Abstract
Fertility preservation is an increasingly important discipline. It requires close coordination between reproductive medicine specialists, reproductive biologists, and oncologists in various disciplines. In addition, it represents a particular health policy challenge, since fertility-protection measures are to be understood as a treatment for side effects of gonadotoxic treatments and would therefore normally have to be reimbursed by health insurance companies. Therefore, it is inevitable that fertility-preservation activities should organise themselves into a network structure both as a medical-logistic network and as a professional medical society. The necessary network structures can differ significantly at regional, national, and international level, as the size of the regions to be integrated and the local cultural and geographical conditions, as well as the political conditions are very different. To address these issues, the current review aims to point out the basic importance and the chances but also the difficulties of fertility-protection networks and give practical guidance for the development of such network structures. We will not only discuss network structures theoretically but also present them based on three established, different sized networks, such as the Danish Network (www.rigshospitalet.dk), representing a centralised network in a small country; the German-Austrian-Swiss network FertiPROTEKT® (www.fertiprotekt.com), representing a centralised as well as decentralised network in a large country; and the Oncofertility® Consortium (www.oncofertility.northwestern.edu), representing a decentralised, internationally oriented network, primarily serving the transfer of knowledge among its members.
Keywords: fertility preservation, network, FertiPROTEKT, Oncofertility Consortium, ovarian tissue, transplantation
Introduction
Since the birth of the first child after ovarian tissue transplantation, fertility protection in women and men has developed into a new and clinically relevant independent field.1 This field has a number of special features.
On one hand, the discipline is interdisciplinary, since fertility protection requires close coordination between reproductive medicine specialists, reproductive biologists, and oncologists in various disciplines.
On the other hand, some fertility-preserving measures such as transplantation of ovarian tissue, in vitro maturation of oocytes, maturation of oocytes from ovarian tissue, and the cryopreservation of testicular tissue from prepubertal boys are still in clinical or even scientific development and therefore require a high degree of specialisation on the part of the centres involved.2-5
In addition, this new specialist field represents a particular health policy challenge, since fertility-protection measures are to be understood as a treatment for side effects of gonadotoxic treatments and would therefore normally have to be reimbursed by health insurance companies, which is not yet the case in many countries.
Due to these special features of this field, it is inevitable that all the scientific, clinical, and, if necessary, even health care policy areas involved organise themselves into a network structure both as a medical-logistic network and as a professional medical society.
The necessary network structures can differ significantly at regional, national, and international level, as the size of the regions to be integrated and the local cultural and geographical conditions, as well as the political conditions are very different.
Therefore, in this article, we would like to point the basic importance and the chances, but also the difficulties, of fertility-protection networks and give practical guidance for the development of such network structures. We will not only discuss network structures theoretically but also present them based on three established different sized networks. These three networks have different goals and different logistic structures and thus cover the possible range of possible network structures.
The selected networks are as follows:
The Danish Network (www.rigshospitalet.dk). This network is a centralised network for the practical implementation of specific fertility-preserving techniques such as the cryopreservation and transplantation of ovarian tissue in a small country.
The German-Austrian-Swiss network FertiPROTEKT (www.fertiprotekt.com). This network is a centralised as well as decentralised network that controls the implementation of fertility-protective techniques in a large country.
The Oncofertility Consortium (www.oncofertility.northwestern.edu). This is a decentralised, internationally oriented network that primarily serves the transfer of knowledge among its members.
Network Structure
The structure of a network depends on the following mostly given and therefore unchangeable conditions and the self-imposed goals:
Given conditions for the establishment of a network
Size of the region to be networked.
Transport-logistical development of the region.
Density and area coverage of reproductive medicine centres.
Willingness of the centres and doctors to cooperate
Political and financial support.
Health care policy conditions.
These conditions are largely unchangeable. Accordingly, the desired network structures must take these conditions into account and integrate them into the network concepts.
Goals for the establishment of a network
A nationwide supply with specialised centres should be established.
Individual, especially not yet fully established or experimental reproductive techniques such as cryopreservation and transplantation of ovarian tissue and cryopreservation of testicular tissue from prepubertal boys should be centralised.
Regular information events should be carried out by the participating centres and associated disciplines.
A data register is to be established.
The establishment of a good regional or national network should try to achieve all these goals. Only partial implementation is possible in some regions and countries.
Structural composition of networks
Networks are often modular; the number of modules depends on their size (Figure 1).
Figure 1.
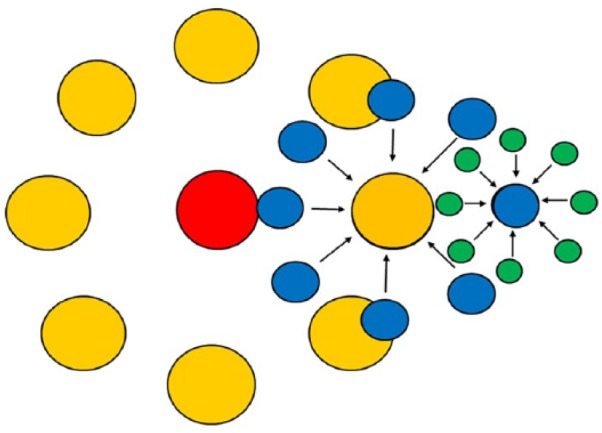
Networks are frequently set up as multimodular structures. Small modules (blue) such as infertility centres or hospitals integrate gynaecologists and oncologists etc. (green). Several of these small modules (blue) are organised as a medium-sized network (orange) such as regional or small national networks. Several of these medium-sized modules (orange) are organised as a large network with a centralised body (red) which organises registries, scientific activities, conferences, and so on.
The conditions and intentions of these modules are different (Table 1). The smallest modular unit is usually a reproductive medicine centre or a clinic that networks regionally or within the clinic with oncologists. Patients are referred to the reproductive medicine centres directly by the oncologists. The therapy decision is often based on direct bilateral communication. The reproductive medicine centre documents the treatments so that the data can later be passed on to a registry.
Table 1.
Characteristics of the different sized networks.
| Characteristics | Local and regional networks | Small national or nationwide networks | Large national networks | Very large networks or continental networks |
|---|---|---|---|---|
| Example | Reproductive medicine centre or clinic | Danish network (www.rigshospitalet.dk) | FertiPROTEKT (www.fertiprotekt.com) | Oncofertility Consortium (www.oncofertility.northwestern.edu) |
| Centralization of facilities (eg storage of gonadal tissue) | Yes | Yes, mostly one facility | Yes, mostly several facilities | Rather no |
| Continuing education | Bilateral exchange | Continuing education | National congresses | International congresses |
| Data collection in registers | Very possible | Quite possible, high data quality | Possible, lower data quality, but high data quantity | Possible to a limited extent |
| Political activities | Not as good | Good | Good | Not as good |
The next medium-sized modular stage is a union of local units into a small national or large regional network. An example of such a network is Denmark (www.rigshospitalet.dk). The centres know each other, and personal communication is possible. Data from the local units are merged into a register, which is relatively easy to create due to its limited size. It is easily possible to establish centralised, highly specialised facilities, for example, cryopreservation of gonadal tissue. The establishment of such centralised facilities allows high-quality fertility-protective techniques, scientific evaluation, good transparency of activities, and thus also health care policy initiatives. Due to short travel distances, shorter training courses can be organised with the help of oncologists. The strengths of these medium-sized networks lie in the possibility of being able to collect high-quality data, as detailed data documentation is usually possible.
In very large regions or larger countries, several network structures are combined into one large network. One such example is the FertiPROTEKT network (www.fertiprotekt.com). Accordingly, there may be several central cryopreservation facilities. Continuing education takes place every 1 to 2 years, mainly with reproductive physicians and biologists, but less with oncologists. These are usually spread over 1 to 2 days because of the longer travelling distances. A register requires good and easy-to-use online input tools to ensure reliable data entry. The strength of these large networks lies in their ability to collect relevant amounts of data. The level of detail of this data is limited by online data collection.
In addition, it is possible to combine several of these networks for data collection and professional exchange. Examples of such international networks are the ‘Oncofertility® Consortium’ (www.oncofertility.northwestern.edu), the Special Interest Group ‘Fertility Preservation’ of the European Society of Human Reproduction and Embryology (ESHRE) (www.eshre.eu/Specialty-groups/Special-Interest-Groups/Fertility-Preservation.aspx), and the ‘International Society for Fertility Preservation’ (ISFP) (www.isfp-fertility.org).
Obstacles to Creating Networks
The explanations have shown that networks are clinically, scientifically, and politically of great importance. In practice, however, they are often difficult to implement.
What are the reasons for this?
In most cases, networks can only be implemented in regions and countries that have good medical, technical, and infrastructure care. Thus, the topic of fertility protection can only be of importance if sufficient oncological care is guaranteed. If these requirements are not met, network-based care with fertility-protective measures is hardly possible.
In industrialised, well-developed countries, there are no infrastructural obstacles to the establishment of networks. Nevertheless, it is often difficult to build networks. The main obstacle is the ‘human factor’ (Table 2).
Table 2.
The human factor as the main obstacle in the establishment of networks.
| Obstacles to establishing networks | Ways to avoid these obstacles |
|---|---|
| Scientific competition | • Consideration of all persons involved as co-authors in publications |
| Lack of time for documentation | • High-quality documentation software • Interfaces with already established national registers to avoid repeated entries |
| Lack of interest | • Sensitisation to the fact that fertility-protective measures are also economically relevant • Use of the network website as an advertising platform for member centres • Democratic voice in decision-making processes • Annual membership meetings at interesting locations with an interesting programme • Introduction of certificates for member centres and their external presentation |
| Lack of awareness | • Development of good websites and linking to member centres to increase Internet presence • Regional and national information events |
| Lack of willingness to cooperate | • Development of political activities to enforce cooperation |
When setting up a network, priority should be given to examining what can motivate active participation. University centres are often more interested in scientific activities and cooperation, private centres more in economic advantages, and the use of networks as an advertising platform. Possible motivations for active participation must be identified and integrated into the network programmes. The willingness to participate in the network can often be increased by a democratic voice. If there is a danger that the right to have a say in the network will hamper its development, a democratically elected network board can also make sense to promote the development and expansion in a targeted manner with a small group of board members.
Financing of Networks
The financing of networks differs regionally and nationally. The start-up financing for setting up a network may differ.
Only a few thousand Euros were initially available from a pharmaceutical company for the FertiPROTEKT network to set up a website. All other costs were covered by the members. Annual continuing education was covered by pharmaceutical companies and participation fees. All other activities were initially performed voluntarily.
In contrast, the United States initially made 22 million dollars available for the founding of the Oncofertility Consortium.
Undoubtedly, generous start-up financing is advantageous for the establishment of a network. Far more decisive is not the amount of funding but the initiative and willingness of a few people.
The following are necessary to start a network:
An initiation meeting with as many reproductive medicine centres as possible.
The development of a network name and logo.
A website that can be created largely free of charge by network members with IT experience.
An online documentation tool which can be created largely free of charge by network members with IT experience.
Regular (eg annual) continuing education events.
Introduction of the Danish Network – Centralised Network for Smaller Countries/Large Cities
History
Inspired by research efforts three decades ago from a large number of people including Roger Gosden, Outi Hovatta, Kutluk Oktay, and David Baird, it became clear that it would be potentially clinically feasible to freeze human ovarian tissue with the intention of replacing it later to restore ovarian function. At the end of the last century, encouraging results emerged from primate studies and from transplantation of human ovarian tissue.6,7 This sparked the first clinical initiatives of freezing ovarian tissue in the United Kingdom and Belgium. In Denmark, our laboratory conducted a number of mouse studies, in which different cryoprotectants and freezing protocols were evaluated.8 However, Danish legislation clearly stated that it was illegal to transplant ovarian tissue to a woman. In 1998, we had a direct correspondence with Danish Minister of Health and he concluded that there were no restrictions on freezing ovarian tissue from a woman as long as only autologous transplantation was considered. There was no time limit on the storage period and normal medical rules applied for use of this technique including replacement of frozen thawed tissue. In addition, the Minister of Health informed that similar rules applied for testicular tissue. This was basically a very liberal rule that placed this new technique in the context of normal medical practice and the concept of ‘do no harm’.
At the end of the last century, our own laboratory had a more than 40-year-old tradition for studying the physiology of and working with human ovaries as the only laboratory in Denmark focussing on female reproduction. We started out with a clinical service freezing ovarian tissue at our local hospital in 1999, and soon thereafter, other parts of Denmark also wanted to start. We shortly realised that it would be more effective if we could centralise this service, which at that time was only performed occasionally. Instead of doing few cases per centre, we reasoned that it would provide a better service if the activity was centralised to the laboratory in which the knowledge and expertise of dealing the human ovaries was already available. On this background, we developed what is now known as the Danish concept for freezing ovarian tissue ‘the woman stays the tissue moves’ (Figures 2 and 3).9-12
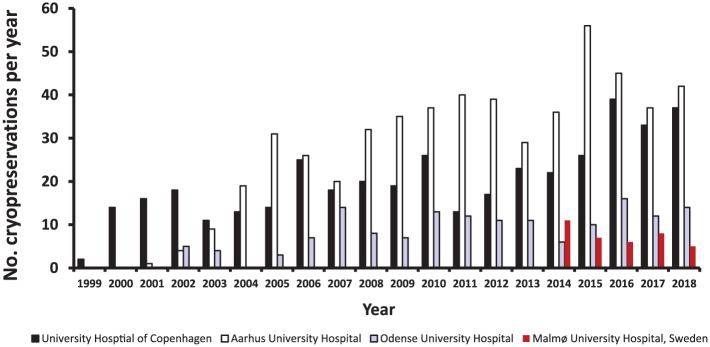
Figure 2.
Number of cryopreservations of ovarian tissue per year in the collaborating centres covering all of Denmark and the very southern part of Sweden.
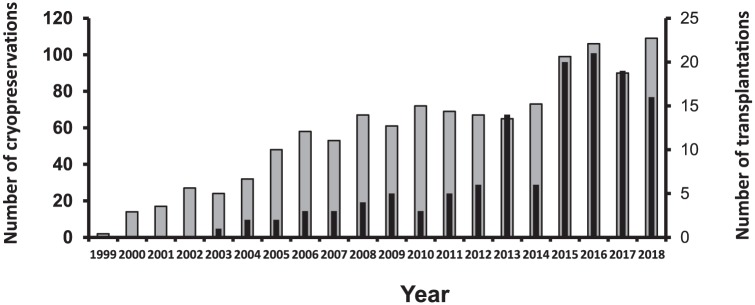
Figure 3.
Yearly number of cryopreservations of ovarian tissue (grey bar) and number of transplantations of frozen/thawed ovarian tissue (black bar).
Structure
Cancer treatment in Denmark is centralised mainly to three university hospitals located in different parts of Denmark. Each of these hospitals also has a fertility clinic which focus on in vitro fertilisation (IVF) treatment and other assisted reproductive technology (ART) procedures. In addition, our network now also includes Skåne Region of Sweden, in which the University Hospital of Lund and Malmø are members. These two regions have entered a formal agreement, allowing patients from one country to be treated in other country in the area of reproductive medicine. The Swedish side also has a fertility clinic that is coordinating the local counselling. The Skåne Region is now connected to Copenhagen via a bridge, and the transport time from the local hospital to the central laboratory is maximally 1 to 2 hours by car and lower from other parts of Denmark. Importantly, all these clinics are public-funded hospitals, and patients receive treatment for free paid for by the taxes, including extraction of tissue, freezing, storage, and transplantation. Furthermore, if ART is needed after transplantation, this will also be covered by the public health care system. Basically, any Danish woman or women in the Skåne Region of Sweden, who potentially may benefit from these procedures and who qualify for these treatments (ie age, diagnosis, and clinical evaluation) should have it offered and then it is her decision to accept or decline the offer.
In each of these fertility clinics, one or two consultants have specialised in fertility preservation. Patients potentially requiring fertility preservation identified in the oncological, haematological, or other departments in which patients are exposed to potential gonadotoxic treatment are referred to the consultants of the fertility clinic where they immediately get a consultation. Here, the different options are discussed with the patient including ovarian stimulation with cryopreservation of mature oocytes, excision, and cryopreservation of ovarian tissue or doing nothing. Depending on the clinical evaluation and the patient’s wishes, a plan for fertility preservation is agreed upon. In case freezing of ovarian tissue is planned, a date will be agreed upon with the central laboratory performing the cryopreservation and the surgeons excising the ovarian tissue at the local hospital.
The surgical intervention to extract the tissue is normally the first operation on that day, and the tissue will be able to reach the central laboratory in maximally 4 to 5 hours. After excision of the ovarian tissue, the surgeon will bring it to the local laboratory, where the tissue is placed in a 50-ml tube with basal medium that goes into a flamingo-box filled with crushed ice to maintain temperatures around 0°C. The box is transported to the central laboratory that checks for the presence of ice and processes the tissue immediately after arrival.
After cryopreservation, the tissue will be stored at the central laboratory and kept in liquid nitrogen until potential use or until the patient decides otherwise for her tissue. The central laboratory is accredited by the Danish authorities to conduct this treatment including a licence according to the European Union (EU) tissue directive. We have collaborated with the competent Danish authorities to formulate guidelines for new clinics and networks starting out to cryopreserved ovarian tissue.13 If the patient request transplantation, the tissue will be transported in liquid nitrogen to the local hospital, where the surgical procedure of replacing the tissue will take place.
The clinical follow-up of transplanted patients is performed by the local hospital including monitoring of whether the patient becomes pregnant, experience relapse and so on.
Representatives from the participating clinics in the network will meet to discuss the service and results to align policies and various other matters when needed. We are setting up a framework for a database containing all information on patients who have ovarian tissue cryopreserved, which will provide valuable information looking forward. The competent Danish authorities secure implementation of the EU tissue directive and also function as advisers to the political system. We have discussions with authorities and have direct political contacts where we try to modify the Danish regulations to allow storage of tissue for social indications and for postponing menopause at the cost of the patient herself.
Financial support
Initially, the project was financed from external funding by the Danish Cancer Society, but around the last 10 years, the treatment has been recognised as an established treatment that the public health care system covers. As the technique is almost exclusively performed in the public system of hospitals that accounts for more than 99% of activity in the medical field anyway, the patient categories who have this technique offered for free have been limited to patients with a risk of iatrogenic-induced follicle loss and infertility plus patients with a genetic condition that may render them infertile prematurely.
Scientific focus
The scientific focus in Denmark has during recent years been on the surplus medulla tissue that contains growing follicles that do not sustain freezing (Table 3). Normally this tissue is discharged, but we have ethical permission to ask women for donation of this surplus tissue for research purposes. Furthermore, we can ask women who have cortical stored and who do not wish to continue storage for permission to use the tissue for research purposes. This has resulted in an unprecedented access to normal ovarian tissue both fresh and frozen from women at various ages and has allowed us to study human folliculogenesis.
Table 3.
Scientific focus and examples of corresponding publications with the participation of the Danish network.
| Scientific focus | Examples of publications |
|---|---|
| • New technologies, options, and issues of importance for fertility preservation and restoration | • Andersen et al14
• Schmidt et al15 • Rosendahl et al16 • Rosendahl et al17 • Schmidt et al18 • Andersen et al19 • Donnez et al20 • Andersen21 • Andersen and Kristensen22 • Kristensen et al23 • Andersen et al13 • Kristensen et al24 |
| • Human small antral follicles | • Andersen and Byskov25
• Andersen et al26 • Nielsen et al27 • Andersen et al28 • Nielsen et al29 • Jeppesen et al30 • Jeppesen et al31 • Jeppesen et al32 • Kristensen et al24 |
| • In vitro follicle growth and follicle activation | • Schmidt et al33
• Meirow et al34 • Yin et al35 • Kristensen et al36 • Kristensen et al37 |
| • Evaluation of potential malignant cell contamination in ovarian tissue | • Rosendahl et al38
• Rosendahl et al39 • Andersen et al10 • Greve et al40 • Greve et al40 • Dolmans et al41 • Ernst et al42 • Sørensen et al43 • Andersen et al44 • El Issaoui et al45 |
| • In vitro maturation | • Wilken-Jensen et al46
• Yin et al47 • Gruhn et al48 |
| • Transplant tissue to restore reproductive and/or endocrine function | • Schmidt et al49
• Schmidt et al50 • Rosendahl et al16 • Andersen et al51 • Ernst et al42 • Greve et al52 • Schmidt et al53 • Greve et al54 • Ernst et al55 • Rosendahl et al56 • Macklon et al57 • Jensen et al11 • Jensen et al58 • Jensen et al59 • Gellert et al60 • Matthwes et al61 • Lunding et al62 |
| • Cryopreservation and transport protocols do not affect tissue quality nor follicle growth | • Schmidt et al15
• Rosendahl et al8 • Kristensen et al63 |
| • Human preantral follicles | • Kristensen et al64
• Markholt et al65 • Kristensen et al66 • Kristensen et al67 |
| • Patient attitudes and effect of cryopreserving one ovary | • Schmidt et al68
• Macklon et al69 |
Lately, we are focussing on establishing an optimised platform for human in vitro maturation (IVM), since the surplus medulla tissue contains immature oocytes which may become a surplus fertility-preservation option in case metaphase II (MII) oocytes can be generated in sufficient numbers with sufficient quality. We are in the situation that IVM is not considered a standard procedure in Denmark, and we are legally unable to use the MII oocytes generated for clinical purposes.47
Key points for success of the network
The most important task of the network has been to establish a patient friendly and patient-oriented treatment offer that provided stable, reliable, and well-documented results in terms of follicle survival in connection with the freezing procedure and renewed and improved ovarian function after transplantation. Our service has provided these results to a large extent:
Following transplantation frozen/thawed ovarian tissue more than a total of 130 times; the tissue has consistently provided renewed and improved ovarian function for variable time periods mainly depending on the initial follicular density, patient age, and amount of tissue transplanted. Except for one case, where a woman in her mid-thirties had only three pieces of cortical tissue frozen, which did not result in ovarian function after transplantation.
It has been a focus area to employ a freezing technique with as good a follicular survival rate as possible. We have recently published a new quantitative method to evaluate follicular survival following a period of freezing and found a survival rate of on average 84%, with a 91% rate of healthy follicles in unfrozen control samples.70 Furthermore, survival rates were constant over a period of freezing lasting 17 years and similar irrespective of whether the tissue was transported or not.
During several different interviews, patients have expressed great satisfaction with the treatment offer and the ability to stay in the local environment at difficult times having just faced a cancer diagnosis. The psychological impact of having tissue stored has a massive positive impact on many patients.
What can be further improved and further challenges
We have now recently documented that the quantitative survival of follicles during the freezing process is very high showing that only a minor part of follicles is lost during the freezing process.70 In contrast, the follicle loss during transplantation due to poor vascularization, ischemia, and reduced oxygen tension accounts for the clear majority of follicle demise. We are therefore now trying to improve follicle survival during the initial stages of revascularization by interfering with the processes that lead to follicle atresia.
Furthermore, one aspect that can be optimised and which is of obvious importance for the patients is the speed at which fertility preservation is executed. They may suffer a potential deadly disease and require in many instances gonadotoxic treatment as fast as possible. We are now trying to monitor the speed at which the procedure is executed and whether there may be cases where we can reduce the time needed to perform the fertility-preservation procedure.
Introduction of FertiPROTEKT Network – a Partly Decentralised Network for Large Countries
History
At the start of this millennium, various scientific endeavours in the field of fertility protection already existed in Germany,71,72 but there was still no coordinated counselling and care of patients. Therefore, at the initiative and invitation of Prof M. von Wolff (then Department of Gynaecological Endocrinology and Fertility Disorders, Heidelberg) and Prof M. Montag (then Department of Gynaecological Endocrinology and Reproductive Medicine, Bonn), 30 university reproductive medical centres met in Heidelberg in May 2006 and founded the FertiPROTEKT network. The members elected a leadership team for 2 years each, in which both physicians (representatives of university and non-university centres) and biologists were represented.73 Since 2008, private centres can also become members. The network now also includes centres from Austria and Switzerland (www.fertiprotekt.de, www.fertiprotekt.ch, www.fertiprotekt.at, and www.fertiprotekt.com).
However, the increasingly well-known national and international network was initially not a founded scientific society and was in danger of, for example, being insufficiently recognised and acknowledged in professional policy discussions or in the development of guidelines by other (including interdisciplinary) scientific societies. Therefore, in 2015, the decision was made to create a scientific society. The FertiPROTEKT Network e.V. was founded by the then management team on November 10, 2015, in Hamburg. The main office is in Germany (Marburg/Lahn).
Centres that would like to become members of the FertiPROTEKT network must prove with their application that they not only advise on fertility protection but that they can also implement all fertility-protective methods themselves or in established cooperations.
Structure
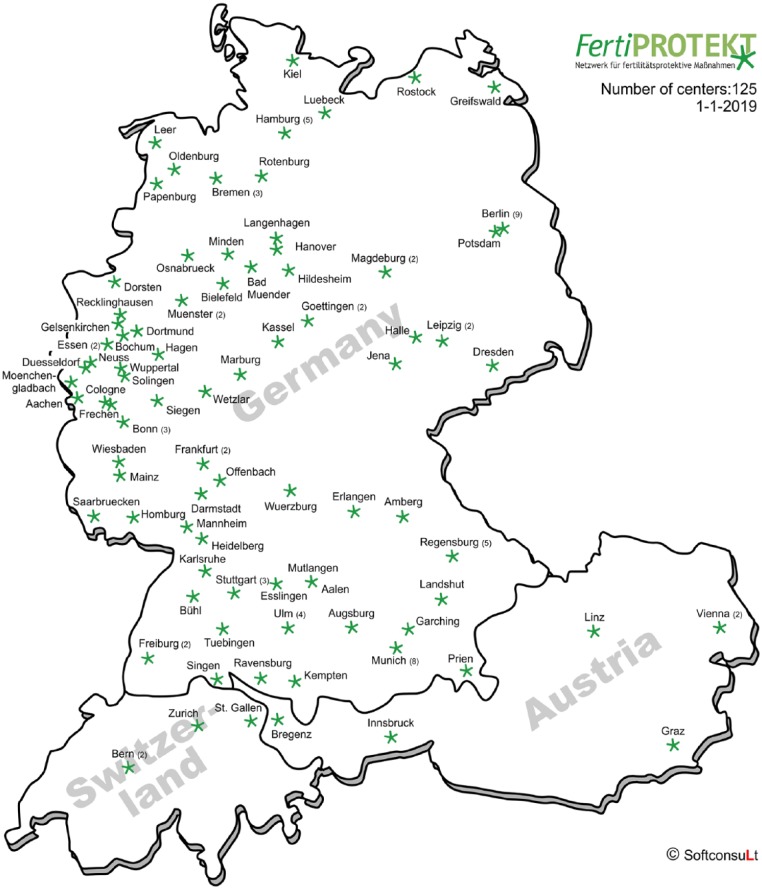
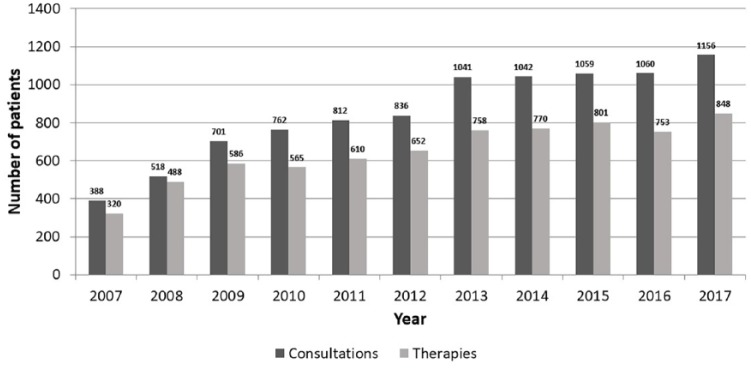
The FertiPROTEKT network comprises 125 centres. In 2017, 1156 consultations and 848 documented fertility-protective therapies were performed (Figures 4 and 5).
Figure 4.
FertiPROTEKT centres in Germany, Austria, and Switzerland.
Figure 5.
Number of documented counsellings and treatments performed by the large German-Austrian-Swiss network FertiPROTEKT.
The structural characteristics shown in Table 1 are represented as follows in the network FertiPROTEKT.
Centralisation of facilities
This applies to the centralization of cryobanks for ovarian tissue in the FertiPROTEKT network. Specialised cryobanks are associated with the university gynaecology clinics in Bonn, Düsseldorf, and Erlangen. There is no compulsory requirement for the member centres to send their samples to these cryobanks; however, they do so with great participation.
The cryobanks have established a transport logistics system which plans that the peripheral member centre providing advice and the indication for cryopreservation of ovarian tissue orders a special transport container from the cryobank and has it transported to the relevant centre. The latter sends the tissue immediately postoperatively in this container in a nutrient medium on ice (ie under defined conditions) to the cryobank overnight, where it is prepared and cryopreserved.
Continuing education
From 2006 to 2018, 2-day working meetings were held annually at different locations, organised logistically and in terms of content by the board of the network and a representative of the member centre of each venue. In addition to individual lectures, the programme included workshops on various fertility-protection methods. From 2018, these meetings will only be held every 2 years because FertiPROTEKT has since then been able to conduct scientific meetings on topics of its own choice at least once a year at their congress(es), thanks to its cooperation with other scientific societies.
Data collection in registers
Data are currently entered in a self-created register, which is accessible to the member centres via the intranet of the FertiPROTEKT homepage. These data are evaluated once a year and is presented to the members. From 2019, FertiPROTEKT will cooperate with the German IVF Register (https://www.deutsches-ivf-register.de/), which has adapted its data collection and evaluation for this purpose. The FertiPROTEKT data will then be integrated into the so-called IVF Yearbook, in which the German IVF Register has presented the results of all German ART cycles for many years. FertiPROTEKT finances this cooperation including programming work and so on via the Society’s account.
Political activities
FertiPROTEKT is a well-known and well-networked contact partner for other scientific societies; patient organisations including self-help groups or political parties due to its long-standing presence at congresses; many publications; and other scientific activities. The most important current focus of political activities is efforts to enforce the financing of fertility-protective services by the health insurance companies.
Financial support
When the FertiPROTEKT network was founded, only a few thousand Euros were available from a pharmaceutical company to set up a website. All other costs were covered by the members. Annual continuing education was covered by pharmaceutical companies and participation fees. All other activities were carried out on a voluntary basis.
Since the founding of the FertiPROTEKT Society, membership fees have been available which are charged annually per centre at a cost of 180 Euro. In addition, there are sponsoring members in the form of pharmaceutical companies who together pay 15 000 Euro annually. These revenues can be used to run an office and fund board meetings as well as a few network activities. The website and the register will continue to be run by the members on a voluntary basis.
Scientific focus
The scientific strengths lie in register analyses, studies with tissue stored in centralised cryobanks, smaller multicentre studies, and the compilation of practically oriented recommendations (Table 4).
Table 4.
Scientific focus and examples of corresponding publications with the participation of the FertiPROTEKT network.
| Scientific focus | Examples of publications* |
|---|---|
| • Efficacy of luteal phase and random-start ovarian stimulation | • von Wolff et al74
• von Wolff et al75 |
| • Effectiveness of ovarian stimulation with various concurrent diseases | • Henes et al76
• Henes et al77 • Henes et al78 • von Wolff et al79 |
| • Transport and transplantation of ovarian tissue | • Dittrich et al80
• Dittrich et al81 • Van der Ven et al2 • Liebenthron et al82 • von Wolff et al83 |
| • Combination of ovarian tissue cryopreservation and ovarian stimulation | • Huober-Zeeb et al84 |
| • Fertility protection in children and adolescents | • Sänger et al85
• Sänger et al86 |
| • Counselling and treatments in the network | • Lawrenz et al87
• von Wolff et al88 • von Wolff et al89 |
| • Recommendations for fertility protection | • von Wolff et al90
• Schüring et al91 • von Wolff et al92 • Dittrich et al93 |
See also keyword «Fertiprotekt» in PubMed (www.ncbi.nlm.nih.gov).
Key points for success of the network
Establishment of numerous and qualified counselling and therapy centres
The greatest merit of the network is the consolidation and coordination of many advisory member centres, which helps patients and oncologists, to find a contact person for advice on fertility-protection measures even at short notice, who can also implement them promptly if they so wish.
Coordination of content of counselling and therapies
Through studies, publications of recommendations and joint exchange among member centres and other specialist areas, the network has created a basis for the content-related consultation and implementation of fertility-protection therapies. The annual 2-day national member meetings, in which approximately 200 persons participate, are an essential element for the coordination of content.
Documentation of consultations and therapies in a register
A register for fertility-protective consultations and therapies has existed since 2007. Due to the high quantity of data and the further improvement in quality in the future (through data entry via the national IVF register), there have been and will be opportunities in the future to clarify scientific questions. In addition, the amount of data is an argumentation aid, for example, in the enforcement of cost absorption by health insurance funds.
Initiation, implementation, and support of studies
The close cooperation and common interests in the network enable a timely agreement on the initiation of studies and their implementation. Scientific questions from member centres can be dealt with conceptually within a short period of time and can be investigated by joint prospective or retrospective studies on larger amounts of data.
Definition of standards and publication of recommendations
Joint working meetings in combination with our own study data and international experience form the basis for the constantly developing standards discussed in the network, as well as published recommendations for counselling and therapy in the context of fertility protection. The first recommendations were published in 2011 with ‘online access’ to make them available to as many readers as possible free of charge.90 The update was carried out in 2018 with a paper on indications based on selected diseases and another on fertility-protective techniques.91,92 The association also financed the online access for these two publications. A German Austrian and Swiss guideline on fertility preservation was prepared and published93 by the AWMF with the contribution of FertiPROTEKT. Furthermore, a practically orientated textbook on fertility preservation has been published by the FertiPROTEKT network. It is currently re-edited and will then not only be available in English but also in German.
What can be further improved and further challenges
At a strategy meeting in April 2018, the executive board of the FertiPROTEKT network intensively discussed topics including cost absorption by health insurance funds, research, marketing, contacts with national, and international scientific societies as well as the documentation of register data and developed a basis for concepts and solutions that will be implemented over the coming years. The main objectives are, on one hand, an even closer oncological link, for example, to oncological societies, to better coordinate the indications for counselling, and for the implementation of fertility-protective measures and, on the other hand, the implementation of cost reimbursement of fertility-protective measures by health insurance companies.
Introduction of the Oncofertility Consortium – A Decentralised Global Network
History
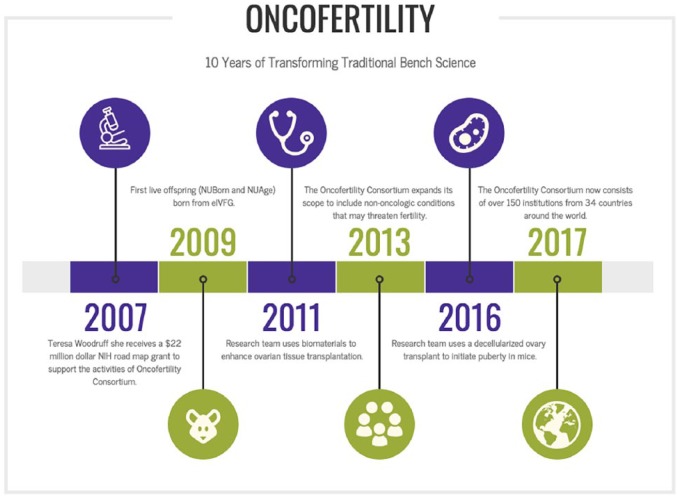
Oncofertility is a discipline that merges oncology with fertility and has moved rapidly from the purview of individual champions to an integrated field that has become standard of care in many institutions.94 Oncofertility as a field has developed in parallel to the many life-preserving advances in oncologic care, including earlier diagnostics and the emergence of targeted cancer therapies, methods to reduce radiation dose and field, and localised surgical procedures. Addressing the complex treatment plans, general health, and quality of life issues that concern young cancer patients whose fertility may be threatened by their disease or its treatment is a priority, and the Oncofertility Consortium has led efforts in this area for almost 15 years.94-104 Northwestern University was first funded as a Specialised Cooperative Centre Programme in Reproductive Research (SCCPIR) in 2003, and the Centre for Reproductive Research focused on understanding structure-function relationships in reproductive science. This centre provided a mechanism to bring new perspectives to reproductive science from ancillary disciplines (eg bioengineering and structure biology). In 2007, we transitioned this fundamental science to a National Institutes of Health (NIH) Roadmap Interdisciplinary Research Consortium – the Oncofertility Consortium – to specifically address the intractable problem of fertility-preservation options for young female cancer patients.94 In 2012, we broadened the scope of the Oncofertility Consortium to include the Centre for Reproductive Health After Disease, whose mission is to protect and preserve the reproductive health – including fertility, endocrine health, sexuality, or the ability to carry an offspring to term – of women at reproductive risk after disease or treatment of disease.
Structure
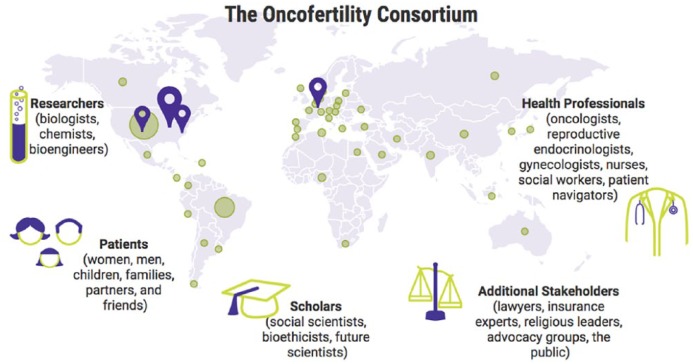
The multidisciplinary, international approach to the Oncofertility Consortium consists of research sites Oncofertility Professional Engagement Network (OPEN) Members, and key members of the multidisciplinary oncofertility team. The Oncofertility Consortium includes stakeholders across the width and depth of the academy, across departments and institutions, and includes expert core facilities and sophisticated human specimen collection and use and disseminates our work to patients, families, and the public. These are meaningful interactions that are fostered by the presence of a strong organisation like the Oncofertility Consortium and through leadership at the academic and staff level.
Centralisation of facilities
The Oncofertility Consortium Network spans six continents, including 40 countries around the globe and 97 sites in the United States (Figure 6). The OPEN works to engage researchers and clinicians both domestically and aboard, and these communities converge at the Annual Oncofertility Consortium Conference held in Chicago, Illinois, each year.
Figure 6.
Decentralised structure of the Oncofertility Consortium.
Purple indicates key research centres and green circles indicate OPEN members, both clinical and research sites around the globe.
Continuing education
Education is a hallmark of the Oncofertility Consortium’s programming. We have created numerous field forming and changing tools that have catalysed the growth of oncofertility. These items include the first comprehensive oncofertility textbook, training videos, and educational materials that are enduring but also updatable. These products are listed below in more detail. In addition to these, we also house institutional review board (IRB) documents that can be used as templates for those wanting to start an oncofertility programme, a Follicle Culture Handbook for basic scientists, and many other materials that enable faster adoption of best practices by members of the broader field. Through the use of Facebook, Twitter, and other social media modalities, the Oncofertility Consortium facilitates the ability of our projects to be communicated in a way that enables the public to see how the work is progressing in a lay-friendly manner.
Annual Oncofertility Consortium Conference
The Oncofertility Consortium hosts an annual conference to convene the field and set priorities for the upcoming year. Oncofertility Consortium focuses on providing attendees at the annual Oncofertility Conference the ability to connect with colleagues from around the world to share research and clinical case studies to facilitate a rapid pace of growth within the field and expand resources to non-malignant conditions. The Oncofertility Conference is a place where the field-wide advances are shared through traditional lectures, hands-on training, and small group sessions. The variety of education settings address the wide range of education levels and backgrounds represented at the conference.
Fellow Education Day Symposium
At the annual Oncofertility Conference, we host an annual Fellow Education Day Symposium. The purpose of the course is to educate fellows on fertility-preservation options and survivorship care for cancer patients across the reproductive life cycle. The course will also model a team approach to fertility-preservation care. The course is comprised of didactic lectures given by leaders in the field interspersed with complex clinical cases that will be reviewed in interdisciplinary teams. Participants are supplementary materials for review before course attendance to facilitate an interactive ‘flipped classroom’ approach to team-based learning. This course uses e-learning modules that were created with the American Society for Reproductive Medicine (ASRM).105
Oncofertility textbooks
Together with other colleagues in the field, we have published seven books to encompass the areas of basic science, ethics religion and the law, medical practice, communication strategies, paediatric and disorders of sexual development populations, and non-oncologic and other non-malignant fertility threatening conditions, as well as the first of its kind Oncofertility Textbook, which includes didactics. The hope is these books, which aggregate everything we know in the field, serve as a starting point for material that will become integrated into the major oncology, internal medicine, and reproductive texts of our professions.
Oncofertility Saturday Academy
The Oncofertility Science Academy was created in 2007 as a way to introduce underserved high school girls from the Chicagoland area to science and medicine by engaging them in hands-on lab and clinical activities on the Northwestern University medical campus. Northwestern’s OSA programme impacted more than 275 students with 5 students securing oncofertility research internships or employment within the Woodruff Lab; a true example of training the next generation of future clinicians and scientists. The Oncofertility Saturday Academy (OSA) model addresses the gap in reproductive science education at the high school level and provides an adaptable education model that can be implemented across multiple institutions. Currently, OSA curricula are available at Northwestern University; University of Pennsylvania; University of California, San Diego; and Oregon National Primate Research Centre and has impacted 545 high school girls nationally. Learning goals of OSA include providing students with hands-on laboratory and clinical activities, incorporating art modules for learning scientific and medical information in a new format and developing relationships with scientists, doctors, and other professionals.106
Data collection in registers
The Oncofertility Consortium does not have any formal registries or patient data collection.
Political activities
As the Oncofertility Consortium is housed within Northwestern University, the university policy limits lobbing activities and the Consortium’s ability to directly contact politicians and participate in many political activities. However, the Oncofertility Consortium works with other politically motivated groups, like the Alliance for Fertility Preservation, to push forward legislative activities in any capacity within its scope. The Oncofertility Consortium is the great convener in the United States and helps to make critical connections among members of the oncofertility community, like lawyers, patients, and politicians, to ensure that fertility-preservation coverage is attainable in each state. Currently, there are five states in the United States that require insurance companies to cover oncofertility and fertility-preservation services for cancer patients.
Financial support
Most of the funding for the Oncofertility Consortium’s activities comes from the NIH and the Eunice Kennedy Shriver Institute for Child Health and Human Development (NIH/NICHD). Its efforts are currently supported by the Centre for Reproductive Health After Disease (P50HD076188) from the NIH National Centre for Translational Research in Reproduction and Infertility (NCTRI). The annual Oncofertility Consortium Conference has been funded for years by an NIH grant (5R13HD063248), as well as institutional funds from the Robert H. Lurie Comprehensive Cancer Centre at Northwestern University and the Office of the President. In addition to these funds, we have secured other funding from industry partners including EMD Serono, Merck, Ferring, Walgreens, and Reprotech Ltd. We have targeted a variety of sources to enable our success and made innovation and invention in communication methods a main mission of our programme.
Scientific focus
Mechanisms underlying the fertility threat of life-preserving cancer drugs (Figure 7, Table 5).
Methods for cryopreservation (freezing), storing, and growing ovarian and gonadal tissue.
In vitro follicle grown and oocyte maturation using a three-dimensional environment.
Communication barriers between cancer patients and health care providers.
Ethical and legal concerns regarding the use of fertility-preservation technologies in cancer patients.
Figure 7.
10 years of transforming traditional bench science in the Oncofertility Consortium network.
Table 5.
Scientific focus and examples of corresponding publications with the participation of the Oncofertility Consortium network.
| Scientific focus | Examples of publications |
|---|---|
| • New technologies for fertility preservation and restoration | • Jakus et al107
• Laronda et al108 • Laronda et al109 • Que et al110 • Rios et al111 • Skory et al112 • Treff et al113 |
| • In vitro follicle growth | • Silva et al114
• Xiao et al115 • Xiao et al116 |
| • In vitro maturation | • Kidder117
• Sowińska et al118 |
| • Transplant tissue to restore reproductive and/or endocrine function | • Lunardi et al119
• Oktay and Buyuk120 • Salama and Woodruff121 • Smith et al122 |
| • Create an embryo from mature follicles within the tissue | • Kizuka-Shibuya et al123 |
| • Cryopreservation and transport protocols do not affect tissue quality nor follicle growth | • Armstrong et al124
• Duncan et al125 • Duncan et al126 |
| • Individual follicles can be cryopreserved and quality assessed | • Duncan et al126 |
| • Primary follicle number may not be a predictor of future fertility and can be cultured | • Bortoletto et al127
• Duncan et al125 • Finlayson et al128 • Kniazeva et al129 • Laronda et al130 • Tagler et al131 |
| • Follicles can be grown in vitro to the large antral stage | • Duncan et al125 |
| • Patient access | • Ataman et al104
• Bortoletto et al127 • Clayman et al132 • Fuchs et al133 • Gargus et al134 • Lawson et al135 • Lawson et al136 • Llarena et al137 • Rashedi et al138 • Rashedi et al139 • Stein et al140 • Smith et al141 • Waimey et al142 |
Key points for success of the network
Because of the intrinsic value in creating diverse networks and collaborations, the Oncofertility Consortium continues its efforts to connect local centres of excellence and create a strong global network of diverse collaborators, many of whom may not have worked together otherwise. The Oncofertility Consortium supports interaction between global and local partners to create momentum for clinical activities (shared protocols and patient case studies, inclusion of allied health professionals), research (sharing results, both failures and successes, in ways that hasten work), and meeting patient needs (educational websites, patient decision tools, and patient navigator). By facilitating these interactions, the Oncofertility Consortium ensures the coordinated effort of the global oncofertility community in conducting cutting-edge research that can continue to be rapidly translated to the clinic and establish an evidence-based standard of care.
What can be further improved and further challenges
There are a number of common barriers that are commonly identified by OPEN members and the Oncofertility Consortium. These barriers include lack of insurance coverage and high out-of-pocket costs for patients, lack of awareness among providers and patients, cultural and religious constraints, and lack of funding to help to support oncofertility programmes. Despite these barriers, many opportunities exist to grow the field of oncofertility. Continuing to engage stakeholders around the globe and expand the efforts of the Oncofertility Consortium will aid in the acceptance of oncofertility on a global level thus accelerating the pace of research from bench to bedside to babies.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MVW designed the manuscript. All authors prepared the manuscript and revised the final version.
ORCID iD: Michael von Wolff  https://orcid.org/0000-0003-4303-2734
https://orcid.org/0000-0003-4303-2734
References
- 1. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. [DOI] [PubMed] [Google Scholar]
- 2. Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31:2031–2041. [DOI] [PubMed] [Google Scholar]
- 3. Shirasawa H, Terada Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod Med Biol. 2017;16:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- 5. Picton HM, Wyns C, Anderson RA, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- 6. Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod. 1995;10:2334–2338. [DOI] [PubMed] [Google Scholar]
- 7. Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. [DOI] [PubMed] [Google Scholar]
- 8. Rosendahl M, Schmidt KT, Ernst E, et al. Cryopreservation of ovarian tissue for a decade in Denmark – an overview of the technique. Reprod Biomed Online. 2011;22:162–171. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt KL, Ernst E, Byskov AG, Andersen AN, Andersen CY. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod. 2003;18:2654–2659. [DOI] [PubMed] [Google Scholar]
- 10. Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595–608. [DOI] [PubMed] [Google Scholar]
- 11. Jensen AK, Kristensen SG, Macklon KT, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30:2838–2845. [DOI] [PubMed] [Google Scholar]
- 12. Kyono K, Hashimoto T, Toya M, et al. A transportation network for human ovarian tissue is indispensable to success for fertility preservation. J Assist Reprod Genet. 2017;34:1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen CY, Bollerup AC, Kristensen SG. Defining quality assurance and quality control measures in connection with ovarian tissue cryopreservation and transplantation: a call to action. Hum Reprod. 2018;33:1201–1204. [DOI] [PubMed] [Google Scholar]
- 14. Andersen CY, Byskov AG, Andersen AN. Cryopreservation of human ovarian tissue. Ugeskrift for Læger. 2001;163:5007–5013. [PubMed] [Google Scholar]
- 15. Schmidt KL, Byskov AG, Andersen AN, Muller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–1164. [DOI] [PubMed] [Google Scholar]
- 16. Rosendahl M, Andersen CY, Ernst E, et al. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow up study. Hum Reprod. 2008;23:2475–2483. [DOI] [PubMed] [Google Scholar]
- 17. Rosendahl M, Andersen CY, Freiesleben NC, Juul A, Løssl K, Andersen AN. The dynamics of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010;94:156–166. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt KTL, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. Brit J Obstet Gynaecol. 2010;117:163–174. [DOI] [PubMed] [Google Scholar]
- 19. Andersen CY, Silber SJ, Berghold SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod Biomed Online. 2012;25:128–132. [DOI] [PubMed] [Google Scholar]
- 20. Donnez J, Dolmans MM, Pellicier A, et al. Fertility preservation for age related fertility decline. Lancet. 2015;385:506–507. [DOI] [PubMed] [Google Scholar]
- 21. Andersen CY. Success and challenges in fertility preservation after ovarian tissue grafting. Lancet. 2015;385:1947–1948. [DOI] [PubMed] [Google Scholar]
- 22. Andersen CY, Kristensen SG. Novel use of the ovarian follicular pool to postpone menopause and delay osteoporosis. Reprod Biomed Online. 2015;31:128–131. [DOI] [PubMed] [Google Scholar]
- 23. Kristensen SG, Georgione V, Humaidan P, et al. Fertility preservation and re-freezing of transplanted ovarian tissue – a potential new way of managing patients with a low risk of malignant cell recurrence. Fertil Steril. 2017;107:1206–1213. [DOI] [PubMed] [Google Scholar]
- 24. Kristensen SG, Liu Q, Mamsen LS, et al. A simple method to quantify follicle survival in cryopreserved human ovarian tissue. Hum Reprod. 2018;33:2276–2284. [DOI] [PubMed] [Google Scholar]
- 25. Andersen CY, Byskov AG. Is oestradiol an important regulator for secretion of anti-Mullerian hormone, inhibin-A and inhibin-B: analysis of fluid from small antral and preovulatory human follicles. J Clin Endocrinol Metab. 2006;91:4064–4069. [DOI] [PubMed] [Google Scholar]
- 26. Andersen CY, Rosendahl M, Byskov AG. Concentration of anti-Mullerian hormone and inhibin-B in relation to steroids and age in follicular fluid from small antral human follicles. J Clin Endocrinol Metab. 2008;93:2344–2349. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen ME, Rasmussen IA, Fukuda M, Westergaard LG, Andersen CY. Concentrations of anti-Müllerian hormone in fluid from small human antral follicles shows a negative correlation with CYP19 mRNA expression in the corresponding granulosa cells. Mol Hum Reprod. 2010;16:637–643. [DOI] [PubMed] [Google Scholar]
- 28. Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–1287. [DOI] [PubMed] [Google Scholar]
- 29. Nielsen ME, Rasmussen IA, Kristensen SG, et al. Expression of androgen-receptor mRNA in granulosa cells from human small antral follicles and the corresponding follicular fluid concentrations of androgens are positively correlated to granulosa cell FSH receptor mRNA expression. Mol Hum Reprod. 2011;17:63–70. [DOI] [PubMed] [Google Scholar]
- 30. Jeppesen JV, Nielsen ME, Kristensen SG, Andersen CY. Concentration of activin A and follistatin in follicular fluid from human small antral follicles associated to gene expression of the corresponding granulosa cells. Mol Cell Endocrinol. 2012;356:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Jeppesen JV, Kristensen SG, Nielsen ME, et al. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeppesen JV, Anderson RA, Kelsey TW, et al. Which follicles make the most anti-Mullerian hormone in humans? evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt KLT, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Müllerian hormone initiates growth of human primordial follicles in-vitro. Mol Cell Endocrinol. 2005;234:87–93. [DOI] [PubMed] [Google Scholar]
- 34. Meirow D, Roness H, Kristensen SG, Andersen CY. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum Reprod. 2015;30:2453–2436. [DOI] [PubMed] [Google Scholar]
- 35. Yin H, Kristensen SG, Jiang H, Rasmussen A, Andersen CY. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term three-dimensional culture. Hum Reprod. 2016;31:1531–1539. [DOI] [PubMed] [Google Scholar]
- 36. Kristensen SG, Pors SE, Andersen CY. Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum Reprod. 2017;32:725–732. [DOI] [PubMed] [Google Scholar]
- 37. Kristensen SG, Pors SE, Andersen CY. Diving into the follicle pool. Curr Opin Obstet Gynecol. 2017;29:112–118. [DOI] [PubMed] [Google Scholar]
- 38. Rosendahl M, Andersen MT, Ralfkjær E, et al. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukaemia. Fertil Steril. 2010;94:2186–2190. [DOI] [PubMed] [Google Scholar]
- 39. Rosendahl M, Wielenga VT, Nedergaard L, et al. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–2161. [DOI] [PubMed] [Google Scholar]
- 40. Greve T, Wielenga VT, Grauslund M, et al. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumor cell contamination. Eur J Cancer. 2013;49:1932–1938. [DOI] [PubMed] [Google Scholar]
- 41. Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99:1514–1522. [DOI] [PubMed] [Google Scholar]
- 42. Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet. 2013;30:975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sørensen SD, Greve T, Wielenga VT, Wallace WHB, Andersen CY. Safety considerations for transplanting frozen/thawed ovarian tissue to restore fertility in patients who have recovered from Ewing sarcoma. Future Oncol. 2014;10:277–283. [DOI] [PubMed] [Google Scholar]
- 44. Andersen CY, Ernst E, Bærentzen S, Birkebæk NH, Clausen N. No malignancy detected in surplus ovarian tissue from a former Ewing sarcoma patient who experienced relapse four years after being grafted with frozen/thawed ovarian tissue. J Assist Reprod Genet. 2014;31:1567–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El Issaoui M, Giorgione V, Mamsen LS, et al. Effect of first line cancer treatment on the ovarian reserve and follicular density in girls under the age of 18 years. Fertil Steril. 2016;106:1757–1762. [DOI] [PubMed] [Google Scholar]
- 46. Wilken-Jensen H, Kristensen SG, Jeppesen JV, Andersen CY. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet Gynecol Scand. 2014;93:32–37. [DOI] [PubMed] [Google Scholar]
- 47. Yin H, Kristensen SG, Jiang H, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gruhn JR, Kristensen SG, Andersen CY, Hoffmann ER. In vitro maturation and culture of human oocytes. Methods Mol Biol. 2018;1818:23–30. [DOI] [PubMed] [Google Scholar]
- 49. Schmidt KLT, Andersen CY, Starup J, Loft A, Byskov AG, Andersen AN. Orthotopic autotransplantation of cryopreserved ovarian tissue to a woman cured of cancer – follicular growth, steroid production and oocyte retrieval. Reprod Biomed Online. 2004;8:448–453. [DOI] [PubMed] [Google Scholar]
- 50. Schmidt KLT, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow up of ovarian function post chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. [DOI] [PubMed] [Google Scholar]
- 51. Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. [DOI] [PubMed] [Google Scholar]
- 52. Greve T, Ernst E, Markholt S, Schmidt KT, Andersen CY. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue. Acta Obstet Gynecol Scand. 2010;89:1589–1591. [DOI] [PubMed] [Google Scholar]
- 53. Schmidt KT, Rosendahl M, Ernst E, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95:695–701. [DOI] [PubMed] [Google Scholar]
- 54. Greve T, Clasen-Linde E, Andersen MT, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120:4311–4316. [DOI] [PubMed] [Google Scholar]
- 55. Ernst E, Kjærsgaard M, Birkebæk NH, Clausen N, Andersen CY. Stimulation of puberty in a girl with chemo – and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49:911–914. [DOI] [PubMed] [Google Scholar]
- 56. Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31:1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jensen AK, Rechnitzer C, Macklon KT, et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod. 2017;32:154–164. [DOI] [PubMed] [Google Scholar]
- 59. Jensen AK, Macklon KT, Fedder J, et al. Erratum to: 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gellert SE, Pors SE, Kristensen SG, et al. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matthews SJ, Picton HM, Ernst E, Andersen CY. Successful pregnancy in a woman previously suffering from β-thalassaemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018;70:432–435. [DOI] [PubMed] [Google Scholar]
- 62. Lunding SA, Pors SE, Kristensen SG, et al. Autotransplantation of fragmented ovarian cortical tissue: a laparoscopic demonstration. Fertil Steril. 2018;110:1181–1183. [DOI] [PubMed] [Google Scholar]
- 63. Kristensen SG, Mamsen LS, Jeppesen JV, et al. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol (Lausanne). 2018;8:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of preantral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–166. [DOI] [PubMed] [Google Scholar]
- 65. Markholt S, Grøndahl ML, Ernst EH, Andersen CY, Ernst E, Lykke-Hartmann K. Global gene analysis of oocytes from early stages in human folliculogenesis shows high expression of novel genes in reproduction. Mol Hum Reprod. 2012;18:96–110. [DOI] [PubMed] [Google Scholar]
- 66. Kristensen SG, Andersen K, Clement AC, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, and the associated SMADs in five isolated size-matched populations of preantral follicles from normal human ovaries. Mol Hum Reprod. 2014;20:293–308. [DOI] [PubMed] [Google Scholar]
- 67. Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol. 2015;401C:189–201. [DOI] [PubMed] [Google Scholar]
- 68. Schmidt KTA, Andersen TN, Greve E, Ernst A, Loft A, Andersen CY. Fertility in cancer patients after cryopreservation of one ovary. Reprod Biomed Online. 2013;26:272–279. [DOI] [PubMed] [Google Scholar]
- 69. Macklon KT, Ernst E, Andersen AN, Andersen CY. Cryobanking of human ovarian tissue: do women still want their tissue stored beyond 5 years? Reprod Biomed Online. 2014;29:452–456. [DOI] [PubMed] [Google Scholar]
- 70. Kristensen SG, Andersen CY. Cryopreservation of ovarian tissue: opportunities beyond fertility preservation and a positive view into the future. Front Endocrinol (Lausanne). 2018;9:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liebermann J, Nawroth F, Isachenko V, et al. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67:1671–1680. [DOI] [PubMed] [Google Scholar]
- 72. Isachenko V, Isachenko E, Rahimi G, et al. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen: negative effects of disaccharides in vitrification solution. CryoLetters. 2002;23:333–344. [PubMed] [Google Scholar]
- 73. Nawroth F, von Wolff M. FertiPROTEKT Netzwerk e.V. – das interdisziplinäre Netzwerk für fertilitätsprotektive Maßnahmen. Gynäkologe 2018;51:951–958. [Google Scholar]
- 74. von Wolff M, Thaler CJ, Frambach T, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. [DOI] [PubMed] [Google Scholar]
- 75. von Wolff M, Capp E, Jauckus J, et al. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146–149. [DOI] [PubMed] [Google Scholar]
- 76. Henes M, Henes JC, Neunhoeffer E, et al. Fertility preservation methods in young women with systemic lupus erythematosus prior to cytotoxic therapy: experiences from the FertiPROTEKT network. Lupus. 2012;21:953–958. [DOI] [PubMed] [Google Scholar]
- 77. Henes JC, Henes M, von Wolff M, et al. Fertility preservation in women with vasculitis: experiences from the FertiPROTEKT network. Clin Exp Rheumatol. 2012;30:S53–S56. [PubMed] [Google Scholar]
- 78. Henes M, Neis F, Krämer B, et al. Possibilities of fertility preservation in young patients with ovarian cancer. Anticancer Res. 2014;34:3851–3854. [PubMed] [Google Scholar]
- 79. von Wolff M, Bruckner T, Strowitzki T, Germeyer A. Fertility preservation – ovarian response to freeze oocytes is not affected by different malignant diseases – an analysis of 992 stimulations. J Assist Reprod Genet. 2018;35:1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dittrich R, Lotz L, Keck G, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. [DOI] [PubMed] [Google Scholar]
- 81. Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103:462–468. [DOI] [PubMed] [Google Scholar]
- 82. Liebenthron J, Montag M, Reinsberg J, et al. Overnight ovarian tissue transportation for centralized cryobanking – a feasible option [published online ahead of print January 19, 2019]. Reprod Biomed Online. doi: 10.1016/j.rbmo.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 83. von Wolff M, Sänger N, Liebenthron J. Is ovarian tissue cryopreservation and transplantation still experimental? it is a matter of female age and type of cancer [published online ahead of print October 5, 2018]. J Clin Oncol. doi: 10.1200/JCO.18.00425. [DOI] [PubMed] [Google Scholar]
- 84. Huober-Zeeb C, Lawrenz B, Popovici RM, et al. Improving fertility preservation in cancer: ovarian tissue cryobanking followed by ovarian stimulation can be efficiently combined. Fertil Steril. 2011;95:342–344. [DOI] [PubMed] [Google Scholar]
- 85. Sänger N, Jarisch A, Ochsendorf F, et al. Fertility preservation in prepubertal and pubertal children and adolescents. Klin Padiatr. 2018;230:122–129. [DOI] [PubMed] [Google Scholar]
- 86. Sänger N, Jarisch A, von Wolff M. Pädiatrische Onkologie: Fertilitätserhalt bei Kindern mit Krebs. Dtsch Arztebl. 2018;115:A-196, B-172, C-172. [Google Scholar]
- 87. Lawrenz B, Jauckus J, Kupka MS, Strowitzki T, von Wolff M. Fertility preservation in >1,000 patients: patient’s characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. 2011;283:651–656. [DOI] [PubMed] [Google Scholar]
- 88. von Wolff M, Dittrich R, Liebenthron J, et al. Fertility-preservation counselling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online. 2015;31:605–612. [DOI] [PubMed] [Google Scholar]
- 89. von Wolff M, Giesecke D, Germeyer A, et al. Characteristics and attitudes of women in relation to chosen fertility preservation techniques: a prospective, multicenter questionnaire-based study with 144 participants. Eur J Obstet Gynecol Reprod Biol. 2016;201:12–17. [DOI] [PubMed] [Google Scholar]
- 90. von Wolff M, Montag M, Dittrich R, et al. Fertility preservation in women – a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schüring AN, Fehm T, Behringer K, et al. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part I: indications for fertility preservation. Arch Gynecol Obstet. 2018;297:241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. von Wolff M, Germeyer A, Liebenthron J, Korell M, Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch Gynecol Obstet. 2018;297:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dittrich R, Kliesch S, Schüring A, et al. Fertility preservation for patients with malignant disease. Guideline of the DGGG, DGU and DGRM (S2k–level, AWMF Registry No. 015/082, November 2017) – recommendations and statements for girls and women. Geburtshilfe Frauenheilkd. 2018;78:567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Woodruff TK. Oncofertility: a grand collaboration between reproductive medicine and oncology. Reproduction. 2015;150:S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gracia C. Oncofertility Medical Practice: Clinical Issues and Implementation (ed. Woodruff TK.). New York, NY: Springer; 2012. [Google Scholar]
- 97. Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. [DOI] [PubMed] [Google Scholar]
- 98. Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15:1124–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Woodruff TK. The Oncofertility Consortium – addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Woodruff TK. Reproductive endocrinology: fertility in female survivors of childhood cancer. Nat Rev Endocrinol. 2013;9:571–572. [DOI] [PubMed] [Google Scholar]
- 101. Woodruff TK, Gosiengfiao YC. Pediatric and Adolescent Oncofertility: Best Practices and Emerging Technologies. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 102. Woodruff TK, Snyder KA. Oncofertility: Fertility Preservation for Cancer Survivors. New York, NY: Springer; 2007. [Google Scholar]
- 103. Woodruff TK. Oncofertility: Ethical, Legal, Social, and Medical Perspectives. New York, NY: Springer; 2010. [PubMed] [Google Scholar]
- 104. Ataman LM, Rodrigues JK, Marinho RM, et al. Creating a global community of practice for oncofertility. J Glob Oncol. 2016;2:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Miller EJN, Cookingham LM, Woodruff TK, et al. Fertility preservation training for obstetrics and gynecology fellows: a highly desired but non-standardized experience. Fertil Res Pract. 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Castle M, Cleveland C, Gordon D, et al. Reproductive science for high school students: a shared curriculum model to enhance student success. Biol Reprod. 2016;95:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jakus AE, Laronda MM, Rashedi AS, et al. ‘Tissue papers’ from organ-specific decellularized extracellular matrices. Adv Funct Mater. 2017;27:1700992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Laronda MM, McKinnon KE, Ting AY, Le Fever AV, Zelinski MB, Woodruff TK. Good manufacturing practice requirements for the production of tissue vitrification and warming and recovery kits for clinical research. J Assist Reprod Genet. 2017;34:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Que EL, Bleher R, Duncan FE, et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2015;7:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rios PD, Kniazeva E, Lee HC, et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115:2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Skory RM, Xu Y, Shea LD, Woodruff TK. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod. 2015;30:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Treff NR, Krisher RL, Tao X, et al. Next generation sequencing-based comprehensive chromosome screening in mouse polar bodies, oocytes, and embryos. Biol Reprod. 2016;94:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Silva GM, Rossetto R, Chaves RN, et al. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote. 2015;23:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction. 2015;150:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiao S, Zhang J, Romero MM, et al. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kidder BL. In vitro maturation and in vitro fertilization of mouse oocytes and preimplantation embryo culture. Methods Mol Biol. 2014;1150:191–199. [DOI] [PubMed] [Google Scholar]
- 118. Sowińska N, Frankowska K, Filipczyk A, et al. The effect of cumulus cells on domestic cat (Felis catus) oocytes during in vitro maturation and fertilization. Reprod Domest Anim. 2017;52:108–113. [DOI] [PubMed] [Google Scholar]
- 119. Lunardi FO, Araújo VR, Bernuci MP, et al. Restoring fertility after ovarian tissue cryopreservation: a half century of research. Zygote. 2013;21:394–405. [DOI] [PubMed] [Google Scholar]
- 120. Oktay K, Buyuk E. The potential of ovarian tissue transplant to preserve fertility. Expert Opin Biol Ther. 2002;2:361–370. [DOI] [PubMed] [Google Scholar]
- 121. Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34:807–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A. 2014;20:3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kizuka-Shibuya F, Tokuda N, Takagi K, et al. Locally existing endothelial cells and pericytes in ovarian stroma, but not bone marrow-derived vascular progenitor cells, play a central role in neovascularization during follicular development in mice. J Ovarian Res. 2014;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Armstrong AG, Kimler BF, Smith BM, Woodruff TK, Pavone ME, Duncan FE. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium’s National Physicians Cooperative. Future Oncol. 2018;14:363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Duncan FE, Pavone ME, Gunn AH, et al. Pediatric and teen ovarian tissue removed for cryopreservation contains follicles irrespective of age, disease diagnosis, treatment history, and specimen processing methods. J Adolesc Young Adult Oncol. 2015;4:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Duncan FE, Zelinski M, Gunn AH, et al. Ovarian tissue transport to expand access to fertility preservation: from animals to clinical practice. Reproduction. 2016;152:R201–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bortoletto P, Confino R, Smith BM, Woodruff TK, Pavone ME. Practices and attitudes regarding women undergoing fertility preservation: a survey of the National Physicians Cooperative. J Adolesc Young Adult Oncol. 2017;6:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Finlayson C, Fritsch MK, Johnson EK, et al. Presence of germ cells in disorders of sex development: implications for fertility potential and preservation. J Urol. 2017;197:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS, Shea LD. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci Rep. 2015;35:17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Laronda MM, Duncan FE, Hornick JE, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tagler D, Makanji Y, Tu T, et al. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2014;111:1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Clayman ML, Harper MM, Quinn GP, Reinecke J, Shah S. Oncofertility resources at NCI-designated comprehensive cancer centers. J Natl Compr Canc Netw. 2013;11:1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fuchs A, Kashanian JA, Clayman ML, et al. Pediatric oncology providers’ attitudes and practice patterns regarding fertility preservation in adolescent male cancer patients. J Pediatr Hematol Oncol. 2016;38:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gargus E, Deans R, Anazodo A, Woodruff TK. Management of primary ovarian insufficiency symptoms in survivors of childhood and adolescent cancer. J Natl Compr Canc Netw. 2018;16:1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lawson AK, Klock SC, Pavone ME, Hirshfeld-Cytron J, Smith KN, Kazer RR. Prospective study of depression and anxiety in female fertility preservation and infertility patients. Fertil Steril. 2014;102:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lawson AK, Klock SC, Pavone ME, Hirshfeld-Cytron J, Smith KN, Kazer RR. Psychological counseling of female fertility preservation patients. J Psychosoc Oncol. 2015;33:333–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107:djv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rashedi AS, de Roo SF, Ataman LM, et al. Survey of fertility preservation options available to patients with cancer around the globe. J Glob Oncol. 2018;4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rashedi AS, de Roo SF, Ataman LM, et al. Survey of third-party parenting options associated with fertility preservation available to patients with cancer around the globe. J Glob Oncol. 2018;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stein DM, Victorson DE, Choy JT, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Smith BM, Duncan FE, Ataman L, et al. The National Physicians Cooperative: transforming fertility management in the cancer setting and beyond. Future Oncol. 2019;14:3059–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Waimey KE, Smith BM, Confino R, Jeruss JS, Pavone ME. Understanding fertility in young female cancer patients. J Womens Health (Larchmt). 2015;24:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Greve T, Schmidt KT, Kristensen SG, Ernst E, Andersen CY. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril. 2012;97:1394–1398. [DOI] [PubMed] [Google Scholar]