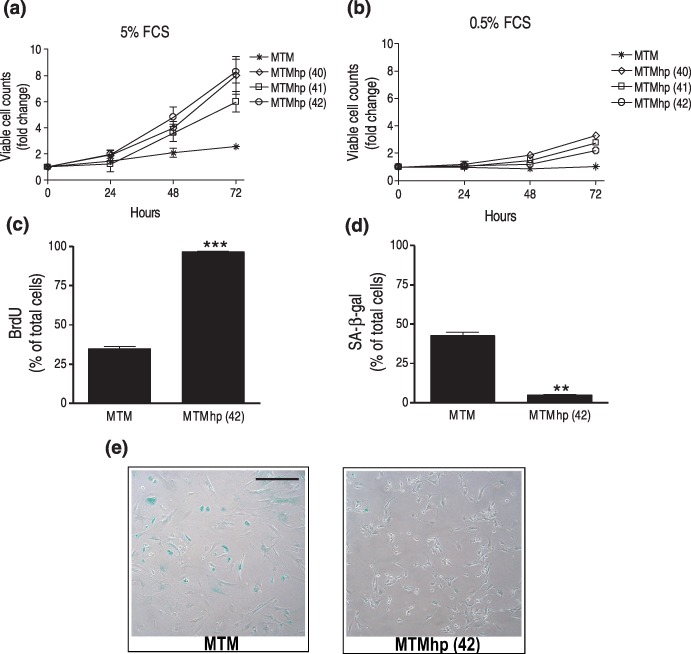
Primary and spontaneously transformed mesothelial cells, cultured for 72 h in (a) normal serum conditions (5% FCS) and in (b) low serum conditions (0.5% FCS), and proliferation assessed by counting viable cells using trypan blue exclusion. Results expressed as mean fold change of viable cell counts ± SEM when compared to cells at time 0. (c) Representative primary and spontaneously transformed mesothelial cells, MTM and MTMhp (42), respectively, were cultured with Bromodeoxyuridine (BrdU) to determine percentage of cells transiting S phase over 24 h. BrdU incorporation was assessed by flow cytometry. Results expressed as the mean percentage of BrdU‐positive cells, ± SEM. ***P < 0.001. (d) Cells were cultured under normal conditions and stained for senescence associated‐β‐galactosidase (SA‐β‐gal) activity. Results expressed and mean percentage of SA‐β‐gal positive cells ± SEM. **P < 0.01. (e) Representative pictures demonstrate SA‐β‐gal staining (blue) in MTM and MTMhp (42) cell lines. Magnification ×100. All results were calculated from triplicate wells of two independent experiments.
Reduced senescence of spontaneously transformed mesothelial cells
To investigate whether spontaneous transformation resulted in the selection of highly proliferating cells, the number of cells transiting S phase within 24 h was determined by analysing uptake of a pulse of BrdU (bromodeoxyuridine) using flow cytometry. Approximately 35% of MTM cells entered S phase of cell cycle within 24 h, in contrast to more than 95% of MTMhp (42) cells (P < 0.001; Fig. 2c). MTM cultures contained approximately 10 times more senescence associated‐β‐gal stained cells than MTMhp (42) cultures (P < 0.01; Fig. 2d), as around 40% of MTM cells were senescent compared to less than 5% of MTMhp (42) cells (Fig. 2e).
Increased DNA content of spontaneously transformed mesothelial cells
MTMhp cell lines displayed aneuploid profiles (Fig. 3b–d) compared to their primary diploid counterparts (Fig. 3a). These transformed cell lines were typically hyperdiploid (1.1–1.8 times the G1 peak of diploid cells), suggesting accumulation of genetic material from diploid or loss of genetic material from tetraploid cells.
Figure 3.
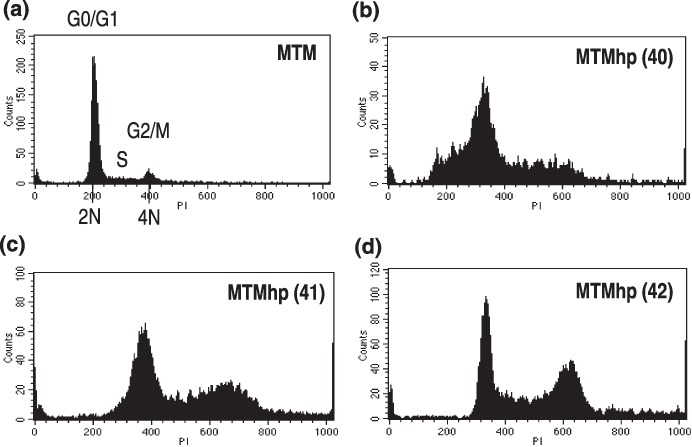
(a) Primary mesothelial cells and spontaneously transformed mesothelial cell lines. (b) MTMhp (40), (c) MTMhp (41), and (d) MTMhp (42) were stained with propidium iodide and their DNA content assessed by flow cytometry. Each histogram plot is representative of data generated from at least three individual experiments.
Spontaneously transformed mesothelial cells exhibited features of malignancy
MTM cells did not form colonies in semisolid medium. However, MTMhp cell lines did, demonstrating their acquired ability for anchorage‐independent growth (Fig. 4a). The majority of colonies were considered small (<20 cells per colony), compared to colonies formed by fully malignant control cell lines (mesothelioma; >50 cells per colony). Cells were injected into syngeneic mice to assess whether they were able to form subcutaneous tumours. The MTMhp (40) cell line formed tumours in four out of four mice, while no tumours were seen when injecting the MTMhp (41) and MTMhp (42) cell lines (P < 0.001; Fig. 4b). To determine whether lack of tumour growth was due to acquired immunogenic properties of these cell lines, they were also injected subcutaneously into athymic (nu/nu) mice. Only the MTMhp (40) cell line formed tumours, demonstrating their malignant phenotype. Growth rate and size of these tumours was consistent with what is seen when injecting asbestos‐induced malignant mesothelioma cells subcutaneously into syngeneic mice.
Figure 4.
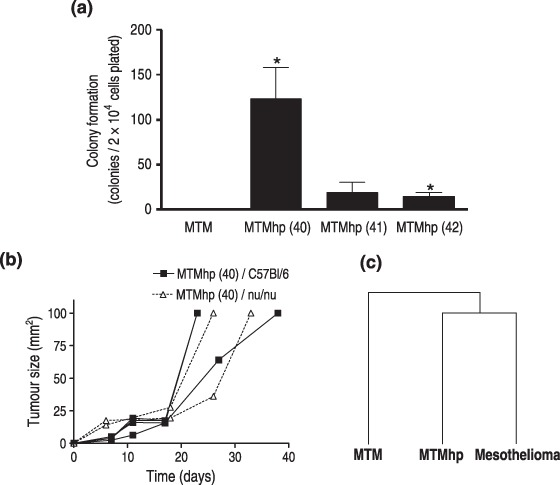
(a) Primary and spontaneously transformed mesothelial cells were cultured from limiting dilution in semisolid medium for 28 days. Colonies were stained with crystal violet and counted manually (>5 cells per colony) using a light microscope. Results were calculated from triplicate wells of two independent experiments and are displayed as the mean number of colonies per 2 × 104 plated cells ± SEM. *P < 0.05. (b) Single cell suspensions (5 × 106 cells) of spontaneously transformed cell lines were injected subcutaneously into syngeneic (C57Bl/6; n = 4) and athymic (nu/nu; n = 2) mice. Tumour growth after injection of the MTMhp (40) cell line is shown, as it was the only cell line that generated tumours. Mice were monitored weekly for tumour formation for up to 100 days. (c) Hierarchical clustering using mean gene expression data from triplicate MTM, MTMhp and mesothelioma cell lines when compared to normal mesothelial cells.
Spontaneously transformed mouse mesothelial cells had gene expression profiles consistent with those from human malignant mesotheliomas
MTM, MTMhp and mesothelioma cell lines were hybridized to 22 K oligonucleotide arrays in comparison to a reference pool of MTM cells. Biological replicate experiments were performed using three cell lines for each condition. Mean gene expression values from each treatment group were calculated and hierarchical clustering analysis was performed. This demonstrated that the gene expression profile of MTMhp cells was more closely related to malignantly transformed cells than to primary mesothelial cells (Fig. 4c). Clustering did not segregate the three MTMhp cell lines, based on their ability to form tumours in vivo. However, cell lines with common morphology (fibroblastic versus epithelioid) were more closely related by this analysis. Mean gene expression profiles were also compared to an independent gene set of genes deregulated in human mesothelioma specimens, when compared to normal lung and pleural tissues (Gordon et al. 2005). Gene Set Enrichment Analysis was used to determine whether spontaneously transformed cell lines were more closely related to the human mesothelioma phenotype than to normal mesothelial cells. Human mesothelioma microarray data were divided into two separate gene sets for this analysis: up‐regulated (Set 1; 328 genes) and down‐regulated (Set 2; 311 genes). Enrichment of differentially expressed genes in MTM cells, when compared to human mesothelioma gene sets, was expected to be low; therefore, the normalized enrichment score for these cells was used as a baseline. As expected, there was no significant enrichment of genes from the MTM data set in the human mesothelioma gene sets (Table 1). When comparing gene expression profiles of mesothelioma cell lines to the human mesothelioma gene sets, mesothelioma cell lines had a score approximately 2.5 times higher than MTM cells. This was highly significant in the down‐regulated human mesothelioma gene set. MTMhp cell lines were enriched for both up‐ and down‐regulated human mesothelioma gene sets with a score approximately three times that of MTM cells. This strongly suggested that the pattern of gene expression in spontaneously transformed cell lines is more closely related to the fully transformed mesothelioma profile than to normal mesothelial cells.
Table 1.
Gene set enrichment analysis
| Size | MTM versus MTM | MTMhp versus MTM | Mesothelioma versus MTM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | NES | NOM p‐val | FDR q‐val | ES | NES | NOM p‐val | FDR q‐val | ES | NES | NOM p‐val | FDR q‐val | ||
| Gordon up | 108 | 0.13 | 0.41 | 1.000 | 1.000 | 0.47 | 1.28 | 0.021 | 0.047 | 0.40 | 1.10 | 0.190 | 0.229 |
| Gordon down | 74 | 0.18 | 0.54 | 0.998 | 1.000 | 0.60 | 1.61 | 0.000 | 0.000 | 0.48 | 1.31 | 0.017 | 0.032 |
GSEA software was used to determine the enrichment of genes from an external data set representing up‐ or down‐regulated genes in human mesothelioma specimens, when compared to normal lung and pleural tissues. ES, enrichment score; NES, normalized enrichment score (bold); NOM p‐val, uncorrected p‐value; FDR q‐val, false discovery rate and multiple testing correction (q‐value).
Tumour suppressor genes commonly deleted in human mesotheliomas were down‐regulated in the MTMhp (40) cell line
Extent of RNA expression of p15INK4b, p16INK4a, p19Arf p21Cip1, p53 and NF2 in MTMhp and MTM cells, was extracted from microarray data (Fig. 5). The malignant spontaneously transformed cell line MTMhp (40) showed low levels of p16INK4a and p21Cip1 expression when compared to normal mesothelial cells and the MTMhp (41, 42) cell lines. This is consistent with a loss of p16INK4a and p21Cip1 expression being an important step in malignant transformation of mesothelial cells. All of the MTMhp cell lines demonstrated lower levels of p15INK4b expression when compared to normal mesothelial cells, while the expression of p19Arf, p53 and NF2 was similar or increased.
Figure 5.
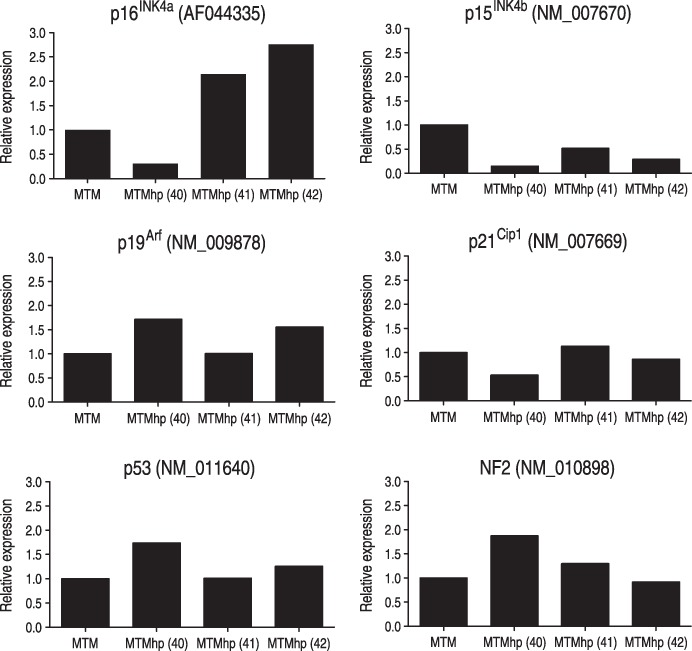
Gene expression data of selected tumour suppressor genes in MTMhp cells relative to normal mesothelial cells. Data from a single probe are shown for each gene.
DISCUSSION
Human mesothelioma develops in the presence of asbestos fibres over a period of around 20 years and, therefore, the earliest stages of transformation are difficult to study in vivo. In this report, we describe an in vitro cell culture system that we have used to analyse phenotypic changes associated with mesothelial cell transformation. Spontaneous transformation of primary cells was an unexpected finding and we have attempted to characterize these cells. We used expression profiling to compare molecular changes that accompany spontaneous in vitro transformation to those that occur in the generation of mesotheliomas in the intact animal. Finally, we performed a meta‐analysis in which these differences were compared to expression profiles of human mesotheliomas.
In humans, mesotheliomas are commonly diagnosed using immunohistochemical techniques, although there is still some disagreement about the specificity of this approach (Robinson et al. 2005a). Nevertheless, there are several antibodies (including calretinin and cytokeratin 5) that are commonly used for the identification of human mesothelial cells; these antibodies differentiate mesothelial cells from fibroblasts and epithelial cells. Unfortunately, these antibodies are not cross‐reactive with mouse mesothelial cells and to our knowledge there are no antibodies for specific identification of mouse mesothelial cells. These cells can only be identified by TEM, by the presence of long, thin microvilli at the cell surface. This definitive characteristic of mesothelial cells distinguishes them from fibroblasts, which are a common contaminant of peritoneal cell cultures. In the current study, we found better than 95% purity of mesothelial cells after approximately three passages (five population doublings) of the in vitro culture.
Mesothelial cells grown from peritoneal cultures had either epithelial or fibroblastic‐like morphology, with random distribution of a few senescing cells. Senescing cells in these cultures are likely to be the result of telomere‐independent replicative senescence that is thought to be due to culture of cells in suboptimal conditions (Ramirez et al. 2001). Murine mesothelial cells constitutively express the enzyme telomerase and, therefore, do not undergo crisis due to shortening of telomeres to a critical length (Sherr & Depinho 2000). The majority of cells continued to proliferate with serial passage, although doubling times progressively increased. By passage 6 (~9 population doublings), most cells had changed morphology, displaying typical characteristics of cell senescence. We found that senescing mesothelial cells remained viable in culture for several weeks. However, if these cultures were maintained at approximately 90–100% confluence, small isolated populations of proliferating cells emerged. These cells were pooled to generate high passage mesothelial cell lines.
Three independently derived cell lines had morphological changes, displayed partial resistance to contact inhibition and a significantly increased rate of proliferation when compared to parental low passage cell lines. These cell lines also grew in low serum conditions. Spontaneous transformation of them has, therefore, led to morphological and proliferative changes that correlate with their loss of dependence on growth factors and other serum components for continued growth in vitro. The three cell lines also demonstrated increased DNA content with no cells retaining a normal diploid profile. Aneuploidy is not a specific feature of spontaneously transformed cell lines, although it has been known for many years that transformed cells can change from a diploid to aneuploid karyotype over time in culture (Todaro & Green 1963). Low passage mesothelial cells display a predominantly diploid profile, but a small proportion of cells are aneuploid even at early stages of culture, probably due to inherent genetic instability of rodent cells.
Spontaneously transformed cell lines may change morphology, lose dependence on growth factors and undergo karyotypic changes; however, they generally retain features of primary cells, such as contact inhibition and anchorage‐dependent growth. Therefore, to assess whether high passage mesothelial cell lines had spontaneously advanced towards neoplastic transformation, we assessed their ability to grow in anchorage‐independent conditions. This assay was considered to be the closest in vitro model to tumour formation. All three cell lines were able to form colonies when grown in soft agarose, but only the MTMhp (40) cell line was able to form tumours after subcutaneous injection into syngeneic and immunocompromised mice. Therefore, this cell line had undergone spontaneous malignant transformation. This was consistent with the ultrastructural findings, showing that MTMhp (40) cells were not as well differentiated as mature mesothelial cells.
An intriguing possibility that the development of senescence contributes to the genesis of malignant change is suggested by the work of Krtolica et al. (2001). The authors found that fibroblast feeder layers made up of as few as 10% senescing cells were able to promote premalignant and malignant, but not normal, epithelial cells to proliferate in culture and form tumours in mice. It is not clear from our study whether the presence of senescing cells contributes to spontaneous mesothelial cell transformation, but it is possible that our normal mesothelial cell lines had sufficient genomic instability to undergo spontaneous immortalization before senescing cells conferred growth advantage and malignant changes in these cultures.
Using gene expression profiling, we have shown that these cell lines are more closely related to malignantly transformed cells than to normal mesothelial cells. This implies that induced molecular and genetic changes that lead to malignant transformation of mesothelial cells in vivo are similar to changes that occur in spontaneous transformation in vitro. Gene set enrichment analysis also demonstrated that deregulated genes in both the malignant and spontaneously transformed cell lines were enriched in gene sets modelled from human mesotheliomas. Human mesotheliomas generally show features of aneuploidy and have discrete structural rearrangements and deletions in chromosomal material, suggestive of a recessive mechanism of oncogenesis (Murthy & Testa 1999). We have shown that spontaneously and malignantly transformed cell lines display a more significant enrichment of genes that are down‐regulated in human mesotheliomas, which suggests that these genes may be key players in the transformation process. The p16INK4a gene is a known tumour suppressor in mesothelioma and is deleted in up to 75% of these tumours (Illei et al. 2003). It is notable that p16INK4a gene expression is lost in the spontaneously transformed line with the malignant phenotype.
These studies highlight the dramatic phenotypic and genotypic changes that occur in rodent mesothelial cells as they bypass senescence. The potential for contamination of transformation studies with spontaneously transformed cells is an important consideration, suggesting that great care should be exercised when interpreting in vitro studies.
ACKNOWLEDGEMENTS
We thank Dr. Pierre Filion and Dr. Robert Cook from the Division for Tissue Pathology, PathWest Laboratory Medicine WA, Perth, Australia, for the TEM. This work was supported by grants from the Western Australian Institute for Medical Research, the Insurance Commission of Western Australia and the National Centre for Asbestos Related Diseases, Australia.
REFERENCES
- Bot J, Whitaker D, Vivian J, Lake RA, Yao V, McCauley R (2003) Culturing mouse peritoneal mesothelial cells. Pathol. Res. Pract. 199, 341–344. [DOI] [PubMed] [Google Scholar]
- Cahill DP, Kinzler KW, Vogelstein B, Lengauer C (1999) Genetic instability and darwinian selection in tumours. Trends Cell Biol. 9, M57–M60. [PubMed] [Google Scholar]
- Davis T, Kipling D (2005) Telomeres and telomerase biology in vertebrates: progress towards a non‐human model for replicative senescence and ageing. Biogerontology 6, 371–385. [DOI] [PubMed] [Google Scholar]
- Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW (1992) Establishment of a murine model of malignant mesothelioma. Int. J. Cancer 52, 881–886. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira‐Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc. Natl. Acad. Sci. USA 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann UK, Osborn RC, Guzman RC, Fajardo LF (1991) Cultured proliferating rat mammary epithelial cells. In Vitro Cell. Dev. Biol. 27A, 749–754. [DOI] [PubMed] [Google Scholar]
- Funaki K, Everitt J, Bermudez E, Walker C (1991) Trisomy of rat chromosome 1 associated with mesothelial cell transformation. Cancer Res. 51, 4059–4066. [PubMed] [Google Scholar]
- Gordon GJ, Rockwell GN, Jensen RV, Rheinwald JG, Glickman JN, Aronson JP, Pottorf BJ, Nitz MD, Richards WG, Sugarbaker DJ, Bueno R (2005) Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large‐scale transcriptional profiling. Am. J. Pathol. 166, 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Huggett AC, Ellis PA, Ford CP, Hampton LL, Rimoldi D, Thorgeirsson SS (1991) Development of resistance to the growth inhibitory effects of transforming growth factor beta 1 during the spontaneous transformation of rat liver epithelial cells. Cancer Res. 51, 5929–5936. [PubMed] [Google Scholar]
- Illei PB, Rusch VW, Zakowski MF, Ladanyi M (2003) Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin. Cancer Res. 9, 2108–2113. [PubMed] [Google Scholar]
- Kaighn ME, Camalier RF, Bertolero F, Saffiotti U (1988) Spontaneous establishment and characterization of mouse keratinocyte cell lines in serum‐free medium. In Vitro Cell. Dev. Biol. 24, 845–854. [DOI] [PubMed] [Google Scholar]
- Ke Y, Reddel RR, Gerwin BI, Reddel HK, Somers AN, McMenamin MG, Laveck MA, Stahel RA, Lechner JF, Harris CC (1989) Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos‐induced mesothelioma. Am. J. Pathol. 134, 979–991. [PMC free article] [PubMed] [Google Scholar]
- Kittrell FS, Oborn CJ, Medina D (1992) Development of mammary preneoplasias in vivo from mouse mammary epithelial cell lines in vitro . Cancer Res. 52, 1924–1932. [PubMed] [Google Scholar]
- Kravchenko IV, Furalyov VA, Vasylieva LA, Pylev LN (1998) Spontaneous and asbestos‐induced transformation of mesothelial cells in vitro . Teratog. Carcinog. Mutagen. 18, 141–151. [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA 98, 12072–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SS, Testa JR (1999) Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell. Physiol. 180, 150–157. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE (2004) The mesothelial cell. Int. J. Biochem. Cell Biol. 36, 9–16. [DOI] [PubMed] [Google Scholar]
- Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, Wright WE (2001) Putative telomere‐independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15, 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel RR, Malan‐Shibley L, Gerwin BI, Metcalf RA, Harris CC (1989) Tumorigenicity of human mesothelial cell line transfected with EJ‐ras oncogene. J. Natl. Cancer Inst. 81, 945–948. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Lake RA (2005) Advances in malignant mesothelioma. N. Engl. J. Med. 353, 1591–1603. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Musk AW, Lake RA (2005) Malignant Mesothelioma. Lancet 366, 397–408. [DOI] [PubMed] [Google Scholar]
- Sedivy JM (1998) Can ends justify the means? telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA 95, 9078–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Depinho RA (2000) Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Suzuki M, Ikawa Y, Aizawa S (1987) Mouse embryonic stem cells exhibit indefinite proliferative potential. J. Cell. Physiol. 133, 197–201. [DOI] [PubMed] [Google Scholar]
- Todaro GJ, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, Gatewood JW, McCormack JM, Walker WS (1991) Immortalization of growth factor‐dependent mouse splenic macrophages derived from cloned progenitors. J. Immunol. Methods 137, 17–25. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon‐Cardo C, Lowe SW (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]


