Summary
Aims
Neurodegenerative disorders are caused by progressive neuronal loss in the brain, and hence, compounds that could promote neuritogenesis may have therapeutic values. In this study, the effects of bis(heptyl)‐cognitin (B7C), a multifunctional dimer, on neurite outgrowth were investigated in both PC12 cells and primary cortical neurons.
Methods
Immunocytochemical staining was used to evaluate the proneuritogenesis effects, and Western blot and short hairpin RNA assays were applied to explore the underlying mechanisms.
Results
B7C (0.1–0.5 μM) induced robust neurite outgrowth in PC12 cells, as evidenced by the neurite‐bearing morphology and upregulation of growth‐associated protein‐43 expression. In addition, B7C markedly promoted neurite outgrowth in primary cortical neurons as shown by the increase in the length of β‐III‐tubulin‐positive neurites. Furthermore, B7C rapidly increased ERK phosphorylation. Specific inhibitors of alpha7‐nicotinic acetylcholine receptor (α7‐nAChR) and MEK, but not those of p38 or JNK, blocked the neurite outgrowth as well as ERK phosphorylation induced by B7C. Most importantly, genetic depletion of α7‐nAChR significantly abolished B7C‐induced neurite outgrowth in PC12 cells.
Conclusion
B7C promoted neurite outgrowth through the activation of α7‐nAChR/ERK pathway, which offers novel insight into the potential application of B7C in the treatment of neurodegenerative disorders.
Keywords: Alpha7‐nicotinic acetylcholine receptor, Bis(heptyl)‐cognitin, Extracellular signal‐regulated kinase, Neurite outgrowth, Neurodegenerative disorders
Introduction
The progressive neuronal loss in specific regions of the brain is regarded as the main cause of neurodegenerative disorders 1, 2, including Alzheimer's, Parkinson's, and Huntington's diseases. Among these, Alzheimer's disease (AD) is the most common form characterized by amyloid plaques and neurofibrillary tangles in the brain. Neuroprotection against neurotoxins in combination with neuritogenesis induced by neurotrophic factors that allow for the replacement of lost neurons have been proposed as a potential therapeutic strategy for these neurodegenerative diseases. However, therapeutic application of neurotrophic factors (e.g. nerve growth factor, NGF) is severely restricted by their poor penetration through the blood‐brain barrier (BBB) and the undesirable apoptotic effect through interaction with the p75NTR receptor. The identification of small molecules that mimic the neurotrophic action to promote neuritogenesis at the impaired sites may have therapeutic values against these devastating neurodegenerative diseases.
The rat pheochromocytoma cell line PC12 is widely used as an in vitro model that differentiates into a neuronal type with extended outgrowth of neurites in response to NGF. The binding of NGF to tyrosine kinase A (TrkA) receptor triggers a canonical signaling cascade: Raf→mitogen‐activated protein kinase kinase (MAPKK) →extracellular signal‐regulated kinase (ERK) 3. Recent studies have also indicated the involvement of alpha7‐nicotinic acetylcholine receptor (α7‐nAChR) in neuritogenesis 4, 5. Upon α7‐nAChR activation, PC12 cells display a neuron‐like morphology and upregulate ERK phosphorylation 4, 6, while α7‐nAChR knockout may result in a lack of maturation of dendritic neurons in hippocampus 7. Therefore, α7‐nAChR is essential for neuritogenesis and is considered as an important therapeutic target for neurodegenerative disorders.
Cortical neurons constitute the brain's largest region, including the two hemispheres of the cerebral cortex that are commonly affected by AD and other related neurodegenerative diseases. Most of the complex activities of the brain that enable thinking, perception, and voluntary movement are connected to the activity of these neurons. The primary cortical neurons have therefore been used extensively to study developmental or pathological neurobiology, particularly regarding neurite outgrowth 8, 9, 10. Hence, primary cortical neurons are utilized as the paradigm in our study.
Bis(heptyl)‐cognitin (B7C) (Figure 1A), a dimeric tacrine analog linked by 7 methylene groups in our laboratory, has been demonstrated as a promising neuroprotectant against neurodegenerative disorders on the basis of its inhibitory effect on acetylcholinesterase (AChE) 11, neuronal nitric oxygen synthase (nNOS), and N‐methyl‐D‐aspartate (NMDA) receptor 12. Moreover, B7C was reported to reduce scopolamine and middle cerebral artery occlusion‐induced brain damage in rats 13. Most encouragingly, B7C is highly lipophilic and can readily cross the BBB 14, suggesting that B7C has the potential to be developed as a central nervous system drug. However, there is no knowledge about its neuritogenesis activity. In this study, we extended our effort in evaluating the effects and underlying mechanisms of B7C on neuritogenesis in both PC12 cells and primary cortical neurons, and examining whether B7C could prevent the reduction of neurite length in differentiated PC12 cells when induced by Aβ, a neurotoxin associated with AD. Our results provided novel molecular insight into the potential of B7C in the treatment of AD and other related neurodegenerative disorders.
Figure 1.
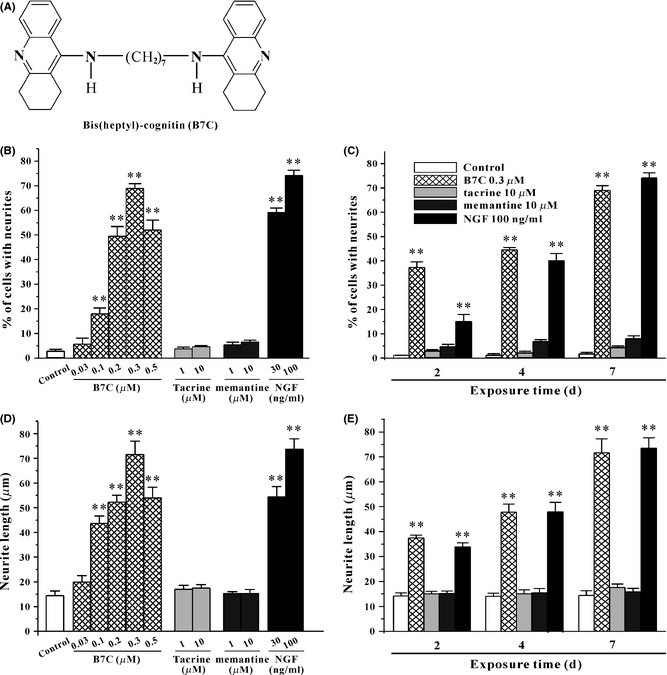
B7C robustly induces neurite outgrowth in PC12 cells. (A) The structure of B7C. (B, D) B7C dose‐dependently induced a robust neurite outgrowth in PC12 cells. 24 h after seeding, cell were incubated in low‐serum medium containing various compounds for 7 days, and then the percentage of cells with neurites as well as the neurite length for identified neurite‐bearing cells was quantified using Image J software. **P < 0.01, compared to control group. (C, E) B7C time‐dependently induced neurite outgrowth in PC12 cells. 24 h after seeding, cells were incubated in low‐serum medium containing 0.3 μM B7C, 10 μM tacrine, 10 μM memantine, or 100 ng/mL NGF for different lengths of time (2, 4, or 7 days), and the percentage of cells with neurites was then calculated and the neurite length measured. **P < 0.01, compared to control group.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM), horse serum (HS), and fetal bovine serum (FBS) were obtained from Gibco (Carlsbad, CA, USA). PD98059, U0126, SP600125, atropine, mecamylamine, methyllycaconitine (MLA), K252a, and SB203580 were purchased from Calbiochem (San Diego, CA, USA). 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), PNU‐120596, tacrine, and tubocurarine were from Sigma Chemicals (St Louis, MO, USA). Purified synthetic Aβ1‐42 was from GL Biochem (Shanghai, China). Recombinant nerve growth factor (NGF) was from R&D Systems (Minneapolis, MN, USA). Antibodies against phospho‐ERK, ERK, βIII‐tubulin were from Cell Signaling Technology (Cell Signaling Technology Inc, Beverly, MA, USA). Antibodies against growth‐associated protein (GAP‐43), α7‐nAChR, and β‐actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of Aβ1‐42
Aβ1‐42 peptide preparation was carried out as we previously reported 15. Briefly, the lyophilized Aβ was dissolved in 100% 1,1,1,3,3,3,‐hexafluoro‐2‐propanal (HFIP) to produce monomeric Aβ, sonicated in a water bath for 10 min, and subsequently stored in small aliquots at −80°C. The HFIP‐treated monomeric Aβ was then dried in a fume hood, dissolved in sterilized distilled water, and aged by incubating at 37°C for 6 days.
PC12 Cell Culture
PC12 cells were maintained in DMEM containing 6% FBS, 6% HS, and 2 mM glutamine at 37°C under an atmosphere of water‐saturated 5% CO2.
PC12 Cell Viability
PC12 cell viability was examined by MTT reduction assay as we previously described 16. Briefly, after various treatments, MTT solution was added to cells in each well in 96‐well plates at a final concentration of 5 mg/mL, and the plates were then incubated at 37°C for 4 h. All culture medium was then removed from the cells, and the formazan produced by live cells was resuspended in 150 μL DMSO. Cell viability was evaluated by observing colorimetric changes using a Bio‐Rad Microplate Reader (Model 680, Bio‐Rad Laboratories, Hercules, CA, USA) at a test wavelength of 570 nm with 655 nm as a reference wavelength. Data were expressed as a percentage of untreated control cultures.
Assay of Neurite Outgrowth in PC12 Cells
The quantification of neurite‐bearing cells was carried out as previously described 17 with minor modifications. For the study of neuritogenesis‐promoting activity of various compounds, PC12 cells were seeded onto 6‐well plates at a density of 1 × 104 cells/well. 24 h after incubation, cells were switched to low‐serum medium (DMEM supplemented with 0.5% FBS and 0.5% HS) containing various compounds that were renewed every 2 days. For the study of neuroprotective effects of B7C, PC12 cells were differentiated with 100 ng/mL NGF in low‐serum medium for 7 days. Cells were then washed twice with phosphate‐buffered saline to remove NGF and replaced with 10 μM aged Aβ with or without B7C for 2 days. Pre‐incubation with B7C (0.1, 0.3, 0.5 μM) was conducted 2 h before Aβ addition. After treatment, neurite‐bearing PC12 cells were observed and photographed using a light microscope equipped with a phase‐contrast condenser, a 10 × objective lens and a digital camera. Cells were scored as positive for neurite outgrowth if at least one neurite was longer than the diameter of the cell body. The neurite length was measured for all identified neurite‐bearing cells in a field by tracing the longest length of neurite per cell using Image J software. Approximately 300 cells in 6 randomly chosen visual fields were counted.
Primary Culture of Cortical Neurons and Drug Treatment
Primary rat cortical neurons were prepared from 18‐day‐old SD rat embryos as we previously described 18. Briefly, the freshly dissociated cortical neurons were seeded onto 35‐mm petri dishes at a density of 150 cells/mm2 in neurobasal medium supplemented with 10% FBS and 0.5 mM glutamine. 24 h after plating, half‐medium was removed and replaced with neurobasal medium containing 1% B27 and 0.25 mM glutamine. At 3 days in vitro (DIV), cortical neurons were incubated with B7C for 2 days.
Immunocytochemical Staining
After incubation for 2 days, cortical neurons were fixed in 4% paraformaldehyde containing 10% sucrose and 15 μg/mL Hoechst 33342 for 20 min. After blocking at room temperature in blocking buffer (0.5% bovine serum albumin, 0.1% Triton X‐100, and 5% goat serum) for 1 h, neurons were exposed to mouse anti‐βIII‐tubulin antibody (Cell Signaling Technology) at 4°C overnight, followed by addition of anti‐mouse secondary antibody for 1 h. The extended neurites were visualized using a fluorescence microscope (Nikon Instruments Inc., Melville, NY, USA).
α7‐nAChR Knockdown
To reduce endogenous α7‐nAChR expression, short hairpin RNA (ShRNA) was performed as we previously reported 19. A pGPU6‐GFP‐neo ShRNA expression vector containing DNA oligonucleotides (21 bp) specially targeting sequences (5′‐GCAGTGCAAACTGAAGTTTGG‐3′) of rat α7‐nAChR (GenePharma, Shanghai, China) was transiently transfected into PC12 cells. Briefly, 24 h after seeding, PC12 cells were transfected with the plasmids in serum‐free DMEM for 6 h using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) at the ratio of 1:2–2.5 (plasmids: liposome). Thereafter, the culture medium was removed and the cells were incubated in DMEM containing 6% FBS, 6% HS, and 100 μg/mL G418 for 24 h. B7C was then added into cultures for 2 days.
Neuronal Imaging and Analysis
Images were captured by a Nikon ECLIPSE Ti‐U microscope (Nikon Instruments Inc) at ×200 magnifications. The ratio of neurite length to the number of neuron was measured using NeuriteTracer, a neurite tracing plugin for the image‐processing program Image J as previously described 20. Briefly, given a directory containing pairs of images corresponding to nuclear (Hoechst 33342) and neurite marker (βIII‐tubulin) images, the plugin opened the images in a nuclear and neuronal stack. Images were first preprocessed to optimize uniformity of illumination and contrast in the input images. These preprocessing steps were performed on a small subset of images to allow user to choose the threshold to be applied to the neuronal images. The plugin then cued the user to input the threshold and image scale, and the remaining images were processed. Following preprocessing, the images were processed by thresholding. Neuronal nuclei were selected using “Image Calculator” command to identify pixels that were present in both the nuclear and neuronal marker images. The neuronal stack was then skeletonized. Six or more randomly captured images were analyzed for each group.
Western Blotting
Western blotting assay was carried out as we previously described 16. Briefly, after treatment, cells were harvested and lysed. The resultant proteins collected by centrifugation were separated on a 12% SDS‐PAGE gel and then transferred to polyvinylidene fluoride membranes. The membranes were immersed in blocking buffer (5% nonfat milk in Tris‐buffer saline/0.1% Tween‐20) for 2 h, followed by addition of anti‐phospho‐ERK, anti‐ERK, anti‐GAP‐43, anti‐α7‐nAChR, and β‐actin antibodies overnight at 4°C. After 3 washes with PBS, the membranes were probed with secondary antibodies, developed with an ECL plus kit (Thermo Scientific, Rockford, IL, USA), and finally exposed to autoradiographic film.
Statistical Methods
Data were shown as mean ± SEM of three independent experiments. Analysis of variance with Dunnett’ s test was employed to test for the differences. P < 0.05 or less was considered to be statistically significant.
Results
B7C, but not Tacrine or Memantine, Induces Robust Neurite Outgrowth in PC12 Cells
To examine the proneurogenesis effect, PC12 cells were incubated for different lengths of time with various compounds that were renewed every 2 days. PC12 cells exposed to NGF differentiated into neurite‐bearing morphology (Figure 2A), and the percentage of cells with neurites after 7 days of incubation with NGF was up to 74.0 ± 2.2% (Figure 1B and C), which is in consistent with findings of several previous studies 6, 21. B7C induced robust neurite outgrowth in PC12 cells at concentrations of 0.1–0.5 μM that did not affect cell viability (Figure 4A), and with an efficacy similar to that of NGF (Figure 1B and C), while tacrine (an AChE inhibitor) and memantine (an NMDA receptor antagonist) did not show any effect on neurite outgrowth. The percentages of neurite‐bearing cells after 2 days, 4 days, and 7 days of incubation with 0.3 μM B7C markedly reached to 37.3 ± 2.3%, 44.6 ± 0.9%, and 68.9 ± 2.0%, respectively (Figure 1C). In addition, the maximal neurite length also markedly increased to 71.6 ± 5.5 μm in cells treated with B7C (Figure 1D and E), which was compatible with those treated with 100 ng/mL NGF (73.6 ± 4.2) μm and significantly longer than those of the control (14.4 ± 1.9 μm).
Figure 2.
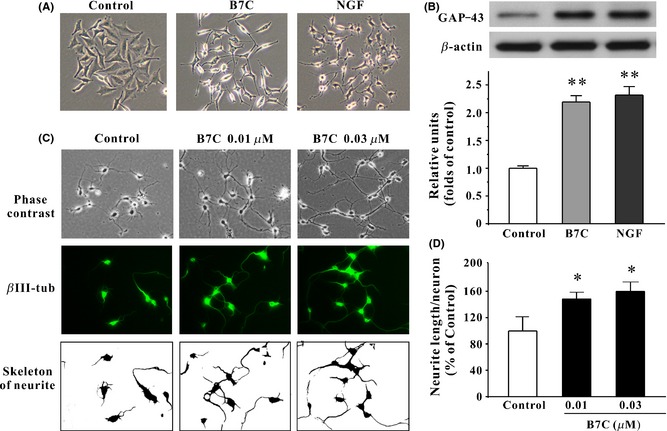
Morphological analysis of neurite outgrowth in PC12 cells and primary cortical neurons induced by B7C. (A) B7C induced PC12 cells differentiation toward a neuronal phenotype. 24 h after seeding, PC12 cells were incubated in low‐serum medium containing 0.3 μM B7C or 100 ng/mL NGF for 7 days, and then, cell morphology was observed using a phase‐contrast microscopy. (B) B7C upregulated the levels of GAP‐43 in PC12 cells. PC12 cells were incubated with compounds shown in (A), and the extracted proteins were subjected to Western blotting analysis using anti‐GAP‐43 and anti‐β‐actin antibodies. **P < 0.01, compared to control group. (C) At 3 DIV, cortical neurons were incubated in the absence or presence of B7C for 2 days. Upper: Phase‐contrast pictures of cortical neurons were obtained using a phase‐contrast microscope. Middle: Neurons were stained with neuronal marker βIII‐tubulin. Lower: βIII‐tubulin‐positive neurites were digitally identified and skeletonized for examination by NeuriteTracer program. **P < 0.01, compared to control group. (D) The calculation of neurite length/neuron stained by βIII‐tubulin as in (C). *P < 0.05, compared to control group.
Figure 4.
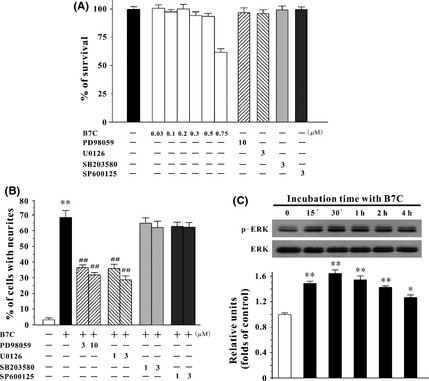
B7C induces neurite outgrowth in PC12 cells by activating ERK pathway. (A) B7C and the selected specific inhibitors, at their effective concentrations, did not affect PC12 cell viability. PC12 cells were incubated with B7C and various inhibitors for 7 days, then subjected to MTT assay. (B) ERK inhibitors, but not p38 or JNK inhibitors, markedly attenuated the neurite outgrowth in PC12 cells induced by B7C. 24 h after plating, cells were pretreated with PD98059 (3, 10 μM), U0126 (1, 3 μM), SB203580 (1, 3 μM) or SP600125 (1, 3 μM) for 2 h, and then exposed to 0.3 μM B7C for 7 days. The percentage of cells with neurites was calculated. **P < 0.01, compared to control group, ## P < 0.01, compared to B7C group. (C) B7C remarkably upregulated the levels of p‐ERK in PC12 cells. Cells were incubated with 0.3 μM B7C in low‐serum medium for different lengths of time, and the extracted proteins were subjected to Western blotting analysis using anti‐p‐ERK and anti‐ERK antibodies. *P < 0.05 and **P < 0.01, compared to control group.
In addition to morphological evaluation, neuritogenesis can also be measured by a biochemical method in determining the expression of the neuronal marker GAP‐43, which is found almost exclusively in neurons. The levels of GAP‐43 in PC12 cells after 7 days of incubation with 0.3 μM B7C and 100 ng/mL NGF reached approximately 2.2‐ and 2.3‐fold higher than that observed in the control group, respectively (Figure 2B).
B7C Markedly Promotes Neurite Outgrowth in Primary Cortical Neurons
To further confirm the neuritogenesis of B7C, the primary cortical neurons were used in our study. It was found that 2 days of incubation with B7C (0.01 and 0.03 μM) remarkably promoted the outgrowth of neurites in primary cortical neurons, as evidenced by the increase in the length of neurites stained by βIII‐tubulin (Figure 2C and D).
B7C Effectively Blocks Neuronal Loss in PC12 Cells Induced by Aβ
To better understand the potential application of B7C in the treatment of AD, we introduced a cell model in which cytotoxicity was induced in differentiated PC12 cells by Aβ. As shown in Figure 3A, B7C efficiently reversed the morphological changes caused by 2 days of Aβ challenge, including unhealthy cell bodies and broken neuritic network. Moreover, B7C (0.1–0.5 μM) markedly prevented the decrease in cell viability and reduction of neurite length in PC12 cells (Figure 3B and C).
Figure 3.
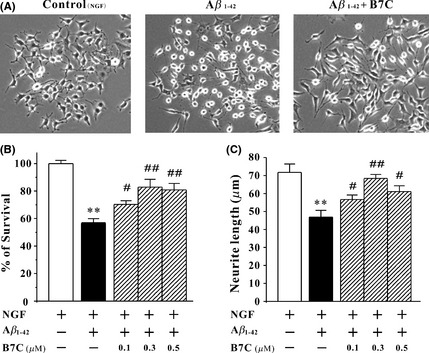
B7C provides neuroprotection against Aβ in differentiated PC12 cells. (A) B7C reversed the morphological changes in PC12 cells induced by Aβ. Differentiated PC12 cells were pretreated with B7C (0.3 μM) for 2 h and then incubated with aged Aβ1‐42 for 2 days, and cell morphology was then observed using a phase‐contrast microscope. (B) B7C markedly increased PC12 cell viability. Differentiated PC12 cells were pretreated with B7C (0.3 μM) for 2 h, incubated with aged Aβ1‐42 for 2 days, and then subjected to MTT assay. **P < 0.01, compared to control group. # P < 0.05; ## P < 0.01, compared to Aβ1‐42 group. (C) B7C reversed the shortening of neurite length caused by Aβ1‐42. Differentiated PC12 cells were pretreated with B7C (0.3 μM) for 2 h, then incubated with aged Aβ1‐42 for 2 days. The neurite length was measured using Image J software. **P < 0.01, compared to control group. # P < 0.05; ## P < 0.01, compared to Aβ1‐42 group.
B7C Promotes Neurite Outgrowth by Activating ERK Pathway in PC12 Cells
It is widely accepted that activation of mitogen‐activated protein kinase family (ERK, p38, JNK) is a critical underlying mechanism in the mediating of neuritogenesis. To examine whether these pathways are involved in B7C‐induced neurite outgrowth, PC12 cells were pretreated for 2 h with specific inhibitors including MEK1/2 inhibitors (PD98059 and U0126), p38 inhibitor (SB203580), and JNK inhibitor (SP600125) and then incubated with 0.3 μM B7C for 7 days. The selected specific inhibitors, at their effective concentrations, did not affect PC12 cell viability (Figure 4A). It was evident that PD98059 (3, 10 μM) and U0126 (1, 3 μM), but not SB203580 or SP600125, significantly blocked the neurite outgrowth‐promoting activity induced by B7C in PC12 cells (Figure 4B).
To further confirm that B7C promoted neuritogenesis by activating ERK signaling pathway, the expression of phospho‐ERK was evaluated by Western blotting analysis. It was observed that the elevated level of phospho‐ERK occurred at 15 min, peaked at 30, min and returned to the basal level at 4 h after B7C treatment (Figure 4C).
Pharmacological Blockage of α7‐nAChR Blocks the Neurite Outgrowth and ERK Activation Induced by B7C in PC12 Cells
It has been demonstrated that agonists of TrkA and acetylcholine receptors (AChRs) could promote neuritogenesis by activating ERK pathway 3, 5. To examine which receptor was involved in B7C‐induced neurite outgrowth, PC12 cells were pretreated for 2 h with specific inhibitors of TrkA (K252a, 0.1 and 0.3 μM), muscarinic AChR (mAChR) (atropine, 10 μM), and nicotinic AChR (nAChR) (tubocurarine and mecamylamine, 10 μM) and then incubated with 0.3 μM B7C. The selected specific inhibitors, at their effective concentrations, did not affect PC12 cell viability (Figure 5C). It was found that tubocurarine and mecamylamine, but not K252a or atropine, significantly attenuated the neuritogenesis‐promoting activity induced by B7C (Figure 5A and B). Furthermore, MLA, which is a specific α7‐nAChR antagonist, partially abolished B7C‐induced neurite outgrowth in PC12 cells (Figure 5B and D). Consistently, pretreatment with MLA and PD98059 abrogated the elevated phospho‐ERK induced by B7C (Figure 5E). To confirm the role of activation of α7‐nAChR in neurite outgrowth, PNU‐120596, a potent positive allosteric modulator (PAM) of α7‐nAChR, was used in our cell model. It was found that PNU‐120596 effectively promoted neurite outgrowth in PC12 cells (Figure 5B and D).
Figure 5.
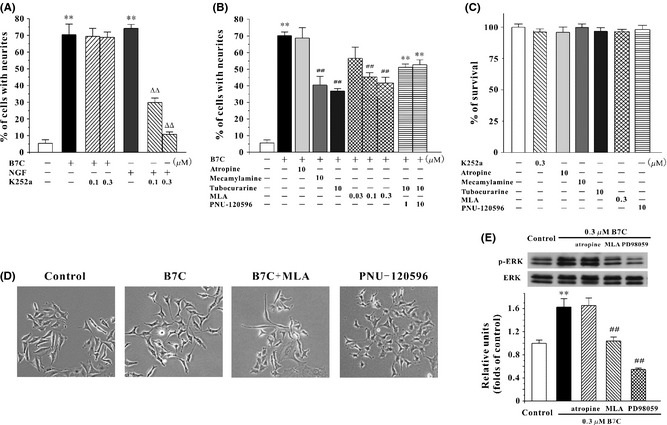
Pharmacological blockage of α7‐nAChR partially abolishes the neurite outgrowth as well as ERK activation in PC12 cells induced by B7C. (A) B7C exerted proneuritogenesis activity in PC12 cells through a TrkA‐independent pathway. Cells were pretreated with K252a (0.1, 0.3 μM) for 2 h before the addition of 0.3 μM B7C or 100 ng/mL NGF. 7 days after treatment, the percentage of cells with neurites was calculated. **P < 0.01, compared to control group; ∆∆ P < 0.01, compared to NGF group. (B) Effects of α7‐nAChR antagonist and PAM on neurite outgrowth in PC12 cells. For the study of the effects of α7‐nAChR antagonist on the neurite outgrowth induced by B7C, cells were pretreated with atropine (10 μM), mecamylamine (10 μM), tubocurarine (10 μM), or MLA (0.03, 0.1, 0.3 μM) for 2 h before the addition of 0.3 μM B7C. 7 days after treatment, the percentage of cells with neurites was calculated. For the study of neuritogenesis‐promoting activities of α7‐nAChR PAM, cells were incubated in low‐serum medium containing PNU‐120596 (1 or 10 μM) for 7 days, and then, the percentage of cells with neurites was quantified using Image J software. **P < 0.01, compared to control group. (C) The selected inhibitors and α7‐nAChR PAM, at their effective concentrations, did not affect PC12 cell viability. PC12 cells were incubated with various inhibitors or PNU‐120596 for 7 days and then subjected to MTT assay. (D) Morphological characteristics of neurite outgrowth in PC12 cells treated with α7‐nAChR antagonist and PAM. Cells were pretreated with MLA for 2 h in low‐serum medium and then incubated with 0.3 μM B7C for 7 days. Or cells were incubated with 10 μM PNU‐120596 for 7 days. After drug treatment, cell morphology was observed using a phase‐contrast microscopy. (E) α7‐nAChR antagonist significantly attenuated ERK activation induced by B7C in PC12 cells. Cells were pretreated with atropine (10 μM), MLA (0.3 μM) and PD98059 (10 μM) for 2 h before the addition of 0.3 μM B7C. 30 min after treatment, the extracted proteins were subjected to Western blotting analysis using anti‐p‐ERK and anti‐ERK antibodies. **P < 0.01, compared to control group. ## P < 0.01, compared to B7C group.
Genetic Depletion of α7‐nAChR Abolishes the Neurite Outgrowth in PC12 Cells Induced by B7C
To confirm the involvement of α7‐nAChR in the proneuritogenesis activity of B7C, short hairpin (ShRNA)‐mediated α7‐nAChR depletion was performed. Inhibition of α7‐nAChR by ShRNA (Shα7‐nAChR) caused an approximate 50% decrease in the expression of endogenous α7‐nAChR in PC12 cells. B7C was no longer able to induce neurite outgrowth in PC12 cells where endogenous α7‐nAChR was inhibited, in contrast to the unchanged neuritogenesis‐promoting activity observed in the negative control ShRNA (ShNC) or the vector‐treated PC12 cells (Figure 6).
Figure 6.
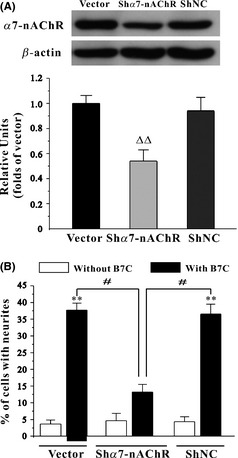
Genetic depletion of α7‐nAChR partially abolishes the neurite outgrowth in PC12 cells induced by B7C. (A) PC12 cells were transiently transfected with pGPU6‐GFP plasmid (vector), pGPU6‐GFP plasmid encoding α7‐nAChR ShRNA (Shα7‐nAChR), or pGPU6‐GFP plasmid encoding negative control ShRNA (ShNC). The extracted proteins were subjected to Western blotting analysis using anti‐α7‐nAChR and anti‐β‐actin antibodies. (B) α7‐nAChR depletion abolished the neuritogenesis‐promoting activity in PC12 cells induced by B7C. PC12 cells transfected with vector or Shα7‐nAChR were incubated with 0.3 μM B7C for 2 days, and the percentage of cells with neurites was calculated. ∆∆ P < 0.05, compared to vector group; **P < 0.01, compared to control group; # P < 0.05, compared to Shα7‐nAChR group.
Discussion
Progressive neuronal loss might be the fundamental mechanism underlying AD and other related progressive neurodegenerative disorders 22, 23. The identification of proneuritogenesis molecules would therefore be a major breakthrough in the treatment of these diseases. Herein, we provided evidence that B7C is a robust neuritogenesis inducer with therapeutic potential.
PC12 cells are widely accepted as a useful model system for the study of neuritogenesis 24. In response to NGF, PC12 cells differentiate into a neuron‐like phenotype, extend long neurites and express neuronal markers including GAP‐43 and βIII‐tubulin. Under a condition of low‐serum medium that is to induce transition from a proliferative phase to differentiation stage, PC12 cells themselves did not sprout neurites. B7C (0.1–05 μM) provided robust proneuritogenesis activity in PC12 cells with an efficacy similar to NGF, as evidenced by the condensed cell body and long and branching neurites (Figure 2A).
In addition to morphological observation, biochemical examination is also an effective method for evaluating neurite outgrowth. GAP‐43 is a neuron‐specific protein that exhibits elevated synthesis and fast axonal transport during neuronal development. Overexpression of GAP‐43 in PC12 cells and transgenic mice was reported to potentiate NGF‐induced neurite outgrowth 25, 26, 27 and induce neuronal sprouting 28, respectively. On the contrary, depletion of GAP‐43 remarkably resulted in shortened neurites, collapsed growth cone, and abnormal axonal pathfinding 29, 30. Thus, GAP‐43 is considered a major and useful indicator of PC12 cell neuritogenesis. In our study, with the assay of Western blotting analysis, the protein expression of GAP‐43 in PC12 cells treated by B7C increased by approximately 100%, an observation comparable to that provided by NGF (Figure 2B), again suggesting that B7C has a robust proneurogenesis effect. Most encouragingly, we further confirmed the neuritogenesis‐promoting activity of B7C in primary cortical neurons, which is an important primary cell model frequently employed in earlier neuritogenesis studies 8, 31. We have selected βIII‐tubulin as our primary marker for cortical neuronal morphology, because it is expressed throughout axons, dendrites, and the cell body and delivers consistently high contrast images that are easily analyzed by ImageJ software. In addition, neurite outgrowth in primary cortical neurons has been extensively evaluated through examination of morphological changes and by immunocytochemistry for βIII‐tubulin 20, 32, 33, 34. Using this primary culture model, we found that B7C markedly accelerated the outgrowth of neurites as shown by the increase in length of βIII‐tubulin‐positive neurites (Figure 2C and D). As suggested by earlier studies 35, 36, neurite outgrowth and guidance to the proper target require the coordination of filamentous actin (F‐actin) and microtubes, the dynamic cytoskeletal polymers that promote shape change and locomotion. Developing neurites that are not yet synaptically connected have highly dynamic, motile structures at their leading edge. These structures are called growth cones that contain F‐actin bundles. It is therefore important to evaluate the length of neurites with the stain of F‐actin bundles. Experiments such as confocal microscopic analysis of phalloidin‐stained F‐actin bundles will be carried out in future studies.
In particular, to better understand the potential application of B7C in the treatment of AD, we introduced a widely used cell model in which cytotoxicity was induced by Aβ in differentiated PC12 cells 37, 38. Using this model, we found that B7C provided neuroprotection against Aβ insult, as evidenced by the reversal of the decrease in cell viability and the shortening of neurite length caused by Aβ (Figure 3). The neuroprotective and neuritogenesis‐promoting effects described herein, together with the inhibitory activities of nNOS and NMDA receptor, may enable B7C to become a promising agent for the treatment of AD, by concurrently acting through multiple mechanisms.
We then explored the mechanisms underlying the proneuritogenesis activity of B7C. As B7C is an NMDA receptor antagonist, it is reasonable to ask whether B7C promotes neurite outgrowth through inhibiting NMDA receptor. It turned out that memantine, another NMDA receptor antagonist, failed to induce neurite outgrowth in PC12 cells even when the concentration was up to 10 μM (Figure 1B and C), suggesting that the proneuritogenesis effect of B7C may be independent of its inhibition of NMDA receptor.
Instead, our study indicated that B7C promoted neurite outgrowth in PC12 cells through activating ERK pathway, a conclusion supported by the evidence that MEK1/2 inhibitors (PD98059 and U0126) markedly blocked the neurite outgrowth‐promoting activity as well as the observed sustained ERK activation (Figure 4B and C). A noticeable increase in phospho‐ERK occurred 15 min after B7C administration, and a maximal response was observed 30 min after B7C administration, which is analogous with that reported by NGF 39. However, the activation kinetics of ERK phosphorylation was slightly different. NGF caused a maximal effect at 5–30 min and then returned to its basal level 2 h after treatment. However, B7C showed a more sustained activation of ERK phosphorylation that lasted for 4 h after induction. The different activation kinetics suggested that the intracellular activation of ERK pathway induced by B7C and that by NGF might be from the modulation of different receptors on the membrane. We then examined which receptors were involved in the B7C‐induced neuritogenesis. It turned out that B7C exerted its neuritogenic activity via a TrkA‐independent pathway as the TrkA‐specific inhibitor failed to block the outgrowth of neurites in PC12 cells induced by B7C.
Recently, increasing lines of evidence strongly suggested that activation of AChRs, α7‐nAChR in particular, is an important underlying mechanism in mediating various cellular responses including neuritogenesis 40, 41. α7‐nAChR activation allows the influx of Ca2+, which stimulates the downstream signaling pathways including ERK pathway. As B7C is a superior AChE inhibitor and can indirectly enhance the concentration of ACh in synaptic cleft, we analyzed whether the activation of AChRs, including mAChR and nAChR, was involved in the proneuritogenesis activity of B7C using specific inhibitors. It was evident that nAChR antagonists, but not mAChR antagonist, abrogated the neurite outgrowth induced by B7C. Our findings that pharmacological blockage and genetic depletion of α7‐nAChR partially abolished the neurite outgrowth as well as ERK activation induced by B7C not only confirm the primary importance of α7‐nAChR in mediating neuritogenesis, but also provide novel insights into the potential application of B7C against neurodegenerative diseases. Although tacrine is also an AChE inhibitor and may mimic the function of ACh, most of its effects on neuronal activity were independent of α7‐nAChR as suggested by earlier studies 42, 43, which may explain its limited effects on neuritogenesis.
The development of nicotinic acetylcholine receptor activator pharmacophore started in 1970 when it was proposed that the binding of ligands to the receptor was dependent on a positively charged nitrogen atom on the ligands. Molecular docking studies revealed that the hydrogen bond, formed between the cationic center (positively charged nitrogen atom) of the ligands and the main‐chain carbonyl oxygen atom of the highly conserved Trp‐149 or the side‐chain hydroxyl oxygen atom of Tyr‐123 in the receptors, is a hallmark of nicotinic ligands binding to α7‐nAChRs 44. Besides, π−π interactions between π rings located within the benzene rings of ligands and the aromatic side chain of the receptors also contribute to the binding of ligands and α7‐nAChRs. B7C (Figure 1A), as well as other α7‐nAChR activators (agonists and PAMs) including acetylcholine, nicotine, PNU‐282987, and PNU‐120596, contain one or more nitrogen atoms that are likely to be positively charged. Moreover, B7C possesses a benzene ring in each of its arm. The chemical structure of B7C suggests that B7C may act as a ligand of α7‐nAChRs. In addition to the similarity in chemical structure, there are also some interesting parallels between the biological effects, such as neuroprotection, induced by B7C and other α7‐nAChR activators. For instance, B7C 45, 46 as well as PNU‐120596 and PNU‐282987 47 prevented loss of retinal ganglion cells in a glaucoma model.
More noticeably, pharmacological inhibition (Figure 5B, D, and E) and genetic depletion (Figure 6) of α7‐nAChRs could not completely abolish the neuritogenesis‐promoting effects in PC12 cells produced by B7C. There could be several reasons for this phenomenon: First, potent PAMs of α7‐nAChR could be an inducer of neurite outgrowth, a conclusion supported by the evidence that PNU‐120596 (a potent PAM of α7‐nAChR) promoted effective neuritogenesis activity in our PC12 cell model (Figure 5B). B7C might act as a potent PAM of α7‐nAChR more than conventional α7‐nAChR agonists. PAM might differentially modify the biophysical properties of α7‐nAChR via an allosteric transmembrane site without interacting at the orthosteric binding site at which conventional α7‐nAChR agonists target as suggested by earlier studies 48, 49. MLA, being a α7‐nAChR antagonist and acting competitively with conventional α7‐nAChR agonist, might therefore partially abolish the neurite outgrowth‐promoting activity of B7C. Second, we could not rule out other possible contributing targets in the membrane, cytoplasm, or nucleus, such as G‐protein‐coupled receptor or cyclic adenosine monophosphate. To verify the hypothesis that B7C directly acts on α7‐nAChR and elucidates the detailed binding sites between B7C and α7‐nAChR, more molecular biological assays, including whole‐cell electrophysiological analysis, receptor‐ligand binding assay, and molecular dynamics simulation, are being undertaken by our team. The exact mechanism underlying the activation of α7‐nAChR by B7C will be revealed in future studies.
In conclusion, B7C promotes robust neurite outgrowth in PC12 cells and primary cortical neurons through activation of the α7‐nAChR/ERK signaling pathway. Given that considerable literature has revealed that multifunctional compounds might provide greater therapeutic efficacy against neurodegenerative disorders by concurrently and synergistically acting on different targets in the brain, the results described herein and published previously may lead us to conjecture that B7C, which possesses anti‐AChE, anti‐NMDA receptor, anti‐nNOS, and proneuritogenesis activities, may have great potential for treatment of the pathological processes of neurodegenerative disorders.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by grants from the Research Grants Council of Hong Kong (PolyU5610/11M, 15101014), The Hong Kong Polytechnic University (G‐YM32, G‐SB10, G‐UC15 and G‐YZ15), Ningbo International Science and Technology Cooperation Project (No. 2014D10019), University of Macau (MYRG2015‐00172‐ICMS‐QRCM) and the National Natural Science Foundation of China (81202510). We sincerely thank Ms. Josephine Leung for proofreading our manuscript.
References
- 1. Faure A, Verret L, Bozon B, et al. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1‐Ki mouse model of Alzheimer's disease. Neurobiol Aging 2011;32:407–418. [DOI] [PubMed] [Google Scholar]
- 2. Greffard S, Verny M, Bonnet AM, et al. Motor score of the unified parkinson disease rating scale as a good predictor of lewy body‐associated neuronal loss in the substantia nigra. Arch Neurol 2006;63:584–588. [DOI] [PubMed] [Google Scholar]
- 3. Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002;296:1648–1649. [DOI] [PubMed] [Google Scholar]
- 4. Utsugisawa K, Nagane Y, Obara D, Tohgi H. Overexpression of alpha7 nicotinic acetylcholine receptor prevents G1‐arrest and DNA fragmentation in PC12 cells after hypoxia. J Neurochem 2002;81:497–505. [DOI] [PubMed] [Google Scholar]
- 5. Nordman JC, Kabbani N. An interaction between alpha7 nicotinic receptors and a G‐protein pathway complex regulates neurite growth in neural cells. J Cell Sci 2012;125:5502–5513. [DOI] [PubMed] [Google Scholar]
- 6. Cui W, Cui GZ, Li W, et al. Bis(12)‐hupyridone, a novel multifunctional dimer, promotes neuronal differentiation more potently than its monomeric natural analog huperzine A possibly through alpha7 nAChR. Brain Res 2011;1401:10–17. [DOI] [PubMed] [Google Scholar]
- 7. Morley BJ, Mervis RF. Dendritic spine alterations in the hippocampus and parietal cortex of alpha7 nicotinic acetylcholine receptor knockout mice. Neuroscience 2013;233:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wasilewska‐Sampaio AP, Silveira MS, Holub O, et al. Neuritogenesis and neuronal differentiation promoted by 2,4‐dinitrophenol, a novel anti‐amyloidogenic compound. FASEB J 2005;19:1627–1636. [DOI] [PubMed] [Google Scholar]
- 9. Ould‐yahoui A, Tremblay E, Sbai O, et al. A new role for TIMP‐1 in modulating neurite outgrowth and morphology of cortical neurons. PLoS ONE 2009;4:e8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi DH, Lee KH, Kim JH, Kim MY, Lim JH, Lee J. Effect of 710 nm visible light irradiation on neurite outgrowth in primary rat cortical neurons following ischemic insult. Biochem Biophys Res Commun 2012;422:274–279. [DOI] [PubMed] [Google Scholar]
- 11. Li WM, Kan KK, Carlier PR, Pang YP, Han YF. East meets West in the search for Alzheimer's therapeutics ‐ novel dimeric inhibitors from tacrine and huperzine A. Curr Alzheimer Res 2007;4:386–396. [DOI] [PubMed] [Google Scholar]
- 12. Li W, Xue J, Niu C, et al. Synergistic neuroprotection by bis(7)‐tacrine via concurrent blockade of N‐methyl‐D‐aspartate receptors and neuronal nitric‐oxide synthase. Mol Pharmacol 2007;71:1258–1267. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Y, Li W, Chow PC, et al. Bis(7)‐tacrine, a promising anti‐Alzheimer's dimer, affords dose‐ and time‐dependent neuroprotection against transient focal cerebral ischemia. Neurosci Lett 2008;439:160–164. [DOI] [PubMed] [Google Scholar]
- 14. Yu H, Li WM, Kan KK, et al. The physicochemical properties and the in vivo AChE inhibition of two potential anti‐Alzheimer agents, bis(12)‐hupyridone and bis(7)‐tacrine. J Pharm Biomed Anal 2008;46:75–81. [DOI] [PubMed] [Google Scholar]
- 15. Hu S, Wang R, Cui W, et al. Inhibiting beta‐amyloid‐associated alzheimer's pathogenesis in vitro and in vivo by a multifunctional dimeric Bis(12)‐hupyridone derived from its natural analogue. J Mol Neurosci 2015;55:1014–1021. [DOI] [PubMed] [Google Scholar]
- 16. Hu SQ, Cui W, Xu DP, et al. Substantial neuroprotection against K+ deprivation‐induced apoptosis in primary cerebellar granule neurons by novel dimer bis(propyl)‐cognitin via the activation of VEGFR‐2 signaling pathway. CNS Neurosci Ther 2013;19:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Maiwulanjiang M, Lam KY, et al. A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: A signaling mediated by protein kinase A. J Agric Food Chem 2014;62:1890–1897. [DOI] [PubMed] [Google Scholar]
- 18. Fu H, Li W, Lao Y, et al. Bis(7)‐tacrine attenuates beta amyloid‐induced neuronal apoptosis by regulating L‐type calcium channels. J Neurochem 2006;98:1400–1410. [DOI] [PubMed] [Google Scholar]
- 19. Cui W, Zhang Z, Li W, et al. Unexpected neuronal protection of SU5416 against 1‐Methyl‐4‐phenylpyridinium ion‐induced toxicity via inhibiting neuronal nitric oxide synthase. PLoS ONE 2012;7:e46253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pool M, Thiemann J, Bar‐Or A, Fournier AE. NeuriteTracer: A novel ImageJ plugin for automated quantification of neurite outgrowth. J Neurosci Methods 2008;168:134–139. [DOI] [PubMed] [Google Scholar]
- 21. Daniele S, Lecca D, Trincavelli ML, Ciampi O, Abbracchio MP, Martini C. Regulation of PC12 cell survival and differentiation by the new P2Y‐like receptor GPR17. Cell Signal 2010;22:697–706. [DOI] [PubMed] [Google Scholar]
- 22. Gorman AM. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J Cell Mol Med 2008;12:2263–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Golde TE. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol Neurodegener 2009;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim HS, Yumkham S, Kim SH, et al. Secretin induces neurite outgrowth of PC12 through cAMP‐mitogen‐activated protein kinase pathway. Exp Mol Med 2006;38:85–93. [DOI] [PubMed] [Google Scholar]
- 25. Yankner BA, Benowitz LI, Villa‐Komaroff L, Neve RL. Transfection of PC12 cells with the human GAP‐43 gene: Effects on neurite outgrowth and regeneration. Brain Res Mol Brain Res 1990;7:39–44. [DOI] [PubMed] [Google Scholar]
- 26. Anderson KD, Morin MA, Beckel‐Mitchener A, et al. Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP‐43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J Neurochem 2000;75:1103–1114. [DOI] [PubMed] [Google Scholar]
- 27. Anderson KD, Sengupta J, Morin M, Neve RL, Valenzuela CF, Perrone‐Bizzozero NI. Overexpression of HuD accelerates neurite outgrowth and increases GAP‐43 mRNA expression in cortical neurons and retinoic acid‐induced embryonic stem cells in vitro. Exp Neurol 2001;168:250–258. [DOI] [PubMed] [Google Scholar]
- 28. Aigner L, Arber S, Kapfhammer JP, et al. Overexpression of the neural growth‐associated protein GAP‐43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 1995;83:269–278. [DOI] [PubMed] [Google Scholar]
- 29. Ma YZ, Ning N, He WB, et al. Claulansine F promotes neuritogenesis in PC12 cells via the ERK signaling pathway. Acta Pharmacol Sin 2013;34:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu Q, Julien JP. A key role for GAP‐43 in the retinotectal topographic organization. Exp Neurol 1999;155:228–242. [DOI] [PubMed] [Google Scholar]
- 31. Jin Y, Sui HJ, Dong Y, et al. Atorvastatin enhances neurite outgrowth in cortical neurons in vitro via up‐regulating the Akt/mTOR and Akt/GSK‐3beta signaling pathways. Acta Pharmacol Sin 2012;33:861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Day JS, O'Neill E, Cawley C, et al. Noradrenaline acting on astrocytic beta(2)‐adrenoceptors induces neurite outgrowth in primary cortical neurons. Neuropharmacology 2014;77:234–248. [DOI] [PubMed] [Google Scholar]
- 33. Goldshmit Y, Greenhalgh CJ, Turnley AM. Suppressor of cytokine signalling‐2 and epidermal growth factor regulate neurite outgrowth of cortical neurons. Eur J Neurosci 2004;20:2260–2266. [DOI] [PubMed] [Google Scholar]
- 34. Wu H, Mahmood A, Qu C, Xiong Y, Chopp M. Simvastatin attenuates axonal injury after experimental traumatic brain injury and promotes neurite outgrowth of primary cortical neurons. Brain Res 2012;1486:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 2011;3:a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsumoto Y, Inden M, Tamura A, Hatano R, Tsukita S, Asano S. Ezrin mediates neuritogenesis via down‐regulation of RhoA activity in cultured cortical neurons. PLoS ONE 2014;9:e105435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dong YL, Wang YM, Yang HJ, Yang XZ, Yu HJ. Phytoestrogen alpha‐Zearalanol exerts antiapoptotic effects in differentiated PC12 cells via oestrogen receptor alpha. Basic Clin Pharmacol Toxicol 2015;116:110–114. [DOI] [PubMed] [Google Scholar]
- 38. Shen JN, Wang DS, Wang R. The protection of acetylcholinesterase inhibitor on beta‐amyloid‐induced the injury of neurite outgrowth via regulating axon guidance related genes expression in neuronal cells. Int J Clin Exp Pathol 2012;5:900–913. [PMC free article] [PubMed] [Google Scholar]
- 39. Morooka T, Nishida E. Requirement of p38 mitogen‐activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem 1998;273:24285–24288. [DOI] [PubMed] [Google Scholar]
- 40. Resende RR, Alves AS, Britto LR, Ulrich H. Role of acetylcholine receptors in proliferation and differentiation of P19 embryonal carcinoma cells. Exp Cell Res 2008;314:1429–1443. [DOI] [PubMed] [Google Scholar]
- 41. Gardiner NJ, Fernyhough P, Tomlinson DR, Mayer U, von der Mark H, Streuli CH. Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol Cell Neurosci 2005;28:229–240. [DOI] [PubMed] [Google Scholar]
- 42. Takada‐Takatori Y, Kume T, Sugimoto M, et al. Neuroprotective effects of galanthamine and tacrine against glutamate neurotoxicity. Eur J Pharmacol 2006;549:19–26. [DOI] [PubMed] [Google Scholar]
- 43. Takada‐Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of Alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3‐kinase cascade. Neuropharmacology 2006;51:474–486. [DOI] [PubMed] [Google Scholar]
- 44. Kombo DC, Bencherif M. Comparative study on the use of docking and Bayesian categorization to predict ligand binding to nicotinic acetylcholine receptors (nAChRs) subtypes. J Chem Inf Model 2013;53:3212–3222. [DOI] [PubMed] [Google Scholar]
- 45. Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)‐tacrine against glutamate‐induced retinal ganglion cells damage. BMC Neurosci 2010;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li JB, Lu ZG, Xu L, Wang Q, Zhang ZH, Fang JH. Neuroprotective effects of bis(7)‐tacrine in a rat model of pressure‐induced retinal ischemia. Cell Biochem Biophys 2014;68:275–282. [DOI] [PubMed] [Google Scholar]
- 47. Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Invest Ophthalmol Vis Sci 2014;55:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, Gopalakrishnan M. Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: Ligand interactions with distinct binding sites and evidence for a prominent role of the M2‐M3 segment. Mol Pharmacol 2008;74:1407–1416. [DOI] [PubMed] [Google Scholar]
- 49. Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 2011;108:5867–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]


