Abstract
Objectives
Cardiac atrial appendage stem cells (CASCs) have recently emerged as an attractive candidate for cardiac regeneration after myocardial infarction. As with other cardiac stem cells, CASCs have to be expanded ex vivo to obtain clinically relevant cell numbers. However, foetal calf serum (FCS), which is routinely used for cell culturing, is unsuitable for clinical purposes, and influence of long‐term in vitro culture on CASC behaviour is unknown.
Materials and methods
We examined effects on CASC biology of prolonged expansion, and evaluated a culture protocol suitable for human use.
Results
In FCS‐supplemented medium, CASCs could be kept in culture for 55.75 ± 3.63 days, before reaching senescence. Despite a small reduction in numbers of proliferating CASCs (1.37 ± 0.52% per passage) and signs of progressive telomere shortening (0.04 ± 0.02 kb per passage), their immunophenotype and myocardial differentiation potential remained unaffected during the entire culture period. The cells were successfully expanded in human platelet plasma supernatant, while maintaining their biological properties.
Conclusions
We successfully developed a protocol for long‐term culture, to obtain clinically relevant CASC numbers, while retaining their cardiogenic potential. These insights in CASC biology and optimization of a humanized platelet‐based culture method are an important step towards clinical application of CASCs for cardiac regenerative medicine.
Introduction
Over the past decade, discovery of resident cardiac stem cells (CSCs) has raised high hopes for regenerating functional myocardium and restoring cardiac function after myocardial infarction (MI). CSCs can be found clustered in niches located in specific areas of the adult heart, such as the atria, apex and epicardium 1, 2, 3; they are self‐renewing, clonogenic and multipotent 4, 5, 6. Data from preclinical animal models have provided strong support that CSCs play a fundamental role in cardiac regeneration after ischaemic insult 7, 8. They are tissue‐specific, pre‐committed to cardiac fate and can be isolated from a target patient population making them suitable for autologous use. As a consequence, CSCs have recently been investigated in phase I clinical trials in which MI patients were transplanted with autologous CSCs to assess their safety, feasibility and efficacy 9, 10.
Very recently, our research group described the isolation of cardiac atrial appendage stem cells (CASCs) from ischaemic heart disease (IHD) patients 11. The isolation procedure relies on elevated activity of aldehyde dehydrogenase (ALDH), a well‐known feature of several types of stem cell, such as haematopoietic, mesenchymal (MSC) and neural stem cells 12, 13, 14. In native tissue, CASCs are typically ALDH+, CD34+, CD45− and c‐kit−, but CD34 expression is lost during cell culture. The cells are clonogenic, express a number of pluripotency associated genes, and display functional cardiogenic differentiation in co‐culture with neonatal rat cardiomyocytes (NRCMs). In addition, autologous injection of expanded CASCs into the peri‐infarct zone has resulted in successful engraftment and cardiac differentiation in a minipig MI model 11. Results of a functional repair follow‐up study in this preclinical animal model, have shown the potential of CASCs for clinical use in cardiac regenerative medicine (article submitted).
Many key questions regarding the biological properties of CASCs, which may directly affect their clinical success in IHD patients, remain unanswered. In addition, as for other CSCs, CASCs are found in relatively low frequency in the human heart (0.9 ± 0.8% total heart cell population). Thus, patient application of CASCs requires their isolation and subsequent large‐scale ex vivo expansion, to obtain sufficient relevant cell quantities for clinical use. Moreover, CASCs have to be collected from elderly patients, which might constrain their cardiac regeneration potential as they may already display signs of cell ageing, such as telomere shortening and dysfunction, causing cells to enter a crisis phase. In addition, long‐term in vitro culture might exacerbate these ageing processes resulting in early senescence and loss of differentiation potential. Consequently, these concerns need to be addressed before CASCs can be applied in regenerative medicine.
Traditional cell culture protocols use foetal calf serum (FCS) as a nutritional supplement. Considering clinical application, however, FCS is a less suitable additive as it has a number of disadvantages, such as potential risk of transmitting infectious agents, inducing xenogeneic immune reactions and high cost 15, 16, 17. In this context, human‐derived platelet lysate (PL) and platelet plasma supernatant (PPS) have been suggested to be adequate alternatives for FCS in large‐scale cell culture for clinical settings 18, 19, 20. PPS can be easily collected from common platelet units, while PL can be simply generated from the same units by freeze–thaw procedures.
The goal of the present study was to examine biological characteristics of CASCs over long‐term ex vivo expansion. Thus, we evaluated CASC proliferation, ageing, immunophenotype and differentiation characteristics at various time points during culture. In addition, with a view to future transplantation studies, we evaluated suitability of a CASC culture method based on blood platelet‐derived supplements.
Material and methods
All procedures were carried out in accordance with the principles set forth in the Helsinki Declaration. Approval by the Jessa Institutional Review Board and informed consent from each patient were obtained. All animal studies were approved by the Hasselt University Institutional Animal Care and Use Committee.
Preparation of platelet plasma supernatant
Outdated platelet concentrates obtained by platelet aphaeresis were provided by the blood bank of the Jessa Hospital, maximum 1 week after collection. Platelet concentration was in the order of 1 × 109/ml. Platelet concentrates were centrifuged for 15 min at maximum speed (3600 g), PPS was collected and aliquots were stored at −20 °C until use. The platelet pellet was suspended in X‐Vivo 15 medium at 5.7 × 109 platelets/ml and platelets were either snap frozen in liquid nitrogen or frozen at −80 °C followed by rapid thawing at 37 °C to lyse them. This freeze/thaw cycle was repeated twice. The suspension was centrifuged for 15 min at maximum speed (3600 g) and obtained PL suspension was collected and aliquots which were stored at −20 °C until use.
Isolation and expansion of CASCs
Cardiac atrial appendage stem cells were isolated from atrial appendages obtained from IHD patients undergoing routine cardiac surgery, as previously described by Koninckx et al. 11. Cells were seeded in fibronectin (8–24 μg/ml)‐coated culture plates (Becton & Dickinson, Franklin Lakes, NJ, USA) and expanded at 37 °C in humidified atmosphere containing 5% CO2. After the first cell passage, 20% FCS was reduced to 10% FCS or alternatively, replaced by 10% PL or different concentrations of PPS, in the presence of 2% penicillin–streptomycin–amphotericin B (Lonza) and 5 IU/ml heparin (Leo). For comparison of 10% FCS and 7.5% PPS, two different platelet units were tested for every CASC culture. Average values of these replicates were calculated and used for further analysis. Each time 80–85% confluence was reached, cells were replated at 5 × 103 cells/cm². Medium was changed twice a week. CASCs were uninterruptedly maintained in long‐term in vitro cultures until a state of proliferative arrest was reached. This was characterized by population doubling (PD) of less than one, which leads to stabilization of the population doubling level (PDL) and cumulative cell number.
Determination of growth kinetics
Cells were counted, following trypan blue exclusion testing, at every passage. Total number of cells at each passage was calculated as ratio of total number of cells harvested at the current passage to total number of cells seeded at the previous passage, multiplied by total number of cells harvested at the previous passage. PDs were calculated using the formula:
where A 0 represents initial cell number and A represents cell harvest number 21. To calculate cumulated PDL at a particular passage, calculated PD for this passage was added to PDs of previous passages. Population doubling time (PDT) was obtained from the formula:
where A 0 signifies initial cell number, A is cell number at time point ‘t’ and t represents time (in days) since the last passage 22.
Cell cycle analysis
Cell cycle distribution was analysed using the BD Cycletest™ Plus DNA Reagent Kit (Becton & Dickinson) according to the manufacturer's instructions. To this end, 2.5 × 105 CASCs were cultured under standard culture conditions and 10 ng/ml KaryoMAX® Colcemid™ solution (Invitrogen) was added the following day, for 48 h, to obtain mitotic arrest in metaphase. Cells were then harvested after trypsinization, frozen in buffer solution and stored until analysis. Cell DNA content was analysed using FACSCanto® (Becton & Dickinson) apparatus. CASCs in S and G2M phase were defined as actively proliferating cells. DNA QC particles (Becton & Dickinson) were used for quality control according to the manufacturer's protocol. Normal peripheral blood cells collected from healthy individuals was utilized as diploid DNA reference for calibration and had <0.5% proliferating cells. Mitotic indices were calculated with ModFit LT software 3.0 (Verity Software House, Topsham, ME, USA).
Immunophenotyping by flow cytometry
Antigenic expression profile of CASCs was determined by flow cytometry. 5 × 104 CASCs/tube were incubated for 20 min in the dark with human monoclonal antibodies as recommended by the manufacturer. Fluorescence minus one combined with mouse IgG isotype control was used for correct gating and to identify non‐specific staining. All antibodies were purchased from Becton & Dickinson, except for CD105‐FITC (Serotec, Kidlington, UK).
Analysis of aldehyde dehydrogenas expression
Flow cytometric analysis of ALDH expression in CASCs was performed using Aldefluor™ kit (Aldagen, Inc., Durham, NC, USA). For this, 4 × 104 cells were incubated in 500 μl Aldefluor assay buffer containing 1.5 μm activated Aldefluor® reagents (Aldagen, Inc). ALDH+ and ALDH− CASC populations were flow sorted and separately seeded in 96‐well plates in X‐Vivo 15 medium supplemented with 20% FCS and 2% P/S, to evaluate their continued growth. In addition, a fraction of both populations was flow sorted into FACS tubes containing Aldefluor assay buffer. Subsequently, cytospin centrifugation was performed for microscope visualization of the green fluorescent reaction product using an Axiovert 200M microscope (Zeiss, Oberkochen, Germany). Alternatively, ALDH expression was directly analysed on cultured CASCs by seeding 1 × 104 cells/well in 24‐well plates and performing similar incubation steps in the well plates. After incubation at 37 °C for 30 min, cells were washed and kept in Aldefluor assay buffer for microscope visualization of green fluorescent reaction product. For all microscopy data acquisitions, exposure times were kept constant during each recording.
Production of green fluorescent protein lentiviruses and transduction of CASCs
Prior to setting up co‐culture differentiation assays, CASCs were labelled with green fluorescent protein (GFP). Production of GFP‐containing lentiviruses and transduction of CASCs was performed as previously described 23.
Functional cardiomyogenic differentiation assays
To stimulate cardiomyogenic differentiation, co‐culture systems were set up between GFP+ CASCs and NRCMs as previously described. After 1 week, cardiac differentiation was evaluated by immunofluorescence for cardiac troponin (cTn)T and I 11.
Telomere length measurement with real‐time PCR
Genomic DNA was isolated from CASCs by ethanol precipitation or using QIAamp DNA mini kit (Qiagen Ltd., Venlo, The Netherlands), according to the manufacturer's instructions. The 1301 cell line (T‐cell lymphoblastic leukaemia), which has been reported to have long and constant telomeres, was used as positive control 24.
Singleplex real‐time PCR reactions were carried out in duplicate with Rotor‐Gene Q (Qiagen Ltd.), based on methods of Cawthon et al. 25. Final reaction master mix composition consisted of 1X Platinum® SYBR® Green qPCR SuperMix‐UDG (Invitrogen), 1 M Betaine (Sigma‐Aldrich, St. Louis, MO, USA), 900 nm albumin or 450 nm telomere primer, respectively, and 20 ng DNA. PCR reaction consisted of the following steps: 95 °C for 15 min, 2 cycles of 94 °C for 15 s and 50 °C for 15 s, 32 cycles of 94 °C for 15 s, 62 °C for 10 s, 74 °C for 15 s (acquisition of telomere product fluorescence), 84 °C for 10 s and 86 °C for 15 s (acquisition of albumin product fluorescence), followed by melt curve analysis. To quantify absolute telomere length (aTL), standard curves of albumin and telomere plasmids were generated as previously described 26.
Telomerase expression with real‐time RT‐PCR
Total RNA was extracted from CASCs using RNeasy Micro Kit Isolation System (Qiagen Ltd.), according to the manufacturer's instructions. RNA quality and integrity were analysed on an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA was eluted in RNase‐free H2O and stored at −80 °C. cDNA was synthesized using Superscript™ First‐Strand cDNA Synthesis System (Invitrogen), according to the provider's protocol. Real‐time RT‐PCR reactions were performed in duplicate on Rotor‐Gene Q (Qiagen Ltd.). Reaction conditions consisted of 1X Absolute qPCR Sybr Green Mix (Thermo Scientific, Waltham, MA, USA), 300 nm human telomerase reverse transcriptase (hTERT) primers or 450 nm Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) primers and 1 μl of cDNA. As previously described by Anedchenko et al., hTERT primers were designed specifically to amplify the only functional full‐length hTERT isoform 1: hTERT forward primer 5′‐CTGTACTTTGTCAAGGTGGATGTGA‐3′ and hTERT reverse primer 5′‐ GTACGGCTGGAGGTCTGTCAAG‐3′ 27. The single copy gene (scg) primers were designed in‐house by primer express 3.0 with: GAPDH forward primer 5′‐AGTCAACGGATTTGGTCGTATTG‐3′ and GAPDH reverse primer 5′‐ATCTCGCTCCTGGAAGATGGT‐3′. PCR reaction was performed as previously described: 5 min at 95 °C, 50 cycles at 95 °C for 15 s, 60 s at the corresponding annealing temperature (AT) (57 °C for GAPDH and 59 °C for hTERT) and 72 °C for 20 s, which was followed by melt curve analysis 27.
Statistical analysis
To analyse whether starting number correlated to maximal cumulative number of CASCs, CASC populations were categorized into two groups: CASCs with <4 × 104 initial cells (A 0low; n = 10) and CASCs >4 × 104 initial cells (A 0high; n = 10). Comparisons between groups were performed in GraphPad version 5.01 using Mann–Whitney testing. Linear mixed models were used for data measured at repeated time points during the follow‐up period (patient evolution data). Linear mixed models were used to compare aTL per passage (random intercept) as well as number of proliferative CASCs per passage (random intercept and slope), after outlier detection and removal, with software ‘R’ version 2.10.1 (Vienna, Austria). For comparison of CASC aTL in FCS and PPS, a treatment–time interaction was included. All statistical analyses for linear mixed models were performed in SAS version 9.2 (SAS Institute, Cary, NC, USA). For each test, at least three patients per passage were analysed, unless stated otherwise.
Results
Isolation and long‐term in vitro expansion of CASCs
Growth properties of CASC cultures from different IHD patients (n = 21; Table 1) were evaluated over the entire expansion period. Cultures that did not grow beyond P5 were excluded from further analysis (n = 4). In general, CASC cultures had an early, exponential growth phase (P2–P5), followed by a late phase (P6–P9) characterized by slow decay in growth rate. Cells remained in culture for 51.88 ± 2.60 days on average and performed 9.04 ± 0.98 PDs until ultimately reaching a state of proliferative arrest. This was accompanied by apparently enlarged volume morphology and formation of cell structures resembling apoptotic blebs and necrotic cell debris (data not shown). In terms of growth and in vitro lifespan, a wide inter‐individual variability was observed among CASC cultures. This was clearly reflected in the maximum calculated cumulative number of cells, which ranged between 0.34 × 106 and 11.07 × 109 (Fig. 1a). However, maximal cumulative number of cells did not correlate with their starting number as initially harvested from tissue specimens (P = 1.00, data not shown). Furthermore, early phase CASCs had an average of 2.01 ± 0.12 PDs, which reduced slightly to 1.60 ± 0.15 PDs for late phase cells. CASCs had average PDL of 6.02 ± 0.60 at P5 and 13.65 ± 1.89 at P9 (Fig. 1b). According to PDT data, 52.9% of CASCs had high initial growth rate, slowing towards the end of the culture period, while 41.2% of the populations displayed variable growth rates, with alternating periods of slow and fast expansion. In 5.9% of CASC cultures, a rather slow expansion rate was observed during early phases which accelerated towards the end of the culture period, prior to reaching growth arrest. PDT measured in early phase CASCs was 3.58 ± 0.45 days, increasing to 6.54 ± 0.79 days in late phase CASCs. Average PDT commonly increased throughout all CASC cultures.
Table 1.
Patient characteristics
| CASC group (N = 21) | |
|---|---|
| Age | 71.71 ± 9.95 |
| Male | 16 (76) |
| Risk factors | |
| Weight (kg) | 80.00 ± 11.78a |
| Body mass index (kg/m²) | 27.54 ± 3.72a |
| Last creatinine level pre‐operation (mg/dl) | 1.01 ± 0.24 |
| Smoker | 10 (50)a |
| Family history of CAD | 5 (25)a |
| Diabetes | 5 (25) |
| Hyperlipidaemia | 14 (70) |
| Renal dysfunction | 4 (20) |
| Hypertension | 14 (67) |
| Chronic lung disease | 1 (5) |
| Peripheral vascular disease | 4 (19) |
| Cerebrovascular disease | 6 (29) |
| Pre‐operative cardiac status | |
| Myocardial infarction | 7 (35) |
| Congestive heart failure | 4 (20) |
| Angina | 17 (81) |
| Arrythmia | 4 (19) |
| Classification: NYHA I/II/III/IV | 0/11/4/5a (0/55/20/25)a |
| Pre‐operative medicine | |
| Beta‐blockers | 13 (60) |
| Nitrates PO | 10 (50) |
| Nitrates IV | 5 (25) |
| Diuretics | 2 (10) |
| ACE inhibitors | 6 (30) |
| Ca antagonists | 4 (15) |
| Antiarrythmias | 3 (15) |
| Lipid lowering | 15 (75) |
| Aspirin | 18 (85) |
| Other antiplatelets | 6 (30) |
| N vessels: 0/1/2/3 | 1/0/6/13 (5/0/29/67) |
| Surgical procedure | |
| CABG/valve/other | 19/4/0 (95/20/0) |
Values are expressed as mean ± SD or n (%).
CASC, cardiac atrial appendage stem cell; NYHA, New York Heart Association; ACE, angiotensin‐converting enzyme; Ca, calcium; CABG, coronary artery bypass.
N = 20.
Figure 1.

Cardiac atrial appendage stem cell growth kinetics over long‐term expansion. Graphs represent cumulative cell number (a) and population doubling level (PDL, b). Graphs are displayed in function of passage number (P2–P9) as mean ± SEM. At each passage N ≥ 4.
Additionally, proliferation assays were performed to follow cell cycle progression over long‐term in vitro expansion (n = 13; P4–P9). On average 19.40 ± 1.07% of all CASCs were in mitosis (Fig. 2a). This proliferative percentage ranged from maximum of 22.77 ± 2.45% at P5 to 17.10 ± 2.98% at P9. There was a slight but significant reduction of 1.37 ± 0.52% in the number of proliferating CASCs at each passage (P = 0.046) (Fig. 2b). This analysis revealed also that CASCs showed no signs of aneuploidy over the course of expansion, even at higher passages.
Figure 2.
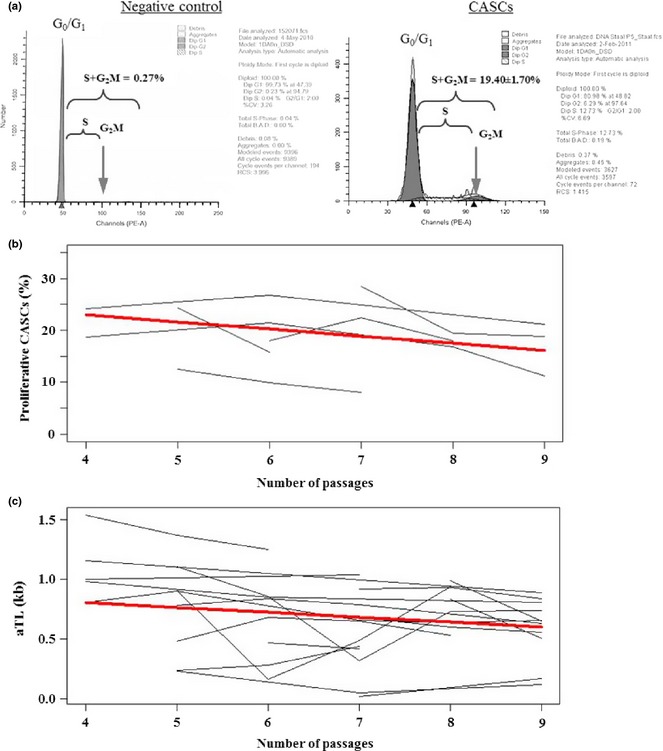
Proliferation and absolute telomere length (aTL) of expanded cardiac atrial appendage stem cells ( CASC s). Representative cytometry output of cell cycle distribution of peripheral blood cells (negative control, a left) and expanded CASCs (P4–P9) (a right). (b) Individual (grey lines) and average (bold red line) linear reduction in percentage of proliferating CASCs with increasing passages (P = 0.046). (c) Individual (grey lines) and average (bold red line) inverse linear relation between aTL and passage number (P = 0.019).
Ageing and cell senescence dynamics of CASCs
To assess the influence of concomitant serial passaging on cell ageing, aTL was determined in CASCs over their expansion period. Cells were collected and expanded from 19 different patients, subsequently harvested at consecutive passages (P4–P9) and aTL was measured with real‐time PCR assay. Expanded CASCs had average aTL of 0.69 ± 0.33 kb for the entire culture period, ranging from 1.10 ± 0.28 kb at P4 to 0.59 ± 0.24 kb at P9. Statistical analysis revealed that CASCs showed overall and significant reduction in aTL with increasing number of passages (P = 0.019) (Fig. 2c). More specifically, at each passage, the aTL declined with 0.04 ± 0.02 kb, on average. These results indicate that, despite inter‐individual variation between patients, change of aTL in CASCs was consistent over time. In agreement with these observations, hTERT transcripts were not expressed in expanded CASC cultures up to P9 (n = 2, data not shown).
Biological characteristics of expanded CASCs
The immunophenotype of CASCs (n = 14, P4–P9) was continuously monitored at different passages throughout the culture period and retained a stable phenotype without any specific alteration. Expanded CASCs remained CD34, CD45 and CD117 negative. In contrast, the cells stably expressed CD29, CD55dim, CD73, CD90 and CD105. For CD90 in particular, two subpopulations were demonstrated to be present, CD90dim and CD90+. Data retrieved from distinct CASC populations are summarized in Fig. 3 (red line). Furthermore, ALDH expression in the cells was monitored over their long‐term expansion period (n = 9). Our data indicate that ALDH activity tended to be constant, with average 50.65 ± 2.24% of ALDH+ CASCs during their expansion from P4 to P9. CASCs displayed no sign of decaying ALDH activity with increasing days in culture (Fig. 4a). Interestingly, when ALDH+ and ALDH− CASC subpopulations were flow sorted and seeded in separate culture plates, only the ALDH+ fraction attached to the culture plate and revealed continued growth and expansion (Fig. 4b). In contrast, ALDH− CASCs remained unattached, undergoing cell death.
Figure 3.
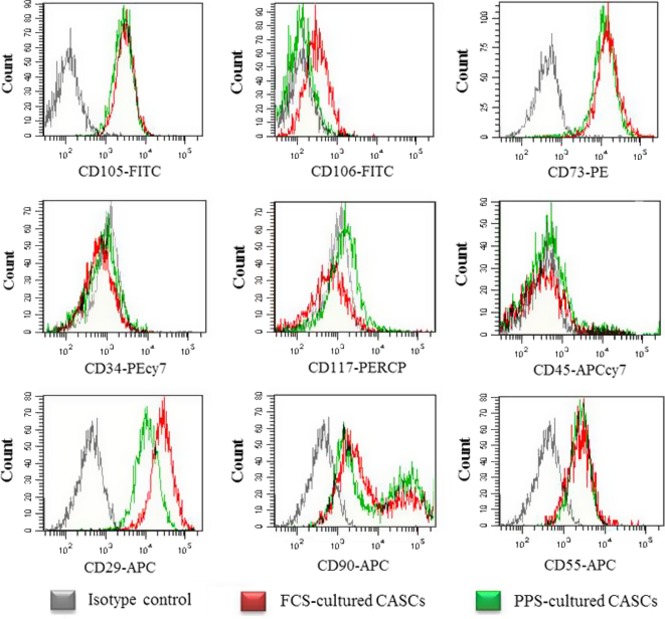
Immunophenotypic analysis of ex vivo expanded cardiac atrial appendage stem cells ( CASC s). Long‐term in vitro expanded CASCs (P4–P9) displayed continuous stable phenotype with expression of CD105, CD73, CD29, CD90 and CD55, while lacking CD106, CD117, CD34 and CD45. N ≥ 3 at each passage for each antigen marker.
Figure 4.
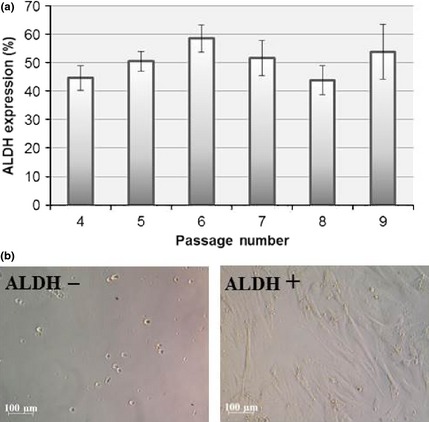
Aldehyde dehydrogenase ( ALDH ) expression of expanded cardiac atrial appendage stem cells. (a) Flow cytometry analysis of ALDH activity in expanded cardiac atrial appendage stem cells (P4–P9), displayed by mean ± SEM. (b) ALDH− (left) and ALDH+ cells (right) in culture. N ≥ 4 per passage.
To evaluate preservation of cardiogenic potential of CASCs (n = 17) over long‐term expansion, GFP+ cells were harvested at different time points and brought into co‐culture with NRCMs. CASC cultures from P4 to P9 maintained their ability to differentiate towards cardiomyocytes, as demonstrated by expression of cardiac‐specific proteins TnT and TnI (Fig. 5a,b).
Figure 5.
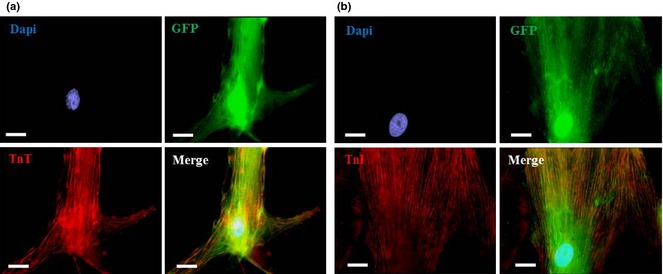
Cardiomyogenic differentiation of expanded cardiac atrial appendage stem cells. Immunofluorescence images illustrating cTnT (red, a) and cTnI staining (red, b) on GFP+ CASCs (green; P4–P9) after 1 week in co‐culture with NRCMs. Nuclei stained with 4′,6′‐diamidino‐2‐phenylindole (DAPI) (blue). N ≥ 3 measurements at each passage. Scale bar = 20 μm.
PPS as a culture supplement for CASC expansion in a clinical setting
For the purpose of a future CASC cell therapy study, we aimed to establish a non‐FCS‐based culture method. To this end, we evaluated the use of human PL and PPS as potential alternative medium supplements for FCS for long‐term in vitro culture of CASCs. Initial experiments showed that CASCs adhere to tissue culture plates within 24 h of seeding, when cultured in the presence of 10% FCS or 10% PPS, but not in 10% PL (n = 4/group; data not shown). Further optimization experiments with 5%, 7.5% and 10% PPS revealed an optimal concentration of 7.5% PPS in the culture medium (n = 4/group; data not shown); this was therefore selected as the medium supplement for further experiments.
Subsequently, biological characteristics of CASCs expanded in medium enriched with 7.5% PPS were compared to those of CASCs cultured in 10% FCS (n = 4/group). In general, CASC growth kinetics in PPS were similar to those of cells cultured in FCS‐based medium (Fig. 6a,b). Maximal cumulative cell number ranged from 1.34 × 106 to 2.56 × 108 when the cells were cultured in medium with FCS, and maximal cumulative CASC numbers in PPS‐supplemented medium ranged from 1.58 × 106 to 6.65 × 107. CASCs cultured in FCS‐ and PPS‐supplemented medium displayed average maximal PDL of 10.43 ± 3.78 and 9.46 ± 2.20 respectively. Furthermore, average aTL of CASCs (P5–P9) cultured in PPS and FCS was similar with values of 0.69 ± 0.30 kb and 0.55 ± 0.16 kb, respectively. Indeed, no significant differences were detected between aTL of CASCs cultured in PPS‐ or FCS‐enriched medium at the same passage (P = 0.134; n = 4/group) (Fig. 6c). Flow cytometry analyses revealed the same antigenic profile for CASCs cultured in PPS‐ compared to FCS‐enriched medium (P2–P9; n = 3/group; Fig. 3 green line). Finally, in comparison with FCS, co‐culturing GFP+ CASCs expanded in PPS‐enriched medium (P4–P9) with NRCMs revealed equally effective cardiomyogenic differentiation potential as demonstrated by expression of cTnT and cTnI (n = 4/group; Fig. 7a,b).
Figure 6.
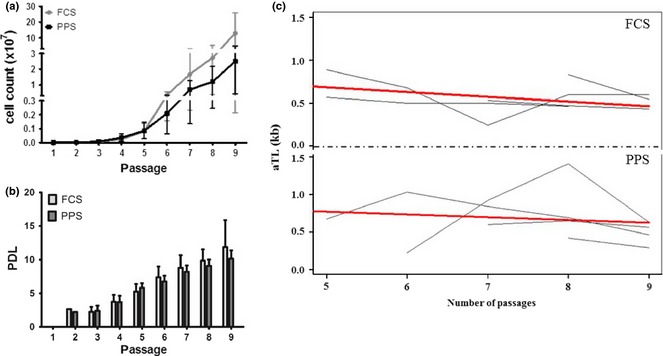
Comparison of growth kinetics and absolute telomere length (aTL) of cardiac atrial appendage stem cells ( CASC s) expanded in medium enriched with platelet plasma supernatant (PPS) or foetal calf serum (FCS). Graphs illustrating cumulative cell count (a) and PDL (b) of expanded CASCs (P2–P9) in medium enriched with FCS and PPS. Data represent mean ± SEM. In general, at each passage N = 4, except for P2 N = 1 and P9 N = 2 or N = 3 for respectively FCS and PPS. Graphs illustrative of individual (grey lines) and average (bold red line) aTL evolutions with increasing passages (c). No significant differences were detected between aTL of CASCs cultured in FCS‐ (top), PPS‐ (bottom) enriched medium of the same passage (P = 0.134).
Figure 7.
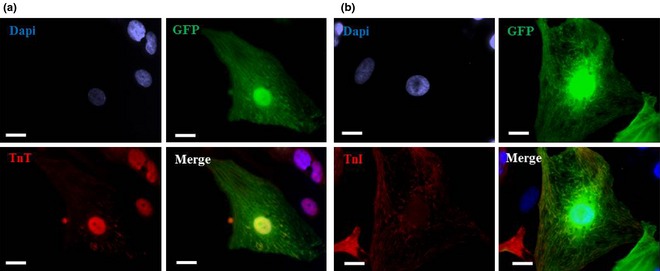
Cardiomyogenic differentiation of cardiac atrial appendage stem cells expanded in platelet plasma supernatant‐supplemented medium. Immunofluorescence images illustrating cTnT (red, a) and cTnI staining (red, b) on platelet plasma supernatant‐cultured GFP+ cardiac atrial appendage stem cells (green; P4–P9) after 1 week in co‐culture with neonatal rat cardiomyocytes. Nuclei stained with DAPI (blue). N = 4. Scale bar = 20 μm.
Discussion
We have recently shown that CASCs may show great promise for cardiac regenerative therapies 11. As for other CSC‐types, ex vivo expansion is an essential step in production of therapeutically meaningful cell numbers. However, long‐term expansion protocols have been reported to negatively impact the differentiation capacity of cells 28. Therefore, the aim of this study was to investigate extensively the influence of prolonged culture, not only on differentiation potential of CASCs but also on their growth, phenotype and ageing processes.
In the present study, we have demonstrated that CASCs isolated from elderly IHD patients had good expansion capacity and could be maintained in culture for approximately 2 months. None of the CASC cultures bypassed the senescence phase. Using this expansion protocol, large quantities of cells were generated, equalling ranges used in previously conducted phase I clinical trials with CSCs 29, 30. Although high inter‐patient variability was observed in growth profiles and expansion potential of CASCs, this is a recurrent observation in most expansion evaluation protocols. Despite observing rather stable growth up to P9, we noticed gradual reduction in proliferative percentage, accompanied by minor but consistent reduction in aTL. This is not remarkable as CASCs lack expression of telomerase catalytic subunits, and these findings are consistent with previous reports describing telomere kinetics in cardiac progenitor cells 31, 32. As telomere shortening is a known trigger for cell senescence and one of the side effects associated with cell proliferation, excessive ex vivo expansion should be kept to a minimum to avoid detrimental effects on telomere maintenance and stem cell ageing. Nevertheless, despite progressive telomere shortening with an accumulating number of passages, cell cycle analysis demonstrated that CASCs did not display any genomic aneuploidy, suggesting the absence of major chromosomal aberrations, although this would have to be investigated in more detail in future experiments. The results here also demonstrate that CASCs can be expanded in the long‐term while retaining their antigenic expression profile, ALDH expression and more importantly their cardiomyogenic differentiation capacity. Interestingly, ALDH expression remained constant during the entire culture period, with an average of 50% of CASCs displaying elevated ALDH levels. Separate flow sorting of ALDH− and ALDH+ subpopulations revealed that only ALDH+ cells could be further expanded. This suggests that these two fractions most likely arose from asymmetrical cell division, in which loss of ALDH expression contributes to a more mature cell fate. Overall, despite a decline in growth rate and progressive telomere shortening at higher passages, CASCs preserved their regenerative capacity through cardiac differentiation until the end of the culture period, indicating that their proliferative arrest over long‐term expansion is most likely due to senescence. Nevertheless, ongoing research in a minipig model of MI will have to determine transplantation efficiency and functional impact of these cells.
Foetal calf serum, which is traditionally used in cell expansion protocols, is less suitable for clinical use as it is a potential source of unknown xenogeneic antigens which might elicit immunological reactions in a recipient 33. Moreover, animal pathogens might cause infection and lead to rejection of transplanted cells 34. In this regard, use of human platelet‐derived growth factors as medium supplement offers great advantages, avoiding risks associated with FCS. Human PL has already been shown to represent an attractive medium supplement for large‐scale expansion of bone marrow MSCs while they maintained stable phenotype and differentiation capacity 19, 35, 36. Noticeably, in this study, CASCs only demonstrated successful growth and expansion in PPS‐supplemented but not PL‐supplemented medium. A reasonable explanation might be that here, medium PL was prepared from expired platelets, which could no longer be used for platelet transfusion. Possibly, spontaneous activation of platelets during this prolonged storage period might have caused release of large contributors to their intragranular growth factor content 37, 38. Our results show that PPS was equally efficient for large‐scale expansion of human CASCs compared to FCS. Moreover, no significant differences between aTL of CASCs cultured in PPS‐, in comparison to FCS‐enriched, medium were observed. Furthermore, CASCs expanded in PPS preserved their antigenic expression profile and their ability to differentiate to cardiomyocytes, even after prolonged culture periods. To our knowledge, we are the first to provide detailed information on the influence of PPS on growth and differentiation of CSCs, in particular CASCs. Our findings are comparable to studies evaluating bone marrow MSCs cultured in platelet‐derived medium supplements 35, 36. Thus, we suggest that replacement of FCS with human platelet‐originating growth factors is very favourable for large‐scale expansion of human CSCs in future clinical settings.
In conclusion, we have demonstrated that CASCs could be successfully isolated then expanded for a prolonged period of time, from elderly IHD patients, producing high cell numbers relevant to therapeutic application. Although serial passaging and concomitant ageing of CASCs resulted in progressive decline of telomere length, the cells cardiomyogenic differentiation potential remained unaffected. Despite this and absence of aneuploidy, more detailed examination on biosafety and tumour formation needs to be performed in a preclinical study to guarantee patient safety. Nevertheless, preservation of CASC biology, reported herein, and use of PPS as efficient replacement for FCS for large‐scale clinical expansion of CSCs represent an important step towards clinical use of CASCs in cardiac regenerative medicine.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was partially funded by a Ph.D. grant of the Agency for Innovation by Science and Technology in Flanders (IWT), and partially by the Limburg Clinical Research Program (LCRP) UHasselt‐Jessa‐ZOL, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Jessa Hospital and Ziekenhuis Oost‐Limburg. We gratefully thank our colleague Ann Creemers, member of the Centre of Statistics (CENSTAT) at Hasselt University and the Flanders Training Network for Methodology and Statistics (FLAMES), for her expertise and kind assistance with the statistical linear mixed model analyses performed in this manuscript.
References
- 1. Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR et al (2007) Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177–182. [DOI] [PubMed] [Google Scholar]
- 2. Itzhaki‐Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, Netser S et al (2009) Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation 120, 2559–2566. [DOI] [PubMed] [Google Scholar]
- 3. Leinonen JV, Emanuelov AK, Platt Y, Helman Y, Feinberg Y, Lotan C et al (2013) Left atrial appendages from adult hearts contain a reservoir of diverse cardiac progenitor cells. PLoS One 8, e59228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A et al (2007) Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 104, 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E et al (2007) Regenerative potential of cardiosphere‐derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908. [DOI] [PubMed] [Google Scholar]
- 6. Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S et al (2005) Postnatal isl1 + cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel‐Latif A et al (2013) Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR et al (2009) Engraftment, differentiation, and functional benefits of autologous cardiosphere‐derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083, 7 p following 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J et al (2012) Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126(11 Suppl 1), S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO et al (2014) Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 63, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koninckx R, Daniels A, Windmolders S, Mees U, Macianskiene R, Mubagwa K et al (2013) The cardiac atrial appendage stem cell: a new and promising candidate for myocardial repair. Cardiovasc. Res. 97, 413–423. [DOI] [PubMed] [Google Scholar]
- 12. Bell GI, Broughton HC, Levac KD, Allan DA, Xenocostas A, Hess DA (2012) Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem Cells Dev. 21, 97–109. [DOI] [PubMed] [Google Scholar]
- 13. Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R et al (2006) Identification of a primitive brain‐derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells 24, 975–985. [DOI] [PubMed] [Google Scholar]
- 14. Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE (2007) Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the ALDH‐bright cell population of cryopreserved, banked UC blood. Cytotherapy 9, 569–576. [DOI] [PubMed] [Google Scholar]
- 15. Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K (2007) No alloantibodies against mesenchymal stromal cells, but presence of anti‐fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 92, 1208–1215. [DOI] [PubMed] [Google Scholar]
- 16. Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U et al (2007) N‐glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells 25, 197–202. [DOI] [PubMed] [Google Scholar]
- 17. Tekkatte C, Gunasingh GP, Cherian KM, Sankaranarayanan K (2011) “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int. 2011, 504723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D et al (2009) Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 27, 2331–2341. [DOI] [PubMed] [Google Scholar]
- 19. Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X et al (2005) Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell‐based therapy applications. J. Cell. Physiol. 205, 228–236. [DOI] [PubMed] [Google Scholar]
- 20. Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E et al (2007) Human platelet lysate can replace fetal bovine serum for clinical‐scale expansion of functional mesenchymal stromal cells. Transfusion 47, 1436–1446. [DOI] [PubMed] [Google Scholar]
- 21. Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC (1998) Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc. Natl. Acad. Sci. USA 95, 10614–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M (2010) Increased proliferation and analysis of differential gene expression in human Wharton's jelly‐derived mesenchymal stromal cells under hypoxia. Int. J. Biol. Sci. 6, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koninckx R, Hensen K, Daniels A, Moreels M, Lambrichts I, Jongen H et al (2009) Human bone marrow stem cells co‐cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy 22, 1–15. [DOI] [PubMed] [Google Scholar]
- 24. Larsson I, Lundgren E, Nilsson K, Strannegard O (1979) A human neoplastic hematopoietic cell line producing a fibroblast type of interferon. Dev. Biol. Stand. 42, 193–197. [PubMed] [Google Scholar]
- 25. Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 37, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Callaghan NJ, Fenech M (2011) A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anedchenko E, Oparina N, Dmitriev A, Krasnov G, Pavlova L, Alexandrova N et al (2008) Activation of the hTERT expression in squamous cell cervical carcinoma is not associated with gene amplification. Oncol. Rep. 20, 469–474. [PubMed] [Google Scholar]
- 28. Bajpai VK, Mistriotis P, Andreadis ST (2012) Clonal multipotency and effect of long‐term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 8, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S et al (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D et al (2012) Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sussman MA, Anversa P (2004) Myocardial aging and senescence: where have the stem cells gone? Annu. Rev. Physiol. 66, 29–48. [DOI] [PubMed] [Google Scholar]
- 32. Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A et al (2005) Myocardial aging – a stem cell problem. Basic Res. Cardiol. 100, 482–493. [DOI] [PubMed] [Google Scholar]
- 33. Selvaggi TA, Walker RE, Fleisher TA (1997) Development of antibodies to fetal calf serum with arthus‐like reactions in human immunodeficiency virus‐infected patients given syngeneic lymphocyte infusions. Blood 89, 776–779. [PubMed] [Google Scholar]
- 34. Halme DG, Kessler DA (2006) FDA regulation of stem‐cell‐based therapies. N. Engl. J. Med. 355, 1730–1735. [DOI] [PubMed] [Google Scholar]
- 35. Gottipamula S, Sharma A, Krishnamurthy S, Majumdar AS, Seetharam RN (2012) Human platelet lysate is an alternative to fetal bovine serum for large‐scale expansion of bone marrow‐derived mesenchymal stromal cells. Biotechnol. Lett. 34, 1367–1374. [DOI] [PubMed] [Google Scholar]
- 36. Perez‐Ilzarbe M, Diez‐Campelo M, Aranda P, Tabera S, Lopez T, del Canizo C et al (2009) Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion 49, 1901–1910. [DOI] [PubMed] [Google Scholar]
- 37. Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J et al (2001) Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 41, 1217–1224. [DOI] [PubMed] [Google Scholar]
- 38. Mirabet V, Solves P, Minana MD, Encabo A, Carbonell‐Uberos F, Blanquer A et al (2008) Human platelet lysate enhances the proliferative activity of cultured human fibroblast‐like cells from different tissues. Cell Tissue Banking 9, 1–10. [DOI] [PubMed] [Google Scholar]


