Abstract
Importance
It is unclear if the associations between fetal growth and later mental health conditions remain after controlling for familial factors and psychiatric comorbidity.
Objective
To examine the associations between fetal growth and general and specific mental health conditions, controlling for familial factors.
Design, Setting, and Participants
This register-based study conducted in Sweden analyzed 546 894 pairs of full siblings born between January 1, 1973, and December 31, 1998. Sibling pairs were followed up through December 31, 2013. First, population-based and within-sibling pair associations (which controlled for time-invariant familial confounders) between fetal growth and the outcomes were estimated. Second, exploratory factor analysis was applied to the outcomes to derive 1 general factor and 4 specific and independent factors. Third, the general and specific factors were regressed on fetal growth. Statistical analysis was performed from March 27, 2017, to October 27, 2018.
Main Outcome and Measures
The outcomes were 11 psychiatric diagnoses (depression, anxiety, obsessive-compulsive disorder, posttraumatic stress disorder, bipolar disorder, alcohol abuse, drug use, attention-deficit/hyperactivity disorder, autism, schizophrenia, and schizoaffective disorder) and court convictions of violent crimes. Birth weight (in kilograms) statistically adjusted for gestational age was the exposure.
Results
The mean (SD) age of the 1 093 788 participants was 27.2 (6.8) years (range, 15.1-40.9 years) and 51.5% were male. Nine outcomes were significantly associated with birth weight in the population at large: depression (odds ratio [OR], 0.96; 95% CI, 0.95-0.98), anxiety (OR, 0.94; 95% CI, 0.92-0.95), posttraumatic stress disorder (OR, 0.91; 95% CI, 0.89-0.93), bipolar disorder (OR, 0.94; 95% CI, 0.89-1.00), alcohol abuse (OR, 0.89; 95% CI, 0.87-0.91), drug use (OR, 0.83; 95% CI, 0.80-0.85), violent crimes (OR, 0.85; 95% CI, 0.83-0.86), attention-deficit/hyperactivity disorder (OR, 0.88; 95% CI, 0.86-0.90), and autism (OR, 0.95; 95% CI, 0.92-0.97). Only depression (OR, 0.95; 95% CI 0.92-0.98), obsessive-compulsive disorder (OR, 0.93; 95% CI, 0.87-0.99), attention-deficit/hyperactivity disorder (OR, 0.86; 95% CI, 0.82-0.89), and autism (OR, 0.72; 95% CI, 0.69-0.76) remained significantly associated within sibling pairs. An exploratory factor analysis indicated that 1 general and 4 specific factors (capturing anxiety, externalizing, neurodevelopmental, and psychotic conditions) fit the outcomes well. Across almost all sensitivity analyses, an increase in birth weight by 1 kg significantly reduced the general (β, −0.047; 95% CI, −0.071 to −0.023) and the specific neurodevelopmental factors (β, −0.159; 95% CI, −0.190 to −0.128) within sibling pairs.
Conclusions and Relevance
Controlling for familial confounders, reduced fetal growth was associated with a small but significant increase in the general factor of psychopathology and a moderate increase in a specific neurodevelopmental factor.
This register-based study of pairs of siblings examines the associations between fetal growth and general and specific mental health conditions, controlling for familial factors.
Key Points
Question
Do the associations between fetal growth and later mental health conditions remain after controlling for familial confounding factors and psychiatric comorbidity?
Findings
This register-based study including more than 1 million participants and using a within-sibling pair design found that higher birth weight (statistically adjusted for gestational age) significantly lowered the risk for attention-deficit/hyperactivity disorder, autism, obsessive-compulsive disorder, and depression. Furthermore, an increase in birth weight by 1 kg significantly decreased a general factor of psychopathology by 0.047 SDs and a specific neurodevelopmental factor by 0.159 SDs.
Meaning
After controlling for familial factors and psychiatric comorbidity, fetal growth was most strongly associated with specific neurodevelopmental disorders.
Introduction
Reduced fetal growth is associated with an increased level of mental health conditions.1 Barring a few replication failures,2,3 reduced fetal growth has been associated with clinical diagnoses of attention-deficit/hyperactivity disorder (ADHD), autism, depression, anxiety, substance abuse, schizophrenia, and bipolar disorder,4,5,6,7,8 as well as with parent-reported, teacher-reported, and self-reported mental health symptoms.9,10,11 These associations are potentially mediated via changes in brain functioning.12,13
Two concerns, however, cloud causal interpretations. First, unmeasured genetic or environmental variables might confound the associations.14 One remedy is to examine whether the associations persist within sibling pairs or twin pairs because they are partly matched on familial time-invariant confounders, including, for example, socioeconomic status and genetics.15 Whereas the associations between fetal growth and ADHD, autism, and obsessive-compulsive disorder remain associated within sibling and twin pairs,16,17,18,19,20 the associations with schizophrenia and bipolar disorder appear to attenuate and sometimes become nonsignificant.18,21,22 Furthermore, the associations between fetal growth and parent-reported externalizing symptoms and substance abuse diagnoses tend to become nonsignificant within sibling pairs.17,18
An additional problem is the high degree of overlap among psychiatric disorders.23,24,25 An observed association between fetal growth and a given psychiatric condition might be attributed to that which is not shared with other psychiatric phenomena (ie, its unique part), or toward that which is shared with other psychiatric phenomena (ie, comorbidity). Research indicates that psychiatric comorbidity can be explained by a general factor of psychopathology with broad effects on virtually all forms of mental health conditions.26,27,28 For example, in a sample of more than 35 000 US adults who were administered a psychiatric interview, a model of comorbidity that included a general factor fit the data significantly better.29 Supporting the effect of fetal growth on psychiatric comorbidity, in a co-twin control study of 745 twin pairs, the twin who weighed less at birth was rated as having a significantly higher score on the total problem scale of the Child Behavior Checklist, which is an approximation of the general factor, in childhood and adolescence.30
However, it remains unknown whether fetal growth is associated with a general factor of psychopathology based on more severe clinical diagnoses, and whether the associations with the unique parts of disorders persist after isolating the general factor. The purpose of this study was to examine the influence of fetal growth on both general and specific mental health conditions, as indicated by clinical diagnoses, across the adult age spectrum in a large population-based sample of full sibling pairs.
Methods
Participants
We linked together all Swedish individuals born between January 1, 1973, and December 31, 1998, from the Swedish Medical Birth Register, Multi-Generation Register, the National Inpatient Register and National Outpatient Register (the inpatient register captures diagnoses since 1973, and the outpatient register captures diagnoses since 2001), and the National Crime Register. The individuals were followed up through December 31, 2013. After excluding individuals who had died or migrated, we selected the oldest full sibling pair (ie, individuals who had the same biological mother and father) within each family to maximize the follow-up time (and thereby power). Because Swedish full siblings almost always grow up in the same household,31 they are more likely to have experienced a similar shared environment. We included only full siblings who were born within 5 years of each other to maximize the probability that the shared environment remained similar for the pairs (see eTable 8 in the Supplement for other age cutoffs). The final sample consisted of 546 894 pairs of full siblings. The mean (SD) age was 27.2 (6.8) years (range, 15.1-40.9 years) and 51.5% were male. This study was approved by the Regional Ethical Review Board in Stockholm. Because the study relied on deidentified registry data, informed consent was not required.
Measures
For the exposure, as a measure of fetal growth, we focused on birth weight (in kilograms) statistically adjusted for gestational age. As covariates, we included sex, date of birth, and birth order. For the outcomes, we included inpatient and outpatient diagnoses of depression, anxiety, obsessive-compulsive disorder, posttraumatic stress disorder, bipolar disorder, alcohol abuse, drug use, ADHD, autism spectrum disorder, schizophrenia, and schizoaffective disorder assigned by the attending psychiatrist in accord with versions 8, 9, or 10 of the International Classification of Diseases (eTable 1 in the Supplement).32 Diagnoses were recorded as lifetime prevalence rates; thus, participants could receive a different diagnosis at any point. We included only diagnoses assigned at age 12 years or later, except for autism and ADHD, which were limited to age 2 years or later. As a marker of antisocial behavior, we included court convictions of violent crimes (eg, unlawful threats, assault, and homicide), which can occur from age 15 years. We counted only the first instance of diagnoses and court convictions (ie, we treated the data as binary). Descriptive statistics about the exposure, covariates, and outcomes are displayed in eTable 2 in the Supplement.
Statistical Analysis
Statistical analysis was performed from March 27, 2017, to October 27, 2018. We analyzed the data in wide format in a Structural Equation Modeling (SEM) framework, which featured several advantages. First, SEM allows for analyzing multiple outcomes simultaneously, such that we could separate specific from general variance at the outcome level. Second, SEM allows for analyzing error-free latent (unobserved) factors rather than partly unreliable outcomes, such that decreases in the fixed-effects estimates cannot be attributed to measurement error in the outcomes. Third, because only pairs who are discordant for the outcomes contribute to the fixed-effect estimates, analyzing latent factors that consist of several variables increases the number of discordant pairs and thereby increases the power. However, SEM is also disadvantageous in that it is a computational challenge to model the hazard ratio of multiple outcomes simultaneously. Therefore, we only modeled whether the outcome had occurred (ie, we treated the data as ordered categories, assuming that an underlying normal and continuous probability function underlay the observed binary outcomes). All analyses were conducted with Mplus software (Muthén and Muthén) using the mean-adjusted and variance-adjusted weighted least square estimator.33 All confidence intervals were 2-sided.
Bivariate Analyses: Random- and Fixed-Effects Regression
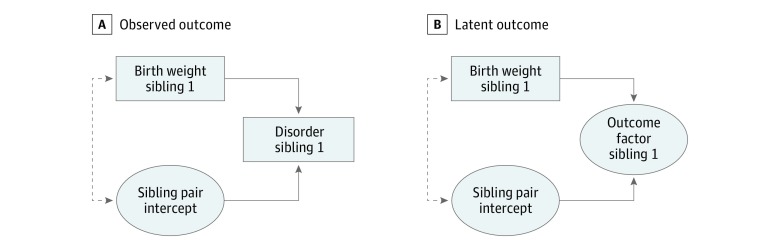
We assumed that part of the variation in the risk for each outcome could be attributed partly to a normally distributed latent sibling pair intercept (ie, a familial effect). We regressed each disorder onto birth weight (adjusted for gestational age) and the covariates in 2 ways following the hybrid approach by Allison34 (Figure 1A). First, to emulate a random-effects model, we forced the latent sibling pair intercept to be unrelated to birth weight and the covariates. This model assumes that the average degree of mental conditions in each sibling pair is unrelated to their birth weight (which would be violated if, for example, poverty or genetics led to both lower birth weight and a higher degree of mental health conditions in the pairs). Second, to emulate a fixed-effects regression model, we allowed the latent sibling pair intercept to correlate with birth weight and the covariates, thereby controlling for all time-invariant unmeasured confounders shared within sibling pairs. In other words, this model estimates whether an individual with low birth weight has an increased (or decreased) risk of developing later mental health conditions, controlling for the overall degree of mental health conditions in the sibling pair. Thus, this model controls for all unmeasured confounders shared between siblings (eg, poverty or genetics) that might lead them to have both lower birth weight and more mental health conditions compared with other sibling pairs.
Figure 1. Observed Outcome and Latent Outcome, Regressed on Birth Weight.
A, Observed outcome. B, Latent outcome. Absence of dashed line indicates random-effects model. Presence of dashed line indicates fixed-effects model.
Multivariate Analyses: Random- and Fixed-Effects Regression
We conducted an exploratory factor analysis on the 12 outcomes to examine whether a fewer number of latent continuous factors could account for their overlap. We used exploratory factor analysis rather than confirmatory approaches because we did not have a strong intuition about how the disorders with psychotic or neurodevelopmental features might pertain to the internalizing and externalizing disorders, and because the outcomes seemed unlikely to have simple structure. We used the scree plot to determine how many factors to extract35 and used the bifactor rotation to isolate general from specific variance.36,37 This generates a single general factor that captures the comorbidity among all the outcomes and a set of specific factors that captures unique variance shared only among a subset of the outcomes. Importantly, the general factor is uncorrelated with the specific factors, such that potential associations between fetal growth and the specific factors cannot be attributed to general comorbidity. We computed the explained common variance index to quantify how much of the reliable variance in the adverse outcomes was attributable to the general factor.38
Second, we regressed the latent and continuous general and specific factors onto birth weight (adjusted for gestational age) and the covariates within the SEM framework.39 We emulated the random and fixed effects by assuming that part of the variation in each latent factor could be partly attributed to a sibling pair intercept (Figure 1B; see eFigure 1 in the Supplement for a more complete figure of the multivariate random and fixed model).37 We allowed the means and intercepts to vary between the older and younger siblings, but held all other parameters (ie, variances and covariances) equal between the siblings. Because past research has indicated that some of the associations between fetal growth and psychiatric disorders appear nonlinear,18 we included both centered birth weight and birth weight squared as the exposures.
Results
Bivariate Regressions
Reduced fetal growth significantly increased the risk for 9 of the 12 outcomes: depression (odds ratio [OR], 0.96; 95% CI, 0.95-0.98), anxiety (OR, 0.94; 95% CI, 0.92-0.95), posttraumatic stress disorder (OR, 0.91; 95% CI, 0.89-0.93), bipolar disorder (OR, 0.94; 95% CI, 0.89-1.00), alcohol abuse (OR, 0.89; 95% CI, 0.87-0.91), drug use (OR, 0.83; 95% CI, 0.80-0.85), violent crimes (OR, 0.85; 95% CI, 0.83-0.86), attention-deficit/hyperactivity disorder (OR, 0.88; 95% CI, 0.86-0.90), and autism (OR, 0.95; 95% CI, 0.92-0.97) (Table 1). When compared within sibling pairs, that is, after controlling time-invariant familial unmeasured confounders, higher birth weight decreased the risk for depression (OR, 0.95; 95% CI, 0.92-0.98), obsessive-compulsive disorder (OR, 0.93; 95% CI, 0.87-0.99), ADHD (OR, 0.86; 95% CI, 0.82-0.89), and autism (OR, 0.72; 95% CI, 0.69-0.76) (Table 1).
Table 1. Psychiatric Disorders and Criminality Regressed on Birth Weight Coded in Kilograms, Controlling for Gestational Age.
| Outcome | Odds Ratio (95% CI) | |
|---|---|---|
| Random Effect | Fixed Effect | |
| Depression | 0.96 (0.95-0.98)a | 0.95 (0.92-0.98)b |
| Anxiety | 0.94 (0.92-0.95)a | 1.00 (0.97-1.03) |
| OCD | 1.03 (0.99-1.07) | 0.93 (0.87-0.99)c |
| PTSD | 0.91 (0.89-0.93)a | 0.98 (0.94-1.02) |
| Bipolar disorder | 0.94 (0.89-1.00)d | 0.98 (0.92-1.05) |
| Alcohol abuse | 0.89 (0.87-0.91)a | 0.97 (0.94-1.01) |
| Drug use | 0.83 (0.80-0.85)a | 0.97 (0.93-1.02) |
| Violent crimes | 0.85 (0.83-0.86)a | 0.97 (0.93-1.00) |
| ADHD | 0.88 (0.86-0.90)a | 0.86 (0.82-0.89)a |
| Autism | 0.95 (0.92-0.97)a | 0.72 (0.69-0.76)a |
| Schizophrenia | 0.97 (0.90-1.04) | 0.89 (0.78-1.02) |
| Schizoaffective disorder | 1.06 (0.96-1.17) | 1.13 (0.89-1.42) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder.
P < .001.
P = .001.
P = .03.
P = .04.
Multivariate Regressions
To examine whether the bivariate associations could be attributed to variance specific to each condition vs that accounted for by the general factor, we examined the multivariate structure of the 12 outcomes. The first 10 eigenvalues are 5.81, 1.35, 1.11, 0.99, 0.60, 0.55, 0.45, 0.34, 0.27, and 0.23, indicating an elbow at 4 factors (see eFigure 2 in the Supplement for the scree plot). However, because we wanted to model the general factor in addition to specific factors, we extracted an additional fifth factor (but we also performed the analyses using a 4-factor solution; eTables 3 and 4 in the Supplement). The 5-factor solution fit the data well (root mean square error of approximation [RMSEA] = 0.005; 90% CI, 0.005-0.006; confirmatory fit index [CFI] = 0.999; Tucker-Lewis index [TLI] = 0.997; χ216 = 545.222; P < .001), which we rotated toward 1 general and 4 specific factors.37,40
As displayed in Table 2,38 all outcomes had large loadings on the general factor (mean loading, 0.65; range, 0.35-0.87). The first specific factor captured anxiety disorders (obsessive-compulsive disorder loading, 0.49; and anxiety loading, 0.33). The second specific factor captured externalizing conditions (violent crimes loading, 0.57; drug use loading, 0.59; and alcohol abuse loading, 0.47). The third specific factor captured neurodevelopmental disorders (ADHD loading, 0.54; and autism loading, 0.59). The fourth specific factor captured psychotic disorders (schizophrenia loading, 0.68; and schizoaffective loading, 0.54).
Table 2. Exploratory Factor Analysis Factor Loadings After Bifactor Rotation.
| Outcome | General Factora | Specific Anxiety Factor |
Specific Externalizing Factor |
Specific Neurodevelopmental Factor |
Specific Psychotic Factor |
|---|---|---|---|---|---|
| Depression | 0.87 | 0.11 | −0.05 | −0.13 | −0.18 |
| Anxiety | 0.79 | 0.33 | 0.06 | −0.09 | −0.16 |
| OCD | 0.59 | 0.49 | −0.15 | 0.11 | 0.02 |
| PTSD | 0.69 | 0.08 | 0.09 | −0.17 | −0.18 |
| Bipolar disorder | 0.78 | −0.07 | −0.05 | −0.10 | 0.00 |
| Alcohol abuse | 0.49 | 0.01 | 0.47 | −0.10 | −0.02 |
| Drug use | 0.67 | 0.03 | 0.59 | −0.05 | 0.00 |
| Violent crimes | 0.35 | −0.10 | 0.57 | 0.10 | 0.05 |
| ADHD | 0.66 | −0.07 | 0.20 | 0.54 | −0.20 |
| Autism | 0.59 | 0.11 | −0.15 | 0.59 | 0.08 |
| Schizophrenia | 0.62 | 0.10 | 0.07 | 0.04 | 0.68 |
| Schizoaffective disorder | 0.74 | −0.24 | −0.09 | −0.16 | 0.54 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder.
Explained common variance = 0.63.38
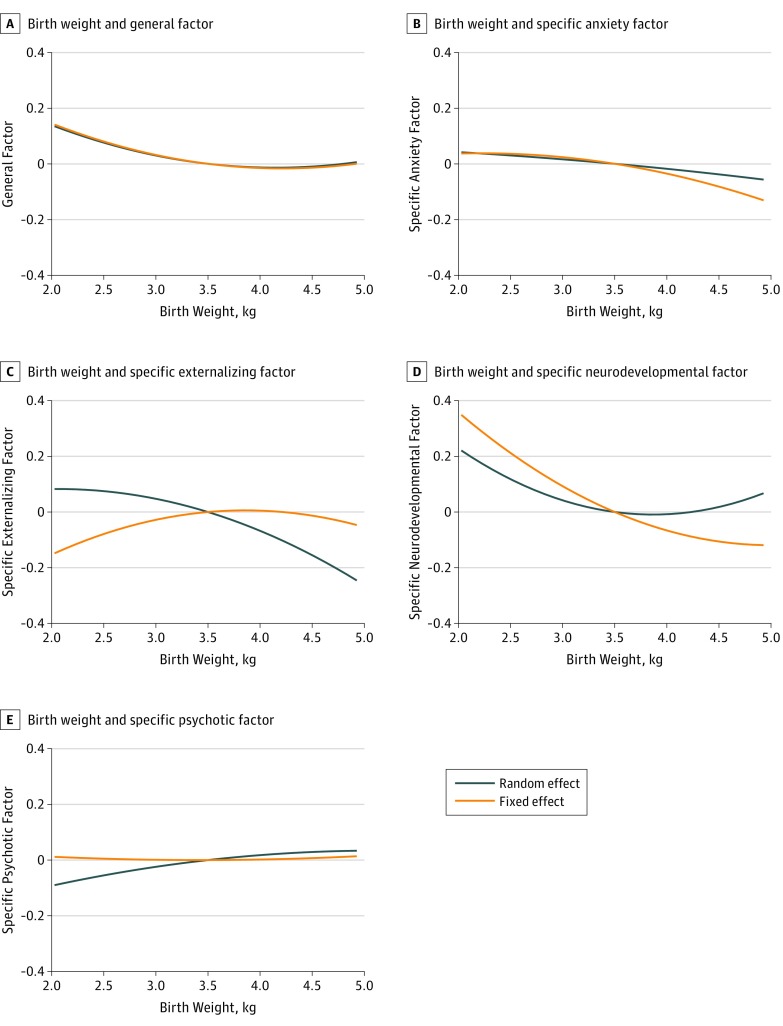
We then regressed the general and specific factors onto birth weight (adjusted for gestational age) and the covariates, which fit the data well (RMSEA = 0.011; 90% CI, 0.011-0.011; CFI = 0.985; TLI = 0.984; χ2511 = 34 688.460; P < .001). Fetal growth was associated with the general and the specific factors (Table 3). The significant effect of birth weight squared indicated that risk was primarily elevated for low birth weight but not for medium or high birth weight (Figure 2).
Table 3. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms, Controlling for Gestational Age.
| Factor | Random Effect (95% CI) | Fixed Effect (95% CI) | ||
|---|---|---|---|---|
| Birth Weight β | Birth Weight Squared β | Birth Weight β | Birth Weight Squared β | |
| General factor | −0.043 (−0.057 to −0.029)a | 0.033 (0.023 to 0.043)a | −0.047 (−0.071 to −0.023)a | 0.033 (0.017 to 0.049)a |
| Specific anxiety factor | −0.034 (−0.063 to −0.005)b | −0.004 (−0.026 to 0.018) | −0.059 (−0.114 to −0.004)c | −0.023 (−0.060 to 0.014) |
| Specific externalizing factor | −0.115 (−0.133 to −0.097)a | −0.040 (−0.054 to −0.026)a | 0.033 (0.002 to 0.064)c | −0.046 (−0.068 to –0.024)a |
| Specific neurodevelopmental factor | −0.050 (−0.068 to −0.032)a | 0.068 (0.056 to 0.080)a | −0.159 (−0.190 to −0.128)a | 0.053 (0.031 to 0.075)a |
| Specific psychotic factor | 0.042 (0.007 to 0.077)b | −0.013 (−0.038 to 0.012) | 0.001 (−0.066 to 0.068) | 0.006 (−0.051 to 0.039) |
P < .001.
P = .02.
P = .04.
Figure 2. Association Between Birth Weight and General and Specific Factors.
A, Association between birth weight and the general factor. B, Association between birth weight and the specific anxiety factor. C, Association between birth weight and the specific externalizing factor. D, Association between birth weight and the specific neurodevelopmental factor. E, Association between birth weight and the specific psychotic factor. All factors are measured in standardized units. See Table 3 for the 95% CIs.
We then controlled time-invariant familial unmeasured confounders, which also fit the data well (RMSEA = 0.011; 90% CI, 0.011-0.011; CFI = 0.986; TLI = 0.983; χ2486 = 34 010.355; P < .001). Within sibling pairs, higher birth weight was significantly associated with lower scores on the general (β, −0.047; 95% CI, −0.071 to −0.023; Figure 2A), specific anxiety (β, −0.059; 95% CI, −0.114 to −0.004; Figure 2B), and specific neurodevelopmental factors (β, −0.159; 95% CI, −0.190 to −0.128; Figure 2D), as well as with higher scores on the specific externalizing factor (β, 0.033; 95% CI, 0.002-0.064; Figure 2C).
Sensitivity Analyses
In the eAppendix in the Supplement, we examined (1) whether the associations with fetal growth remained similar when extracting 4 instead of 5 factors (eTables 3 and 4 in the Supplement); (2) whether changes in diagnostic procedures might have influenced the results by analyzing only individuals born after 1987, such that they were at least 10 years of age when the ICD-10 was introduced (eTable 5 in the Supplement); (3) whether the results were driven primarily by premature births (eTable 6 in the Supplement); (4) whether the results generalized to clinical cutoffs of fetal growth (eTable 7 in the Supplement); (5) whether the results were consistent for siblings born within 2.51, 3, 4, 6, 7, 8, 9, and 10 years apart (eTable 8 in the Supplement); (6) whether the results were similar for same-sex sibling pairs (eTable 9 in the Supplement); and (7) whether the results were comparable among those born within 3 SDs of the mean of birth weight and gestational age (eTable 10 in the Supplement).
Across all 7 sensitivity analyses, restricted fetal growth was significantly associated with higher scores on the specific neurodevelopmental factor within sibling pairs. Likewise, restricted fetal growth was associated with higher scores on the general factor across 6 of the 7 sensitivity analyses (the association approached but failed to reach conventional levels of statistical significance for siblings born within 2.51 years). The observation that reduced fetal growth increased the risk for anxiety but lowered the risk for externalizing conditions failed to emerge consistently across the sensitivity analysis.
Discussion
Reduced fetal growth had a small but significant effect on the risk of developing several psychiatric disorders in adulthood. After controlling for time-invariant unmeasured confounders shared by full sibling pairs, only the associations with ADHD, autism, obsessive-compulsive disorder, and depression remained statistically significant. Depression, however, was subsumed by the general factor, partly in line with past research,28,41 highlighting the importance of taking comorbidity into account when studying mental health conditions.27 In the within-pair multivariate analyses, reduced fetal growth was significantly associated with higher scores on the general and, to a greater extent, the specific neurodevelopmental factors.
Replicating past research, we found that the covariation among psychiatric disorders could be partly accounted for by a general factor.26,27,28,29 Whereas twin, sibling, and molecular genetic investigations have highlighted its genetic cause,31,41,42 our study replicated a co-twin control study, which found an association of fetal growth with total symptom score in adolescence,30 and extended it to a general factor based on more severe mental health conditions in adulthood. Thus, fetal growth seems to mediate environmental effects also to the general factor, albeit weakly. One speculation is that reduced fetal growth compromises brain development during a critical period,43 which in turn slightly increases the risk not only for neurodevelopmental disorders but also for virtually all mental health conditions.
Restricted fetal growth had the strongest association with the specific neurodevelopmental factor. It has been proposed that the association between reduced fetal growth and ADHD are preceded by an insufficient supply of oxygen and nutrients for the developing fetus44; our results imply that this pathway might be shared across the neurodevelopmental spectrum. Multiple studies have found that reduced fetal growth is associated with both autism and ADHD.16,17,18,20 Because several twin studies of neurodevelopmental symptoms have indicated that a portion of their overlap can be attributed to nonfamilial sources,45,46,47 a speculation is that fetal growth is one such source.
Although we failed to identify an association between restricted fetal growth and psychotic disorders within sibling pairs, this null finding is in line with previous co-siblings control studies. For example, in a study of Swedish twins and a study of Danish siblings the within-pair associations between reduced fetal growth and schizophrenia became nonsignificant.21,22 With the caveat that this estimate tends to be relativity imprecise given the rarity of the condition, one speculation is that reduced fetal growth might not be in the causal pathway leading to psychotic disorders after adjusting for comorbidity and familial confounding.
Because the associations remained after controlling for time-invariant familial unmeasured confounders, interventions geared toward increasing fetal growth might reduce future general and specific neurodevelopmental disorders. However, treatment options for fetal growth are limited and tend to have a small effect.48,49,50 Because the effect sizes presented herein were small, in line with past research,17,51 potential interventions are likely to have a relatively small effect on later psychiatric conditions. Nevertheless, given the sheer prevalence of mental health conditions,24 combating maternal malnourishment and improving prenatal care still might influence a significant number of cases.52
Limitations
This study has some limitations. First, it is a computational challenge to model the hazard ratio of multiple outcomes simultaneously within a SEM framework. Therefore, we assumed that the overlap among the diagnoses could be accounted for by unobserved and continuous factors. Thus, we modeled only whether the events occurred, but not their rate of onset as a function of time.
Second, although restricted fetal growth increased the risk for anxiety but lowered the risk for externalizing conditions, the association was not consistent across all sensitivity analyses. Therefore, it remains uncertain whether fetal growth is a risk factor for externalizing and internalizing conditions in this population.
Third, the registers only capture individuals who have been diagnosed by specialists, which usually occurs after referral from the primary care system. Therefore, we were limited to analyzing associations with more severe forms of mental conditions. Less-troubled individuals who do not necessarily warrant referral to specialists might be included in future studies.53
Fourth, although we used siblings born within 5 years of one another to maximize environmental matching, we did not control unmeasured confounders not shared by sibling pairs. Other types of quasi-causal designs relying on different assumptions might begin to address this concern.14 Furthermore, we used full (compared with half) siblings to maximize genetic matching; nevertheless, only identical twins can provide complete genetic matching.
Conclusions
In this population-based sample, reduced fetal growth was associated with a small but significant increased risk for several psychiatric disorders. However, many of these associations became nonsignificant within full sibling pairs. After controlling for comorbidity, restricted fetal growth most strongly increased the risk for neurodevelopmental disorders.
eTable 1. International Classification of Diseases (ICD) Codes
eTable 2. Descriptive Statistics about Exposure, Covariates, and Outcomes
eAppendix. Sensitivity Analyses
eTable 3. EFA Loadings After Bifactor Rotation of Four Factors
eTable 4. Standardized General and Specific Factors Based on Four-Factor Solution Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age
eTable 5. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among Those Born 1987 or Later
eTable 6. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among those Born at Week 37 or Later
eTable 7. Standardized General and Specific Factors Regressed on Small for Gestational Age (SGA) Diagnosis
eTable 8. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Based on Siblings Born Within 2.51, 3, 4, 6, 7, 8, 9, and 10 Years Apart
eTable 9. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Based on Sex-Concordant Sibling Pairs Only
eTable 10. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among Those Born Within Three Standard Deviations of the Mean of Birth Weight and Gestational Age
eFigure 1. Latent Exploratory Factors Regressed on Birth Weight and Covariates (Not Shown)
eFigure 2. Scree Plot of Adverse Outcomes
References
- 1.Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23(7):905-916. doi: 10.1016/j.bbi.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Gunnell D, Harrison G, Whitley E, Lewis G, Tynelius P, Rasmussen F. The association of fetal and childhood growth with risk of schizophrenia: cohort study of 720,000 Swedish men and women. Schizophr Res. 2005;79(2-3):315-322. doi: 10.1016/j.schres.2005.07.022 [DOI] [PubMed] [Google Scholar]
- 3.Wojcik W, Lee W, Colman I, Hardy R, Hotopf M. Foetal origins of depression? a systematic review and meta-analysis of low birth weight and later depression. Psychol Med. 2013;43(1):1-12. doi: 10.1017/S0033291712000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz AP, Bolat GU, Bolat H, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics. 2018;141(1):e20171645. doi: 10.1542/peds.2017-1645 [DOI] [PubMed] [Google Scholar]
- 5.Lampi KM, Lehtonen L, Tran PL, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. 2012;161(5):830-836. doi: 10.1016/j.jpeds.2012.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69(6):E1-E8. doi: 10.1001/archgenpsychiatry.2011.1374 [DOI] [PubMed] [Google Scholar]
- 7.Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67(9):923-930. doi: 10.1001/archgenpsychiatry.2010.100 [DOI] [PubMed] [Google Scholar]
- 8.Gunnell D, Rasmussen F, Fouskakis D, Tynelius P, Harrison G. Patterns of fetal and childhood growth and the development of psychosis in young males: a cohort study. Am J Epidemiol. 2003;158(4):291-300. doi: 10.1093/aje/kwg118 [DOI] [PubMed] [Google Scholar]
- 9.Lyall DM, Inskip HM, Mackay D, et al. Low birth weight and features of neuroticism and mood disorder in 83 545 participants of the UK Biobank cohort. BJPsych Open. 2016;2(1):38-44. doi: 10.1192/bjpo.bp.115.002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathewson KJ, Chow CH, Dobson KG, Pope EI, Schmidt LA, Van Lieshout RJ. Mental health of extremely low birth weight survivors: a systematic review and meta-analysis. Psychol Bull. 2017;143(4):347-383. doi: 10.1037/bul0000091 [DOI] [PubMed] [Google Scholar]
- 11.Wiles NJ, Peters TJ, Leon DA, Lewis G. Birth weight and psychological distress at age 45-51 years: results from the Aberdeen Children of the 1950s cohort study. Br J Psychiatry. 2005;187:21-28. doi: 10.1192/bjp.187.1.21 [DOI] [PubMed] [Google Scholar]
- 12.Drakesmith M, Dutt A, Fonville L, et al. Mediation of developmental risk factors for psychosis by white matter microstructure in young adults with psychotic experiences. JAMA Psychiatry. 2016;73(4):396-406. doi: 10.1001/jamapsychiatry.2015.3375 [DOI] [PubMed] [Google Scholar]
- 13.Haukvik UK, Rimol LM, Roddey JC, et al. Normal birth weight variation is related to cortical morphology across the psychosis spectrum. Schizophr Bull. 2014;40(2):410-419. doi: 10.1093/schbul/sbt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage SH, Munafò MR, Davey Smith G. Causal inference in developmental origins of health and disease (DOHaD) research. Annu Rev Psychol. 2016;67:567-585. doi: 10.1146/annurev-psych-122414-033352 [DOI] [PubMed] [Google Scholar]
- 15.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(suppl 1):S46-S55. doi: 10.2105/AJPH.2013.301252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersson E, Sjölander A, Almqvist C, et al. Birth weight as an independent predictor of ADHD symptoms: a within-twin pair analysis. J Child Psychol Psychiatry. 2015;56(4):453-459. doi: 10.1111/jcpp.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ficks CA, Lahey BB, Waldman ID. Does low birth weight share common genetic or environmental risk with childhood disruptive disorders? J Abnorm Psychol. 2013;122(3):842-853. doi: 10.1037/a0033079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Class QA, Rickert ME, Larsson H, Lichtenstein P, D’Onofrio BM. Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry. 2014;205(5):355-361. doi: 10.1192/bjp.bp.113.143693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brander G, Rydell M, Kuja-Halkola R, et al. Association of perinatal risk factors with obsessive-compulsive disorder: a population-based birth cohort, sibling control study. JAMA Psychiatry. 2016;73(11):1135-1144. doi: 10.1001/jamapsychiatry.2016.2095 [DOI] [PubMed] [Google Scholar]
- 20.Hultman CM, Torrång A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: a prospective Swedish twin study. J Am Acad Child Adolesc Psychiatry. 2007;46(3):370-377. doi: 10.1097/01.chi.0000246059.62706.22 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen PR, Mortensen PB, Dalman C, et al. Fetal growth and schizophrenia: a nested case-control and case-sibling study. Schizophr Bull. 2013;39(6):1337-1342. doi: 10.1093/schbul/sbs148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson E, Stålberg G, Lichtenstein P, Cnattingius S, Olausson PO, Hultman CM. Fetal growth restriction and schizophrenia: a Swedish twin study. Twin Res Hum Genet. 2005;8(4):402-408. doi: 10.1375/twin.8.4.402 [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry. 2011;168(1):29-39. doi: 10.1176/appi.ajp.2010.10030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch Gen Psychiatry. 2005;62(7):709]. Arch Gen Psychiatry. 2005;62(6):617-627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56(10):921-926. doi: 10.1001/archpsyc.56.10.921 [DOI] [PubMed] [Google Scholar]
- 26.Caspi A, Houts RM, Belsky DW, et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119-137. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull. 2017;143(2):142-186. doi: 10.1037/bul0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldman ID, Poore HE, van Hulle C, Rathouz PJ, Lahey BB. External validity of a hierarchical dimensional model of child and adolescent psychopathology: tests using confirmatory factor analyses and multivariate behavior genetic analyses. J Abnorm Psychol. 2016;125(8):1053-1066. doi: 10.1037/abn0000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121(4):971-977. doi: 10.1037/a0028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Os J, Wichers M, Danckaerts M, Van Gestel S, Derom C, Vlietinck R. A prospective twin study of birth weight discordance and child problem behavior. Biol Psychiatry. 2001;50(8):593-599. doi: 10.1016/S0006-3223(01)01085-X [DOI] [PubMed] [Google Scholar]
- 31.Pettersson E, Larsson H, Lichtenstein P. Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol Psychiatry. 2016;21(5):717-721. doi: 10.1038/mp.2015.116 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Vol 2 Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus User's Guide. 7th ed Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- 34.Allison PD. Fixed Effects Regression Models. Thousand Oaks, CA: SAGE Publications; 2009. doi: 10.4135/9781412993869 [DOI] [Google Scholar]
- 35.Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1(2):245-276. doi: 10.1207/s15327906mbr0102_10 [DOI] [PubMed] [Google Scholar]
- 36.Bernaards CA, Jennrich RI. Gradient projection algorithms and software for arbitrary rotation criteria in factor analysis. Educ Psychol Meas. 2005;65(5):676-696. doi: 10.1177/0013164404272507 [DOI] [Google Scholar]
- 37.Jennrich RI, Bentler PM. Exploratory bi-factor analysis. Psychometrika. 2011;76(4):537-549. doi: 10.1007/s11336-011-9218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez A, Reise SP, Haviland MG. Evaluating bifactor models: calculating and interpreting statistical indices. Psychol Methods. 2016;21(2):137-150. doi: 10.1037/met0000045 [DOI] [PubMed] [Google Scholar]
- 39.Muthen BO. Latent variable modeling in heterogeneous populations. Psychometrika. 1989;54(4):557-585. doi: 10.1007/BF02296397 [DOI] [Google Scholar]
- 40.Schmid J, Leiman JM. The development of hierarchical factor solutions. Psychometrika. 1957;22(1):53-61. doi: 10.1007/BF02289209 [DOI] [Google Scholar]
- 41.Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry. 2011;68(2):181-189. doi: 10.1001/archgenpsychiatry.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anttila V, Bulik-Sullivan B, Finucane HK, et al. ; Brainstorm Consortium . Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walhovd KB, Fjell AM, Brown TT, et al. ; Pediatric Imaging, Neurocognition, and Genetics Study . Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci U S A. 2012;109(49):20089-20094. doi: 10.1073/pnas.1208180109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith TF, Schmidt-Kastner R, McGeary JE, Kaczorowski JA, Knopik VS. Pre- and perinatal ischemia-hypoxia, the ischemia-hypoxia response pathway, and ADHD risk. Behav Genet. 2016;46(3):467-477. doi: 10.1007/s10519-016-9784-4 [DOI] [PubMed] [Google Scholar]
- 45.Ronald A, Larsson H, Anckarsäter H, Lichtenstein P. Symptoms of autism and ADHD: a Swedish twin study examining their overlap. J Abnorm Psychol. 2014;123(2):440-451. doi: 10.1037/a0036088 [DOI] [PubMed] [Google Scholar]
- 46.Taylor MJ, Charman T, Robinson EB, et al. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychol Med. 2013;43(8):1735-1746. doi: 10.1017/S003329171200253X [DOI] [PubMed] [Google Scholar]
- 47.Polderman TJC, Hoekstra RA, Posthuma D, Larsson H. The co-occurrence of autistic and ADHD dimensions in adults: an etiological study in 17,770 twins. Transl Psychiatry. 2014;4:e435. doi: 10.1038/tp.2014.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris RK, Oliver EA, Malin G, Khan KS, Meads C. Effectiveness of interventions for the prevention of small-for-gestational age fetuses and perinatal mortality: a review of systematic reviews. Acta Obstet Gynecol Scand. 2013;92(2):143-151. doi: 10.1111/aogs.12029 [DOI] [PubMed] [Google Scholar]
- 49.Taylor LK, Lee YY, Lim K, Simpson JM, Roberts CL, Morris J. Potential prevention of small for gestational age in Australia: a population-based linkage study. BMC Pregnancy Childbirth. 2013;13:210. doi: 10.1186/1471-2393-13-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahlqwist E, Pawitan Y, Sjölander A. Regression standardization and attributable fraction estimation with between-within frailty models for clustered survival data [published online January 1, 2017]. Stat Methods Med Res. doi: 10.1177/0962280217727558 [DOI] [PubMed] [Google Scholar]
- 51.Turkheimer E, Waldron M. Nonshared environment: a theoretical, methodological, and quantitative review. Psychol Bull. 2000;126(1):78-108. doi: 10.1037/0033-2909.126.1.78 [DOI] [PubMed] [Google Scholar]
- 52.Van Lieshout RJ, Krzeczkowski JE. Just DO(HaD) it! testing the clinical potential of the DOHaD hypothesis to prevent mental disorders using experimental study designs. J Dev Orig Health Dis. 2016;7(6):565-573. doi: 10.1017/S2040174416000441 [DOI] [PubMed] [Google Scholar]
- 53.Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry. 2017;17(1):235. doi: 10.1186/s12888-017-1381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. International Classification of Diseases (ICD) Codes
eTable 2. Descriptive Statistics about Exposure, Covariates, and Outcomes
eAppendix. Sensitivity Analyses
eTable 3. EFA Loadings After Bifactor Rotation of Four Factors
eTable 4. Standardized General and Specific Factors Based on Four-Factor Solution Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age
eTable 5. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among Those Born 1987 or Later
eTable 6. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among those Born at Week 37 or Later
eTable 7. Standardized General and Specific Factors Regressed on Small for Gestational Age (SGA) Diagnosis
eTable 8. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Based on Siblings Born Within 2.51, 3, 4, 6, 7, 8, 9, and 10 Years Apart
eTable 9. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Based on Sex-Concordant Sibling Pairs Only
eTable 10. Standardized General and Specific Factors Regressed on Birth Weight Coded in Kilograms Controlling Gestational Age Among Those Born Within Three Standard Deviations of the Mean of Birth Weight and Gestational Age
eFigure 1. Latent Exploratory Factors Regressed on Birth Weight and Covariates (Not Shown)
eFigure 2. Scree Plot of Adverse Outcomes