Abstract
Abstract. An entire mammary epithelial outgrowth, capable of full secretory differentiation, may be comprised of the progeny of a single cellular antecedent. This conclusion is based upon the maintenance of retroviral insertion sites within the somatic DNA of successive transplant generations derived from a single mammary fragment. In addition, dissociation of these clonal dominant glands and implantation of dispersed cells at limiting dilution demonstrated that both duct‐limited and lobule‐limited outgrowths were developed, as well as complete, fully differentiated glands. Thus, transplantation has revealed three distinct mammary epithelial progenitors in the mouse. Similar studies have extended this observation to rat mammary tissue. Recently, using cre‐lox conditional activation of reporter genes, a new epithelial progenitor, specific for mammary secretory epithelium in postlactation females has been uncovered. In situ, these cells were shown to regenerate secretory lobules upon successive pregnancies. In transplant studies, they demonstrated the capacity for self‐renewal and contributed to the new generation of all of the known epithelial cell types among mammary epithelium. In limiting dilution, the parity‐induced progenitors were capable of engendering lobule‐limited and duct‐limited outgrowths in their entirety, but not completely developed glands. Serial transplant studies indicate that these progenitors have a significant but limited capacity for self‐renewal.
INTRODUCTION
It was an interest in premalignant lesions of the breast that led DeOme and colleagues (DeOme et al. 1959) to develop a biological system to recognize, characterize and study hyperplastic nodules (HAN) in the mammary glands of mouse mammary tumour virus (MMTV)‐infected mice. In the quest for a means to demonstrate that these structures were precursors to frank mammary adenocarcinoma, these investigators developed a method for removing the endogenous mammary epithelium from a mammary fat pad. Subsequently, the ‘cleared’ pad was used as a site of implantation where suspected premalignant lesions could be placed, and their subsequent growth and development observed. Using this approach, they were able to show that both premalignant and normal mammary implants could grow and fill the empty fat pad within several weeks. During this growth period, the premalignant implants recapitulated their hyperplastic phenotype, whereas normal implants produced normal branching mammary ducts. Serial transplantation of normal and premalignant outgrowths demonstrated that while normal gland invariably showed growth senescence after several generations, hyperplastic outgrowths did not. It soon became apparent that any portion of the normal mammary parenchyma could regenerate a complete mammary tree over several transplant generations, suggesting the existence of cells capable of reproducing new mammary epithelium through several rounds of self‐renewal. However, it was some time later before this property was recognized as representative of the presence of mammary epithelial stem cells (Williams & Daniel 1983; Smith & Medina 1988).
It was discovered that all portions of the mouse mammary gland appeared competent to regenerate an entire new gland upon transplantation; this triggered a series of papers relating to the reproductive lifetime of mammary cells (Daniel et al. 1968; Daniel & Young 1971; Daniel et al. 1971; Young et al. 1971). The results indicated that no difference existed in the regenerative ability of mammary tissue taken from very old mice vs. that taken from very young mice during serial transplantation. In addition, neither reproductive history nor developmental state had a significant impact on the reproductive longevity of mammary tissue implants. That grafts from old donors could proliferate equivalently to those from young donors in young hosts suggested to these authors that the life span of mammary cells was primarily affected by the number of mitotic divisions, rather than by the passage of chronological or metabolic time. The authors in a series of experiments tested this serially transplanting mammary implants (Daniel & Young 1971). The authors concluded that growth senescence in transplanted mammary epithelium was related primarily to the number of cell divisions. Conversely, mouse mammary epithelium could be transformed to unlimited division potential either spontaneously, by mouse mammary tumour virus (MMTV) infection, or by treatment with carcinogens (Daniel et al. 1975; Medina & Kittrell 1993; Medina 2000). At the time, this observation was taken to signify that ‘immortalization’, i.e. attainment of unlimited division potential, was an important early step in malignant transformation. In recent reports, it has conclusively been shown that accelerated senescence of mammary stem cells, in situ, results in increased refractoriness to MMTV‐induced neoplastic transformation (Boulanger & Smith 2001; Buggiano et al. 2001).
Several recent studies have demonstrated that the multipotent cells in mammary epithelium reside within the luminal cell population in human and mouse (Stingl et al. 1998; Pechoux et al. 1999; Smalley et al. 1999; Stingl et al. 2001; Gudjonsson et al. 2002). However, no specific molecular signature for mammary epithelial stem cells has been revealed. In 1988, (Smith & Medina 1988) an earlier marker was identified that held promise for distinguishing mammary stem cells by their ultrastructural appearance. In this study, it was discovered that mouse mammary explants, like mammary epithelium in situ, contained pale or light‐staining cells, and that it was only these cells that entered mitosis when mammary explants were cultured. Undifferentiated (pale) cells were found to exhibit the expected behaviour of stem cells in mammary explants induced in vitro, that of differentiation towards secretory cell fates.
Our laboratory (Chepko & Smith 1997) analysed light cells in the electron microscope, utilizing their ultrastructural features to distinguish them from other mammary epithelial cells. In a retrospective analysis of light and electron micrographs, a careful and detailed scrutiny of mammary tissue was performed to determine the range of morphological features among the cell types that had previously been reported. The samples evaluated included mouse mammary explants, pregnant and lactating mouse mammary glands, and rat mammary gland from 17 stages of development, beginning with nulliparous, and following through pregnancy, lactation and involution (Smith & Vonderhaar 1981; Vonderhaar & Smith 1982; Smith et al. 1984; Chepko & Smith 1997). This ultrastructural analysis strengthened the conclusion that mitosis occurs only in the undifferentiated (light) cells. The undifferentiated cells were found in two easily recognized forms: small light cells (SLC ∼8 µm) and undifferentiated large light cells (ULLC 15–20 µm). Briefly, SLC (Fig. 1) have a basal location and never touch the lumen, and both the nucleoplasm and the cytoplasm are characteristically electron‐lucent (pale). The nucleus contains dense clumps of chromatin and is sometimes indented, and the cytoplasm contains few organelles and little evidence of specialized function (Smith & Chepko 2001). By contrast, ULLC (Fig. 2) are much larger than SLC, may contact both the basement membrane and the lumen, have larger and rounder nuclei than other epithelial cells, and possess a pale staining, fibrillar, euchromatic nucleoplasm. The cytoplasm is electron‐lucent (pale) and contains some mitochondria, sparse endoplasmic reticulum and an inactive Golgi complex (Smith & Medina 1988; Chepko & Smith 1997). Both SLC and ULLC were observed with condensed mitotic chromosomes indicative of their replicative competence. Partially differentiated ULLC or differentiating large light cells (DLLC) were observed in rapidly proliferating mammary epithelium during pregnancy, and probably represent transient‐amplifying epithelial cells committed to a secretory fate. Conversely, mitotic chromosomes were never found within the differentiated cells, such as the secretory and myoepithelial cells, suggesting that they were terminally differentiated and out of the cell cycle. Using all of the above features, we were able to develop a more detailed description of the epithelial subtypes that comprise the mammary epithelium (Fig. 3).
Figure 1.
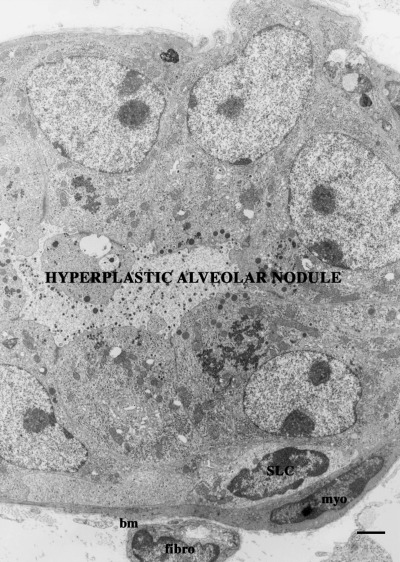
Section from a serially transplanted alveolar outgrowth induced by MMTV. Hyperplastic alveolar outgrowths possess an indefinite replicative lifespan. Consequently, in contrast to normal growth senescent implants, small light cells, (SLC), and undifferentiated large light cells (ULLC) (see Fig. 2) are routinely present. bm, basement membrane; myo, myoepithelial cell; fibro, fibroblast. Bar represents 1 µm.
Figure 2.
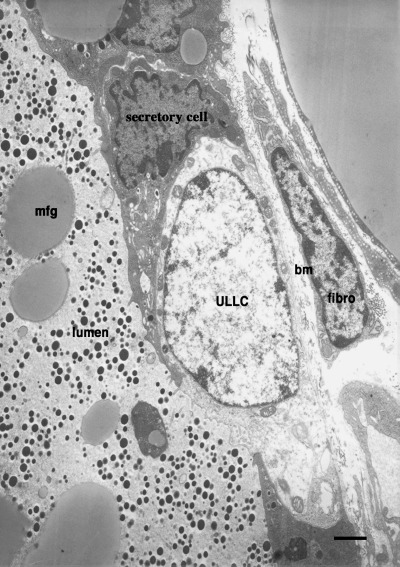
Another portion of the hyperplastic outgrowth shown in Fig. 1, illustrating location and positioning of ULLC. bm, basement membrane; fibro, fibroblast; mfg, milk‐fat globule. Bar represents 1 µm.
Figure 3.
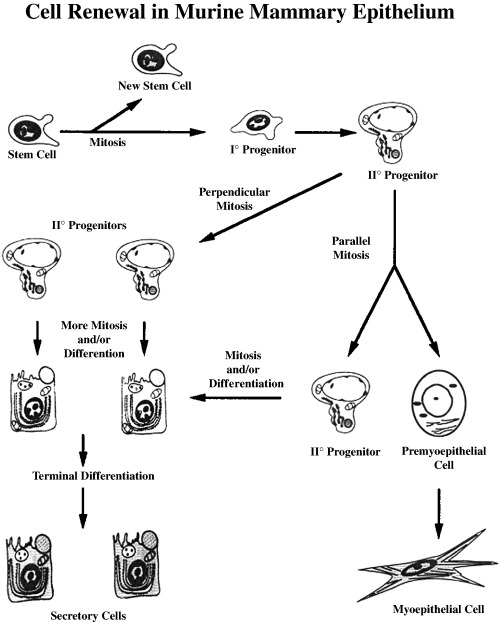
This diagram represents our current model for the ultimate mammary stem cell (SLC), which gives rise to lobular and ductal progenitors that subsequently differentiate into all types representative of mammary epithelium. Adapted from Chepko and Smith (1997).
A total of 3552 cells through 17 stages of rat mammary gland development were counted and the percentage of each morphotype was calculated. A similar analysis was made in the mouse. This evaluation showed that the population density (number of cells/mm) of SLC among mammary epithelium did not change from puberty through postlactation involution. The proportion of SLC in the epithelial population remained unchanged. This means that although the number of mammary epithelial cells increased by 27‐fold during pregnancy in the mouse (Kordon & Smith 1998; Nicoll & Tucker 1965) the percentage of SLC in the population did not change. Therefore the absolute number of SLC increase and decrease at the same relative rate as the expanding epithelial cell population, suggesting that they have a capacity for self‐renewal. In contrast, ULLC numbers were much more variable, perhaps indicative of their transitional nature.
ABSENCE OF SLC AND ULLC IN GROWTH‐SENESCENT MAMMARY TISSUES
Cell and developmental biologists that have examined growing and regenerating tissue by transmission electron microscopy have postulated that the undifferentiated cells observed within these tissues represent tissue‐specific stem or progenitor cells. However, no studies have addressed the issue of whether these undifferentiated, putative stem cells persist in growth‐senescent tissues. Serially transplanted mammary epithelium consistently displays growth senescence, beginning at the third transplant generation. The rate of ageing is not uniform throughout the transplanted population, and complete growth quiescence for all portions of a given outgrowth is reached subsequent to the sixth transplant generation. Mammary epithelial cells bearing the morphological characteristics of undifferentiated stem cells (i.e. SLC and ULLC) likewise disappear from senescent populations simultaneously with growth cessation (Smith et al. 2002). In premalignant mammary epithelial populations, which exhibit indefinitely prolonged growth potential, both of these cell types (SLC and ULLC) are maintained. This observation provides further support for the conclusion that these ultrastructurally distinct mammary cells represent the mammary stem/progenitor cell population.
A recent study (Gudjonsson et al. 2002) of human breast epithelium demonstrated the presence of mammary epithelial cells possessing the ability to regenerate elaborate branching structures, resembling mammary terminal ductal lobular units both in morphology and marker expression, in vivo and in vitro. The authors based their experimental approach upon our ultrastructural studies in the mouse mammary gland (Smith & Chepko 2001), which described SLC and ULLC as putative epithelial stem cells. As SLC and ULLC do not commonly contact the duct or lobule lumen, they predicted that similar cells in the human breast would be negative for sialomucin (a surface marker for luminal epithelial cells) but positive for epithelial‐specific antigen (ESA). Indeed, they found suprabasal breast epithelial cells with these properties and demonstrated that they possessed stem‐cell properties. This discovery lends strong experimental support to the conclusion that the undifferentiated SLC and ULLC described here represent a multipotent epithelial cell population in the mouse, and that a similar epithelial subset exists in the human breast.
EVIDENCE FOR DISTINCT DUCT‐LIMITED AND LOBULE‐LIMITED MAMMARY EPITHELIAL PROGENITORS
We determined the limiting dilution (for FVB/N mice) for obtaining mammary epithelial growth in epithelium‐divested mammary fat pads (Smith 1996), upon inoculation with dispersed mammary epithelial cells from primary cultures. Following this determination, a limiting dilution of WAP‐LacZ mammary epithelial cells was inoculated into a series of cleared mammary fat pads accompanied by 1 × 106 fibroblasts. The hosts were maintained as virgin for 4 weeks and then placed with males to be impregnated. At parturition, the implanted mammary fat pads were collected and evaluated for epithelial growth as whole mounts. Twenty‐two of 34 inoculated fat pads contained positive epithelial growth. The origin of the growth from WAP‐LacZ epithelial cells was confirmed by X‐gal staining (Smith 1996). The results from these transplant studies indicated that there are three separate and distinct epithelial progenitors within the mammary epithelial population. At limiting dilution, three disparate types of outgrowths were observed in the fat pads of postpartum hosts. These were characterized as lobule‐limited outgrowths, duct‐only outgrowth or full development of a complete mammary ductal system, decorated with complete secretory lobule development. Therefore, mammary epithelial progenitors with distinctly limited developmental capacities were demonstrated among the mammary epithelium of nulliparous female mice. Similar experiments with dispersed mammary cells in the rat have also demonstrated lobule‐limited and ductal‐limited epithelial progenitors, within the mammary population (Kamiya et al. 1998; Kamiya et al. 1999).
CLONAL‐DOMINANT MAMMARY OUTGROWTHS COMPRISED OF THE PROGENY OF A SINGLE ANTECEDENT
Limiting dilution transplantation studies identified three distinct multipotent epithelial cells within the mouse mammary gland (Smith 1996). These cells are characterized by their distinctive ability to produce secretory lobules, generate branching mammary ducts or recreate the entire functional (lactating) mammary epithelium upon transplantation into a breeding host. Each appears to have the capacity for self‐renewal, but the lobule‐limited and ductal‐restricted progenitors appear to have a smaller reproductive capacity than the fully competent progenitor. Cap cells and terminal bud formation seem to be within the province of the ductal‐restricted progenitor, whereas lobule development and expansion is absent. The opposite is true for the lobule‐limited progenitor.
Our earlier studies indicate that individual, retrovirally tagged, epithelial stem cells, positioned throughout the mature fully developed mammary gland, have the capacity to produce sufficient differentiated progeny to recapitulate an entire functional gland (Kordon & Smith 1998). Second‐generation outgrowths from the original transplant generation produced mammary populations that exhibited MMTV proviral insertions identical to the original. These second‐generation populations were shown by dissociation and limiting‐dilution transplantation studies to contain all three multipotent mammary cell types described above. We therefore proposed that all three types arise from a single pluripotent precursor and that this precursor was capable of self‐renewal and existed in all epithelial portions of the mouse mammary gland.
Normal mammary transplants in nulliparous hosts show growth senescence during serial propagation. Some implants that showed senescent ductal growth were able to respond to pregnancy, and produce secretory lobules and milk protein in situ (Daniel et al. 1971). From this observation, it seemed to us that both lobule‐committed and ductal‐committed progenitors might exist at any one time within an implant and possess different reproductive capacities. Alternatively, a primary antecedent responsible for the generation of both of these lineage‐committed progenitors loses its capacity to produce one type independent of the other. Originally, we postulated that the entire clonal‐dominant outgrowth was generated from the progeny of one or a few lineally related stem cells, whose predecessor had acquired MMTV proviral insertions. During ductal growth and extension these cells were self‐renewed and distributed throughout the ductal tree. Following this, upon subsequent transplantation the process was repeated and the same proviral tagging pattern was maintained in the subsequent generation. However, because of the existence of multipotent lineage‐limited progenitors within the outgrowths, the pattern of proviral tagging observed in the original outgrowth might result from the sum of several different provirally tagged cellular clones that were maintained at a similar relative fraction throughout the gland. Further, these subpopulations might possess similar or identical growth potentials such that they were maintained in the second outgrowth generation. In an attempt to distinguish between these possibilities, we serially transplanted apparent clonal mammary populations through several generations in breeding recipients (where both ductal and lobular development are supported) to growth senescence (Smith & Boulanger 2002). All subsequent generations were evaluated regarding their proviral content.
The proliferative lifespan of mammary stem cells was examined in serially transplanted clonal‐dominant epithelial populations. Five successive transplant generations were performed. The epithelial cell number in each outgrowth expands ∼500‐fold in nulliparous hosts and ∼10 000‐fold in impregnated hosts. Despite this, all resulting mammary outgrowths showed lineal identity with the original, because all the original MMTV proviral insertions were maintained in the DNA isolated from each generation (Fig. 4). Growth senescence was observed in some implants, beginning at the third generation in impregnated recipients. The ability of an individual implant to support ductal morphogenesis and also secretory lobule development decayed at independent rates. Nevertheless, all proviral insertions were present in these limited populations, indicating that they arise from a common primogenitor (Smith & Boulanger 2002). This supports the conclusion that duct‐ and lobule‐limited epithelial progenitors have a common antecedent. Individual implants from a single clonal‐dominant outgrowth occasionally gave rise to markedly different ductal development within the same host, providing evidence for an epithelial cell autonomous mechanism in ductal patterning. During serial passage, both premalignant and malignant populations appeared focally within the ageing transplants. These transformed populations were also lineally related to the original outgrowth, supporting the conclusion that the primary growth was derived clonally from one or a few lineally related antecedents. Neither the premalignant nor the malignant descendant population exhibited growth senescence, suggesting that they may be supported by a perpetually self‐renewing progenitor. Our serial transplantation of clonal‐dominant outgrowths indicates that a single mammary cell may have the capacity to self‐renew through at least five transplant generations (some sixth‐generation implants showed vigorous growth).
Figure 4.
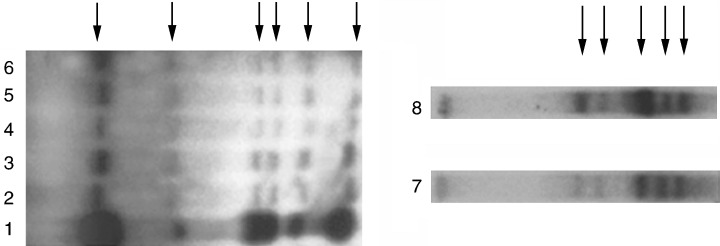
Total DNA from serially transplanted clonal dominant epithelial populations of five successive generations was subjected to digestion with EcoR1 followed by Southern Blot analysis. The subsequent blot was probed with a 32P‐labelled MMTV‐LTR‐specific probe. EcoR1 cuts within the genome of the MMTV, producing two host–viral junction fragments of each provirus insertion. In panel A, five specific host–viral restriction fragments (arrows) were found in the original outgrowth (lane 6), and in all of the succeeding generations. Transplant generations 2 through 5 are represented by the middle four lanes (2–5). The DNA in lane 1 is from the mammary tumour that arose in a fourthth generation outgrowth. In panel B, DNA from a fully developed R12 outgrowth (lane 7) is compared with DNA from a lobule‐limited outgrowth in the contralateral gland at parturition (lane 8). In both cases, all five MMTV‐host restriction fragments are detected.
Recent evidence demonstrates that intestinal stem cells retain the template chromatids during mitosis, passing the newly synthesized strands to their fate‐committed daughters (Cairns 2002; Potten et al. 2002). In this context, our evidence from serial transplantation of MMTV‐infected mammary outgrowths would indicate that this conservation process might occur in mammary epithelial stem cells also. This feature of stem cell asymmetric division would account for the absence of newly acquired MMTV proviral insertions through multiple rounds of stem cell renewal during serial passage of the clonal‐dominant outgrowths.
A NEW, PARITY‐SPECIFIC, SELF‐RENEWING, MULTIPOTENT EPITHELIAL SUBPOPULATION
It is a generally accepted tenet that the remodelled gland in a parous animal resembles that of a mature virgin on the morphological level. However, a number of physiological differences have been noted in comparing the responses of mammary epithelia from nulliparous vs. parous females to hormonal stimulation and carcinogenic agents. In WAP‐Cre/Rosa‐LacZ mice, we (Wagner et al. 2002) present genetic evidence that an involuted mammary gland is fundamentally different from a virgin gland, despite its close resemblance in the morphology. This difference results from the formation of a new mammary epithelial cell population that originates from differentiating cells during pregnancy. In contrast to the majority of fully committed alveolar cells, this epithelial population does not undergo cell death during involution and remodelling following a lactation period. Our experiments have shown that these cells can function as alveolar progenitors in subsequent pregnancies, and can play an important role in functional adaptation in genetically engineered mice, which exhibit a reversion of a lactation‐deficient phenotype. In transplantation studies, this parity‐induced epithelial population shows the capacity for self‐renewal and contributes significantly to the reconstitution of the resulting mammary outgrowth (i.e. ductal morphogenesis and lobulogenesis). We propose that this parity‐induced population contributes importantly to the biological differences between the mammary glands of parous and nulliparous females.
Physiological differences between the mammary epithelium found in nulliparous and in primiparous or parous female mice have been noted earlier (Smith & Vonderhaar 1981; Vonderhaar & Smith 1982; Smith 1987). In explant cultures, the mammary glands of nulliparous mice must synthesize DNA before they become fully responsive to lactogenic hormonal stimuli. In contrast, explants from the mammary glands of parous females do not require new DNA synthesis prior to a lactational response. Subsequent studies ruled out completion of the cell cycle and mitosis as an explanation for this difference between the mammary epithelium of parous and virgin mice (Smith & Vonderhaar 1981). In examining the potential role of the basement‐membrane collagen synthesis, a further difference was noted between virgin and parous mice in cultured explants. When proline was left out of the medium, virgin explants failed to respond even to the extent noted in DNA‐synthesis‐blocked explants, failing to either cytologically differentiate or produce any milk proteins. Again, in contrast, no reduction in the responsiveness of epithelium from parous, nonpregnant mice occurred in the absence of proline (Smith 1987). Therefore, in terms of hormone‐responsiveness in explanted cultures, the mammary epithelium from parous females is distinctly different from that in nulliparous mice. Furthermore, in several species, including mice (Medina & Smith 1999), parous and nulliparous females differ in their propensity to develop mammary cancer. This difference is thought to reflect either systemic change resulting from pregnancy, or more probably, the alteration of the mammary tissue itself. The newly identified mammary epithelial population that encompasses a significant portion of the entire mammary epithelia in parous animals might be a facilitator of the important physiological changes mentioned above.
The multipotent epithelial cells in human and murine glands are associated with cellular populations that express luminal epithelial markers rather than the phenotypic markers associated with myoepithelial cells (Stingl et al. 1998; Smalley et al. 1999; Stingl et al. 2001; Gudjonsson et al. 2002). These findings are consistent with our observations that some WAP‐expressing mammary epithelial cells survive postlactational involution and persist in a luminal niche. Subsequently, upon the succeeding pregnancy, they proliferate to help form new secretory acini. However, in transplants, this parity‐induced epithelial population shows the property of self‐renewal, and these cells maintain themselves at regular intervals among the luminal epithelium of the extending ductal branches. They also orientate themselves within the body of the growing terminal end bud. Thus, these cells express certain features of multipotent stem cells, e.g. the property of self‐renewal. The dissociation of involuted mammary glands from WAP‐Cre/Rosa‐LacZ double transgenic animals, and the transplantation of the dispersed epithelial cells into the cleared fat pad of recipients, demonstrate that this parity‐induced epithelial population can reassociate with other cells and produce complete branching mammary ducts. However, none of the structures produced in these studies were entirely composed of blue cells, therefore these cells may not be capable of producing all of the epithelial cell types in mammary ducts by themselves. Hence, these parity‐induced mammary epithelial cells may lack the most important feature of mammary stem cells; the innate capacity to produce diverse progeny such as the cap cells of the growing terminal end buds.
The appearance of epithelial cells committed to secretory differentiation and capable of proliferation among the parous mammary epithelium provides a buffer population that may protect against depletion of primary mammary stem cells from the population as a result of mitotic activity. Serial transplantation shows evidence that mammary epithelium from an aged, multiparous female is equivalent to that from young pubertal females with respect to longevity and growth potential (Young et al. 1971). The ‘rescue’ of normal secretory development and lactation in the PRL‐R heterozygous knockout mouse is an example of the buffering capacity provided by the survival and proliferative capacity of the parity‐induced epithelial population.
Limiting dilution transplantation of mammary epithelial cells dispersed from parous WAP‐Cre/Rosa‐LacZ Stop females produced interesting results (Smith, unpublished data). Although WAP‐Cre‐activated (LacZ +) cells were a minority (< 20%) of the epithelial cells in the inoculums, all positive takes (12 of 23) contained the LacZ + progeny of WAP‐Cre‐activated cells. Among the positive takes, both lobule‐limited and duct‐limited outgrowths entirely comprised of LacZ + cells were produced. These structures contained LacZ + myoepithelial cells, and luminal cells that were both positive and negative for oestrogen and progesterone receptors. The cap cells in the duct‐limited outgrowth were also LacZ +. Importantly, no outgrowths were found entirely composed of LacZ − cells, even though they represented the vast majority (∼80%) of the epithelial cells injected. Complete outgrowths with full ductal development and associated secretory lobules were composed of a mixture of LacZ + and LacZ − epithelial cells. Where terminal end buds could be distinguished in these fully developed positive takes, none of the cap cells were LacZ +, even though nests of LacZ + cells were found in the body of the end bud. However, in these mixed outgrowths, the subtending regions of the duct were decorated with secretory acini entirely assembled from LacZ + epithelium including oestrogen/progesterone receptor‐positive and ‐negative luminal epithelium, and the associated myoepithelial cells (Fig. 5). These findings indicate that the WAP‐Cre‐activated cells that survive involution subsequent to the cessation of lactation include a population of self‐renewing, multipotent luminal epithelial cells.
Figure 5.
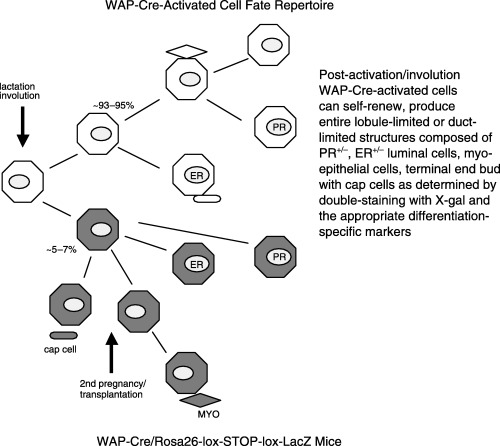
Diagram of the cell fate potential of WAP‐Cre‐activated cells upon subsequent pregnancies or in transplants.
A question of interest is the significance of the appearance of partially proliferation‐competent cells in the parous mammary gland, and the increased resistance to mammary tumorigenesis compared with the nulliparous gland. Women, regardless of ethnicity, who have undergone a full‐term pregnancy before 20 years of age have half the risk of developing breast cancer as nulliparous women (MacMahon et al. 1970). Rats and mice also have a greatly reduced susceptibility to chemically induced mammary tumorigenesis compared with their nulliparous siblings (Medina & Smith 1999). The mechanism(s) for this protective effect have not been defined. The most widely accepted explanation, offered by Russo and Russo (Russo & Russo 1996), is that the protection is afforded by the pregnancy‐induced differentiation of the target structures for carcinogenesis, the terminal end buds and duct termini. More recently, it has been suggested that the hormonal milieu of pregnancy affects the developmental state of a subset of mammary epithelial cells and their progeny, which results in persistent differences in their response to carcinogenic challenge. These changes are reflected in the muted proliferative response to carcinogen exposure by the affected cells and the appearance of a sustained expression of nuclear p53 in the hormone‐treated epithelium. The proliferation block and the induction of p53 occur both in rats and in mice, and support the generality of this hypothesis (2001, 1998).
The ability to identify and subsequently isolate cells from the parity‐induced subpopulation of epithelial cells from multiparous mice offers a unique opportunity to evaluate various aspects of the differences between parous and nulliparous mouse mammary glands. These populations offer the opportunity to examine the contributions of cellular signalling pathways to stem‐cell renewal, asymmetric division, and the potential role of stem cells in the development of premalignant and malignant mammary lesions. Preliminary results from WAP‐Cre/Rosa‐LacZ mice bearing transgenes targeted to WAP‐expressing mammary epithelial cells and/or infected with MMTV (Smith, unpublished data) have provided early insights into the mechanisms underlying senescence, immortalization and tumorigenesis in the murine mammary gland.
REFERENCES
- Boulanger CA, Smith GH (2001) Reducing mammary cancer risk through premature stem cell senescence. Oncogene 20, 2264. [DOI] [PubMed] [Google Scholar]
- Buggiano V, Schere‐Levy C, Abe K, Vanzulli S, Piazzon I, Smith GH, Kordon EC (2001) Impairment of mammary lobular development induced by expression of TGFβ1 under the control of the WAP promoter does not suppress tumorigenesis in MMTV‐infected transgenic mice. Int. J. Cancer 92, 568. [DOI] [PubMed] [Google Scholar]
- Cairns J (2002) Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc. Natl Acad. Sci. USA 99, 10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepko G, Smith GH (1997) Three division‐competent, structurally‐distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 29, 239. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Aidells BD, Medina D, Faulkin LJ (1975) Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen‐induced transformation. Fed. Proc. 34, 64. [PubMed] [Google Scholar]
- Daniel C, Deome K, Young L, Blair P, Faulkin L (1968) The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc. Natl Acad. Sci. USA 61, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Young LJ, Medina D, Deome KB (1971) The influence of mammogenic hormones on serially transplanted mouse mammary gland. Exp. Gerontol. 6, 95. [DOI] [PubMed] [Google Scholar]
- Danielc W, Young LJ (1971) Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo . Exp. Cell Res. 65, 27. [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ, Bern HA, Blair PB (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland‐free mammary fat pads of female C3H mice. J. Natl Cancer Inst. 78, 751. [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov‐Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002) Normal and tumor‐derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 115, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Gould MN, Clifton KH (1998) Quantitative studies of ductal versus alveolar differentiation from rat mammary clonogens. Proc. Soc. Exp. Biol. Med. 219, 217. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Higgins PD, Tanner MA, Gould MN, Clifton KH (1999) Kinetics of mammary clonogenic cells and rat mammary cancer induction by X‐rays or fission neutrons. J. Radiat Res. (Tokyo) 40 (Suppl.), 128. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Trichopoulos D, Valaoras VG, Yuasa S (1970) Lactation and cancer of the breast. A summary of an international study. Bull. World Health Organ. 42, 185. [PMC free article] [PubMed] [Google Scholar]
- Medina D (2000) The preneoplastic phenotype in murine mammary tumorigenesis. J. Mammary Gland Biol. Neoplasia 5, 393. [DOI] [PubMed] [Google Scholar]
- Medina D, Kittrell FS (1993) Immortalization phenotype dissociated from the preneoplastic phenotype in mouse mammary epithelial outgrowths in vivo . Carcinogenesis 14, 25. [DOI] [PubMed] [Google Scholar]
- Medina D, Smith GH (1999) Chemical carcinogen‐induced tumorigenesis in parous, involuted mouse mammary glands. J. Natl Cancer Inst. 91, 967. [DOI] [PubMed] [Google Scholar]
- Nicoll CS, Tucker HA (1965) Estimates of parenchymal, stromal, and lymph node deoxyribonucleic acid in mammary glands of C3H/Crgl‐2 mice. Life Sci. 4, 993. [DOI] [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov‐Jessen L, Bissell MJ, Petersen OW (1999) Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev. Biol. 206, 88. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J (1996) Mammary gland neoplasia in long‐term rodent studies. Environ. Health Perspect. 104, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman L, Conneely OM, Medina D, O'Malley BW (2001) p53 is a potential mediator of pregnancy and hormone‐induced resistance to mammary carcinogenesis. Proc. Natl Acad. Sci. USA 98, 12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman L, Stephens LC, Markaverich BM, Clark JA, Krnacik S, Conneel OM, O'Malley BW, Medina D (1998) Hormone‐induced refractoriness to mammary carcinogenesis in Wistar–Furth rats. Carcinogenesis 19, 1573. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O'Hare MJ (1999) Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J. Histochem. Cytochem. 47, 1513. [DOI] [PubMed] [Google Scholar]
- Smith GH (1987) Functional differentiation of virgin mouse mammary epithelium in explant cultures is dependent upon extracellular proline. J. Cell. Physiol. 131, 190. [DOI] [PubMed] [Google Scholar]
- Smith GH (1996) Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 39, 21. [DOI] [PubMed] [Google Scholar]
- Smith GH, Boulanger CA (2002) Mammary stem cell repertoire: new insights in aging epithelial populations. Mech. Aging Dev. 123, 1505. [DOI] [PubMed] [Google Scholar]
- Smith GH, Chepko G (2001) Mammary epithelial stem cells. Microsc Res. Techn 52, 190. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90, 173. [DOI] [PubMed] [Google Scholar]
- Smith GH, Strickland P, Daniel CW (2002) Putative epithelial stem cell loss corresponds with mammary growth senescence. Cell Tissue Res. 310, 313. [DOI] [PubMed] [Google Scholar]
- Smith GH, Vonderhaar BK (1981) Functional differentiation in mouse mammary gland epithelium is attained through DNA synthesis, inconsequent of mitosis. Dev. Biol. 88, 167. [DOI] [PubMed] [Google Scholar]
- Smith GH, Vonderhaar BK, Graham DE, Medina D (1984) Expression of pregnancy‐specific genes in preneoplastic mouse mammary tissues from virgin mice. Cancer Res. 44, 3426. [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63, 201. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandich I, Emerman JT (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 67, 93. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK, Smith GH (1982) Dissociation of cytological and functional differential in virgin mouse mammary gland during inhibition of DNA synthesis. J. Cell Sci. 53, 97. [DOI] [PubMed] [Google Scholar]
- Wagner K‐U, Boulanger CA, Henry MD, Sagagias M, Hennighausen L, Smith GH (2002) An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377. [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW (1983) Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 97, 274. [DOI] [PubMed] [Google Scholar]
- Young LJ, Medina D, Deome KB, Daniel CW (1971) The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol. 6, 49. [DOI] [PubMed] [Google Scholar]


