Abstract
Plant lectins, a group of highly diverse carbohydrate‐binding proteins of non‐immune origin, are ubiquitously distributed through a variety of plant species, and have recently drawn rising attention due to their remarkable ability to kill tumour cells using mechanisms implicated in autophagy. In this review, we provide a brief outline of structures of some representative plant lectins such as concanavalin A, Polygonatum cyrtonema lectin and mistletoe lectins. These can target autophagy by modulating BNIP‐3, ROS‐p38‐p53, Ras‐Raf and PI3KCI‐Akt pathways, as well as Beclin‐1, in many types of cancer cells. In addition, we further discuss how plant lectins are able to kill cancer cells by modulating autophagic death, for therapeutic purposes. Together, these findings provide a comprehensive perspective concerning plant lectins as promising new anti‐tumour drugs, with respect to autophagic cell death in future cancer therapeutics.
Introduction
Plant lectins are a class of highly diverse non‐immune origin and carbohydrate‐binding proteins that have at least one non‐catalytic domain. This enables them to selectively recognize and reversibly bind to specific free sugars or glycans, present on glycoproteins and glycolipids, without altering structures of carbohydrates. Thus, they can specifically recognize and bind to various sugar structures, triggering several important cellular processes 1. According to their carbohydrate‐binding specificities, plant lectins can be divided into 12 different families, including (i) Agaricus bisporus agglutinin homologues; (ii) amaranthins; (iii) class V chitinase homologues with lectin activity; (iv) the cyanovirin family; (v) EEA family; (vi) GNA family; (vii) proteins with hevein domains; (viii) jacalins; (ix) proteins with legume lectin domains; (x) LysM domain; (xi) nictaba family (formerly cucurbitaceae phloem lectins); and (xii) the ricin‐B family 2. Among these families, proteins with legume lectin domains (the GNA family and the ricin‐B family) have been widely reported to exhibit a number of links to many pathological processes, such as malignancy 3.
In the last two decades, some plant lectins have been used as labelling tools to differentiate between malignant and benign tumours, and degree of glycosylation associated with cancer metastasis 4; recently they have been developed in sophisticated microarrays for better understanding of malignant tumours for diagnosis, and for prognosis by identifying different cancer developmental stages. In addition, some plant lectins such as concanavalin A (ConA) from proteins with legume lectin domains, Polygonatum cyrtonema lectin (PCL) from the GNA family and mistletoe lectins (MLs) from the ricin‐B family, exhibit their remarkable anti‐tumour potentials by targeting both apoptosis and autophagic cell death 5. Distinct from apoptosis, autophagy [a term from Greek ‘auto’ (self) and ‘phagy’ (to eat)], refers to an evolutionarily conserved, multi‐step lysosomal degradation process in which cells degrade long‐lived proteins and damaged organelles 6. Moreover, autophagy is highly regulated by a limited number of autophagy‐related (Atg) genes that may play their key roles in autophagosome formation and autophagy regulation, with numerous links to cancers 7. Recently, activity of ConA, PCL and MLs have been reported to lead to autophagic cell death by targeting some key autophagic signalling pathways in several types of cancer cells. Thus, here we summarize updated research concerning representative plant lectins that may target autophagic cell death pathways which may in turn provide more potential new anti‐tumour agents for drug discovery.
Structure of plant lectins
Concanavalin A
Concanavalin A (ConA) was the first protein with legume lectin domains to be purified and crystallized, and later, primary and three‐dimensional structures of ConA were also revealed 8. The ConA monomer (sometimes termed the ‘jelly roll’ motif), is composed of two anti‐parallel β sheets. A curved ‘front’ β sheet of seven strands aligns with a flat ‘back’ β sheet of six strands, and these two are connected by another five‐stranded ‘roof’ β sheet from the front to the back. ConA dimer, considered to be the canonical legume lectin dimer, involves alignment of two back sheets of the anti‐parallel aspect, side by side 10. Further, two ConA monomers may also lie adjacent, anti‐parallel and back‐to‐back to form a dimer, two side by side dimers of ConA being connected back to back to form the ConA tetramer. Structural organization of ConA is pH dependent; when pH is above 5.5 Con A exists as a tetramer, otherwise it is a dimer (Fig. 1a).
Figure 1.
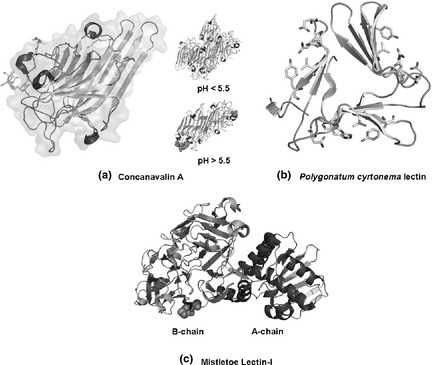
Molecular structures of three representative plant lectins. (a) Concanavalin A; (b) Polygonatum cyrtonema lectin; (c) Mistletoe lectin‐I.
Polygonatum cyrtonema lectin
Polygonatum cyrtonema lectin (PCL), a mannose/sialic acid plant lectin belonging to the GNA‐related lectin family, was firstly isolated from rhizomes of Polygonatum cyrtonema Hua, a traditional Chinese medicinal herb 11. Its nucleic acid sequence and important physiological and biochemical characteristics have been sequentially demonstrated 12. Recently, PCL has been reported to be synthesized as a 160‐residue polypeptide with 28‐residue N‐terminal signal sequence and 22‐residue C‐terminal cleavage polypeptide; the amino acid sequence of PCL is the mature 110‐residue polypeptide. Secondary structure of PCL is typically built from β‐sheets connected by turns and loops, creating tight structural scaffold; crystal three‐dimensional structures of ligand‐free PCL have also been reported 13. By using a potential bivalent mode, subunits of PCL can bind mannose, in which sugar‐binding site I (SBS I) adopts conserved mannose‐binding motif QXDXNXVXY (X being one of many amino acid residues) as observed in other structurally characterized GNA‐related lectins, while SBS II and III adopt modified motifs with some replaced residues respectively (Fig. 1b). As a result, SBS II and III are units for mannose‐binding but they may bind other types of sugar such as sialic acid 14. In addition, ligand‐free PCL is dimeric with both subunits adopting the beta‐prism II fold 15 (Fig. 1b).
Mistletoe lectins
Mistletoe lectins were first isolated from European mistletoes and so far they have been divided into three major types including ML‐I, ML‐II and ML‐III 16. They are hetero‐dimeric glycoproteins, composed of an A‐chain containing three distinct individual domains and a B‐chain of two domains of similar configuration 17. Of the three types of ML, differences in sugar‐binding specificity of their B‐chains can play an important role in determining selective cytotoxicity for tumour cells, by interacting with putative cell surface receptors, while the A‐chain inhibits protein synthesis intracellularly by interacting with the 28S ribosome. As a result, cell cytotoxicity of the lectin seems to require both A‐ and B‐chains 18. Accordingly, recognition and internalization of the B‐chain by putative receptors might be a prerequisite for the A‐chain to exert cytotoxic activity against cancer cells (Fig. 1).
Plant lectin: from recognition tool to anti‐cancer agent
Due to their binding specificities, plant lectins have been used as recognition tools to study subtle distinctions between malignant and non‐malignant cells 19 and a recent advance has been their introduction in the form of microarrays, as a unique protocol for high throughput analysis of protein glycosylation, and for profiling global changes in mammalian and bacterial cell surface glycomes 20. Now, there is extensive literature reporting activity of plant lectins in different tissues and processes, illustrating their widespread importance of potential therapeutic agents, specially with respect to cancer.
Many reports have indicated that plant lectins such as ConA, PCL and the MLs can cause cells to die by apoptosis, a complex but highly defined cell programme of cell death, useful as an anti‐tumour property. Apoptosis occurs through two major pathways, one extrinsic (triggered by Fas family death receptors), and the mitochondria‐dependent intrinsic pathway – that brings about release of cytochrome c as well as activates death signals under appropriate stimuli 21. Numerous studies have demonstrated that ConA can induce apoptotic cell death via the mitochondrial pathway in diverse types of cancer cells, including human melanoma A375 cells and human hepatocellular carcinoma HepG2 cells 22, 23, and recently, ConA has been reported to up‐regulate COX‐2 and down‐regulate Akt expression via the IKK/NF‐κB‐dependent pathway in U87 glioblastoma cells 24. ConA can also induce apoptosis by inhibiting the survival Akt pathway as well as by activating FoxO1a‐Bim signalling, in both ovarian cancer SKOV3 cells and Li‐Fraumeni syndrome MDAH041 cells 25.
Polygonatum cyrtonema lectin has drawn much interest for its anti‐tumour activities on HeLa, MCF‐7, A375 and L929 cells; PCL‐induced apoptotic death was firstly confirmed to be via the mitochondria‐mediated ROS‐p38‐p53 pathway 26. Subsequently the Ras‐Raf pathway and class I PI3K‐Akt signalling pathway have been found to be key negative regulators in apoptosis after PCL treatment 27.
Of the three types of ML, ML‐I can induce apoptosis by breakdown of mitochondrial membrane potential (MMP) and caspase‐3 activation, dependent on the apoptosis‐associated factor‐1 (Apaf‐1) pathway 28. Furthermore, ML‐I can activate JNK to promote translocation of pro‐apoptotic proteins including Bax and Bad. Also, ML‐I can induce apoptosis by down‐regulation of Bcl‐2 and activation of TNF‐α 29. Besides apoptosis‐inducing activities of ML‐I, ML‐II has also been found to possess marked apoptosis‐inducing properties, by activating MAPK signalling, SEK/JNK signalling, SAPK/JNK and p38 pathways, as well as by inhibiting the ERK1/2 pathway in human monoblastic leukaemia U937 cells and in human hepatocarcinoma cells 30.
Autophagic cell death mechanisms of plant lectins in cancer
In contrast to apoptosis, autophagy is an important physiological mechanism that may serve as a means of temporary survival of cancer cells; if cell stress results in continuous or excessively induced autophagy, cell death would ensue 31. Autophagic cell death is an evolutionarily conserved mechanism for degradation and renovation, sometimes known as type II programmed cell death. On the one hand, oncogenic pathways involved in PI3KCI, Akt, mTORC1, Ras, BCR‐ABL, Bcl‐2 and Bcl‐XL can play their important roles in deciding cancer cell survival, but on the other, tumour suppressive pathways (including various regulators, such as Beclin‐1 and p53) can exert ambiguous functions for regulation of autophagy, acting as haploid‐sufficient tumour suppressor proteins 32.
Concanavalin A‐induced autophagic pathways
ConA, a Ca2+/Mn2 + ‐dependent and mannose/glucose‐binding legume lectin, has been reported to have both apoptosis‐inducing and autophagy‐inducing properties. Accumulating evidence has revealed that ConA can induce autophagic cell death in a Bcl‐2/adenovirus E1B 19 kDa‐interacting protein 3 (BNIP‐3)‐mediated manner 33. After associating with a mannose moiety residing on the cell membrane glycoprotein, ConA can be preferentially internalized to the mitochondria via clathrin‐mediated endocytosis, initiating autophagic cell death 34. Supportive of this notion, marked morphological changes are observed after ConA intervention, and several characteristics including LC3‐II formation, BNIP‐3 induction, double layer vesicles and acidic vesicular organelles have been detected. Subsequent studies have demonstrated that this autophagic death is BNIP‐3‐mediated mitochondrial autophagy. Moreover, ConA‐treated glioblastoma U87 cells have also demonstrated up‐regulated expression of BNIP3, Atg3, Atg12 and Atg16‐like 1, where respective inductions are reversed when expression of membrane type 1 matrix metalloproteinase (product of the MT1‐MMP gene), a plasma membrane‐anchored matrix metalloproteinase, is silenced 35 (Fig. 2).
Figure 2.
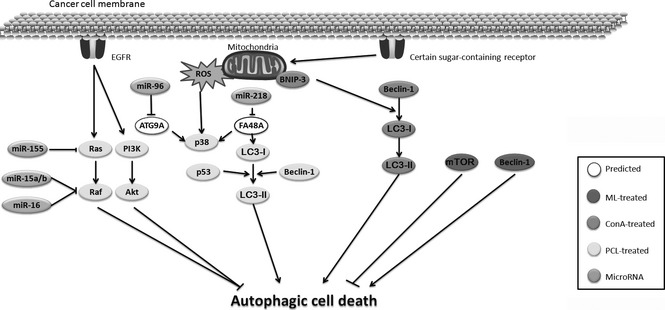
Autophagic mechanisms of plant lectin‐induced cancer cell death.
Polygonatum cyrtonema lectin‐induced autophagic pathways
PCL, a mannose/sialic acid‐binding lectin, has been reported to induce autophagic cell death in various cancer cells. It has been confirmed to be the result of a mitochondria‐mediated ROS‐p38‐p53 pathway in human melanoma A375 cells, similar to being found in PCL‐induced apoptosis 36. In murine fibrosarcoma L929 cells, PCL can induce autophagic cell death by inhibiting Ras, suggesting that the Ras‐Raf signalling pathway can negatively regulate PCL‐induced autophagic cell death. In addition, the class I phosphatidylinositol 3‐kinase (PI3KCI)‐Akt signalling pathway is also a negative regulator in autophagy after PCL treatment 27. The aforementioned evidence has thus demonstrated that PCL can induce autophagy in cancer cells by promoting the ROS‐p38‐p53 pathway, as well as by blocking Ras‐Raf and PI3K‐Akt pathways. Interestingly, PCL‐induced autophagic cell death and apoptosis can connect with each other to participate in leading to cancer cell death by promoting a mitochondria‐mediated ROS‐p38‐p53 pathway, as well as by blocking Ras‐Raf and PI3K‐Akt pathways (Fig. 2).
Mistletoe lectin‐induced autophagic pathways
Mistletoe (Viscum album) lectins, type II ribosome‐inactivating proteins (RIPsII), have received much attention due to their unique anti‐tumour cell mechanisms and therapeutic applications 37. A galactose‐ and N‐acetyl‐d‐galactosamine‐specific lectin (Viscum album L. var. coloratum agglutinin, VCA) isolated from Korean mistletoe, a natural product from the semiparasitic plants, has been used therapeutically to treat some cancers, and it has been reported that European mistletoe lectin (Viscum album L. agglutinin, VAA) also shows anti‐tumour activity through cancer cell‐specific cytotoxicity. However, to date, only one report has demonstrated that one ML alone can induce autophagic cell death in cancer cells. VCA has been known to be toxic to some cancer cells, but it is still confusingly unclear whether VCA has a cytotoxic or indeed a proliferative effect on mesenchymal stem cells (MSCs). Autophagy is regulated by several autophagy key regulators such as mTORC1, PI3KCIII, Beclin‐1, Atg5‐Atg12 and LC3, which interestingly, also act on MSC growth factors to increase cell survival. In addition, it has been found that VCA can increase expression of phosphorylated mTORC1, PI3KCIII, Beclin‐1, Atg12 and active LC3, while phosphorylated mTORC1 and Beclin‐1 in HepG2 cells have also been consistently expressed 38. Thus, VCA can induce survival factor expression thus reducing cell death by autophagic modulation, which may be a useful supplement, capable of enhancing self‐renewal of MSCs (Fig. 2).
How does plant lectin kill cancer cells by autophagic modulation?
How can plant lectins determine ultimate fate of various types of cancer cell? Herein, at least three hypotheses can answer this question: (i) depending on endocytosis they can selectively localize on some organelles; (ii) binding sugar‐containing receptors; and (iii) directly causing ribosome inactivation. ConA can be internalized to mitochondria by clathrin‐mediated endocytosis, after binding a mannose moiety that resides on cell membrane glycoprotein, resulting in reduction of MMP; thus ConA can induce autophagy through the adenovirus BNIP3‐mediated mitochondrial pathway, reduces membrane potential at the molecular level, and thus initiate autophagy after ConA has internalized to the mitochondrial surface 39. For PCL, binding sugar‐containing receptors can inhibit autophagic cell death pathways. Recently, it has been revealed that PCL can bind most (about 95%) unbound EGFR; this is a key sugar‐containing receptor on surfaces of cancer cells, suggesting that PCL may block EGFR‐mediated survival pathways involving BCR‐ABL, PI3K‐Akt‐mTORCI and Ras‐Raf signalling pathways 40. As a member of ricin‐B family, misletoe lectins consist of an A‐chain containing three conserved individual domains and a B‐chain comprising two domains with similar configuration. With sugar‐binding specificity, B‐chains combine with certain sugar chains or sugar‐containing receptors on cell surfaces, and thus transport an A‐chain into the cytoplasm. Thus, A‐chains of ML inhibit protein synthesis by inactivating the 28S ribosome on the endoplasmic reticulum, and blocks autophagic cell death by targeting several autophagic key regulators such as mTOR, PI3KCIII, Beclin‐1, Atg5‐Atg12 and LC3 41.
Accumulating data have revealed that microRNAs (miRNAs) can target several autophagic signalling pathways, and are involved in cancer initiation and progression, which may provide new perspectives for exploring more miRNAs in regulation of autophagy. Several autophagic components can be inhibited by miRNAs including miR‐155 (targeting Ras), miR‐15a/b and miR‐16 (targeting Raf), miR‐96 (targeting Atg9A), miR‐218 (targeting FA48A, which can induce LC3‐I) in treatment by PCL and ConA. Intriguingly, Chinese mistletoe lectin‐1 has been reported for the first time to bear anti‐neoplastic activity to colorectal cancer by down‐regulating miR‐135a/b expression. Accordingly, these findings may provide a new perspective of miRNA‐regulated autophagic pathways in cancer cell death induced by plant lectins, which would uncover multifaceted roles of plant lectins as potent autophagic inducers in cancer therapy 42 (Fig. 2).
From bench to bedside, we still have a long way to go……
Plant lectins have been widely used as candidate anti‐tumour drugs in human cancers in vitro; more importantly, some of them are further applied to pre‐clinical and clinical therapies in treating them 43. ConA, PCL and MLs have been well known to induce both apoptotic and autophagic cell death in vivo. Both ConA and PCL can induce apoptotic cell death in various types of cancer cells; also interestingly, besides the apoptosis‐inducing effect, ConA and PCL induce autophagic cell death in hepatocarcinoma. Accordingly, these results may provide more systematic evidence for better guiding subsequent clinical trials. Moreover, ML‐I has been widely utilized as potential anti‐neoplastic drug or adjuvant therapeutic agent, and it can reduce treatment‐associated side effects during chemotherapy and radiotherapy 44. European mistletoe lectins also have been tested for safety and efficacy, during post‐surgical aftercare of primary intermediate to high‐risk malignant tumour patients. Long‐term treatment with these lectins has been reported to be safe and without any further tumour enhancement. Accordingly, plant lectins have been gradually developed into potential anti‐tumour drugs for cancer therapeutics. However, controversy still exists over such treatment, preventing clinical use of plant lectins, and further studies need to be required before they can be used as anti‐tumour drugs.
Hitherto, plant lectins previously regarded as simple recognition tools for identifying malignant tumours, have become crucial biomarkers for cancer diagnosis and prognosis. Accumulating lines of evidence have recently revealed that targeting autophagic signalling pathways may be a promising avenue for potential therapeutic purposes. These aforementioned oncogenic/tumour suppressive signalling pathways implicate BNIP‐3, ROS‐p38‐p53, Ras‐Raf and PI3KCI‐Akt pathways as well as Beclin‐1 complex. Interestingly, some plant lectins can induce these autophagic pathways as potential therapeutic agents to deal with carcinogenesis. However, as plant lectins have natural toxicity and drug‐resistant production, they need to be modified by direct mutation and thus be designed into a further synthetic ‘ideal’ candidate anti‐tumour drug with higher efficiency and lower toxicity, for therapeutic use 45 (Table. 1).
Table 1.
Characteristics of plant lectins as potential autophagy‐inducing agents in cancer
| Name | Source | Abbreviation | Sugar‐binding type | Structure | Autophagic mechanism | Reference |
|---|---|---|---|---|---|---|
|
Concanavalin A (ConA) |
Jack bean | ConA | Alpha‐d‐mannose | Comprised of two anti‐parallel sheets, a curved ‘front’ sheet of seven strands and a flat ‘back’ sheet of six strands, and a five‐stranded ‘roof’ sheet | ConA induces autophagy through a BNIP3‐mediated mitochondrial pathway | 8, 9, 10, 33, 34, 35, 39 |
| Polygonatum cyrtonema lectin (PCL) | Polygonatum cyrtonema Hua | PCL | Sialic acid alpha‐d‐mannose | The ligand‐free PCL is dimeric, with both subunits adopting the beta‐prism II fold | PCL induces autophagy via ROS‐p38‐p53 pathway, and blocking Ras‐Raf and PI3K‐Akt pathways | 11, 12, 13, 14, 15, 27, 36, 40 |
| Mistletoe lectin (ML) | Mistletoe | ML | N‐acetyl‐d‐glucosamine alpha‐l‐fucose | Consisted of an A‐chain comprising three distinct individual domains and a B‐chain containing two domains | MLs induce autophagy by increasing expression of LC3II and decreasing phosphorylated mTOR | 16, 17, 18, 37, 38, 41 |
All the plant lectins considered here, including ConA, PCL and MLs, hold properties of binding distinct sugar‐containing receptors on surfaces of different cancer cells. Thus, due to their specific structures and sugar receptor binding abilities, we observe that plant lectins can kill many types of cancer cells by targeting autophagic cell death involving many key signalling pathways. With biochemical and molecular complexities of autophagic pathways becoming better understood, new therapeutic strategies will be developed and these in turn will initiate other therapeutic strategies. Together, these findings provide a comprehensive perspective for further elucidating roles of plant lectins that may help them target autophagic cell death pathways as potential agents in cancer pathogenesis and therapeutics.
Acknowledgements
This work was supported in part by grants from the ‘Eleventh Five‐year Plan’ military special fund (no. 08BJ01).
References
- 1. Vijayan M, Chandra N (1999) Lectins. Curr. Opin. Struct. Biol. 9, 707–714. [DOI] [PubMed] [Google Scholar]
- 2. Van Damme EJ, Lannoo N, Fouquaert E, Peumans WJ (2004) The identification of inducible cytoplasmic/nuclear carbohydrate‐binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj. J. 20, 449–460. [DOI] [PubMed] [Google Scholar]
- 3. Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 287, 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Mody R, Joshi S, Chaney W (1995) Use of lectins as diagnostic and therapeutic tools for cancer. J. Pharmacol. Toxicol. Methods 33, 1–10. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B (2012) Plant natural compounds: targeting pathways of autophagy as anti‐cancer therapeutic agents. Cell Prolif. 454, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 7. Liu B, Cheng Y, Liu Q, Bao JK, Yang JM (2010) Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 31, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edelman GM, Cunningham BA, Reeke GN Jr, Becker JW, Waxdal MJ, Wang JL (1972) The covalent and three‐dimensional structure of concanavalin A. Proc. Natl. Acad. Sci. USA 69, 2580–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha S, Gupta G, Vijayan M, Surolia A (2007) Subunit assembly of plant lectins. Curr. Opin. Struct. Biol. 17, 498–505. [DOI] [PubMed] [Google Scholar]
- 10. Li WW, Yu JY, Xu HL, Bao JK (2011) Concanavalin A: a potential anti‐neoplastic agent targeting apoptosis, autophagy and anti‐angiogenesis for cancer therapeutics. Biochem. Biophys. Res. Commun. 414, 282–286. [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Cheng Y, Bian HJ, Bao JK (2009) Molecular mechanisms of Polygonatum cyrtonema lectin‐induced apoptosis and autophagy in cancer cells. Autophagy 5, 253–255. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Peng H, Yao Q, Li J, Van Damme E, Balzarini J et al (2009) Bioinformatics analyses of the mannose‐binding lectins from Polygonatum cyrtonema, Ophiopogon japonicus and Liparis noversa with anti‐proliferative and apoptosis‐inducing activities. Phytomedicine 16, 601–608. [DOI] [PubMed] [Google Scholar]
- 13. Sharon N (2007) Lectins: carbohydrate‐specific reagents and biological recognition molecules. J. Biol. Chem. 282, 2753–2764. [DOI] [PubMed] [Google Scholar]
- 14. Wang SY, Yu QJ, Bao JK, Liu B (2011) Polygonatum cyrtonema lectin: a potential antineoplastic drug targeting programmed cell death pathways. Biochem. Biophys. Res. Commun. 406, 497–500. [DOI] [PubMed] [Google Scholar]
- 15. Ding J, Bao J, Zhu D, Zhang Y, Wang DC (2010) Crystal structures of a novel anti‐HIV mannose‐binding lectin from Polygonatum cyrtonema Hua with unique ligand‐binding property and super‐structure. J. Struct. Biol. 171, 309–317. [DOI] [PubMed] [Google Scholar]
- 16. Strüh CM, Jäger S, Kersten A, Schempp CM, Scheffler A, Martin SF (2013) Triterpenoids amplify anti‐tumoral effects of mistletoe extracts on murine b16. f10 melanoma in vivo. PLoS One 8, e62168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seifert G, Jesse P, Laengler A, Reindl T, Lüth M, Lobitz S et al (2008) Molecular mechanisms of mistletoe plant extract‐induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 264, 218–228. [DOI] [PubMed] [Google Scholar]
- 18. Hoessli DC, Ahmad I (2008) Mistletoe lectins: carbohydrate‐specific apoptosis inducers and immunomodulators. Curr. Org. Chem. 12, 918–925. [Google Scholar]
- 19. Siegle I, Fritz P, McClellan M, Gutzeit S, Mürdter TE (2001) Combined cytotoxic action of Viscum album agglutinin‐1 and anticancer agents against human A549 lung cancer cells. Anticancer Res. 21, 2687–2691. [PubMed] [Google Scholar]
- 20. Pusztai A, Bardocz S, Ewen SW (2008) Uses of plant lectins in bioscience and biomedicine. Front. Biosci. 13, 1130–1140. [DOI] [PubMed] [Google Scholar]
- 21. Liu JJ, Lin M, Yu JY, Liu B, Bao JK (2011) Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 300, 105–114. [DOI] [PubMed] [Google Scholar]
- 22. Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK (2009) Antiproliferative activity and apoptosis‐inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 482, 1–6. [DOI] [PubMed] [Google Scholar]
- 23. Liu ZY, Li XF, Ding XP, Yang Y (2010) In silico and experimental studies of Concanavalin A: insights into its antiproliferative activity and apoptotic mechanism. Appl. Biochem. Biotechnol. 162, 134–145. [DOI] [PubMed] [Google Scholar]
- 24. Sina A, Proulx‐Bonneau S, Roy A, Poliquin L, Cao J, Annabi B (2010) The lectin Concanavalin‐A signals MT1‐MMP catalytic independent induction of COX‐2 through an IKKγ/NF‐κB‐dependent pathway. J. Cell Commun. Signal. 4, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruhul Amin AR, Paul RK, Thakur VS, Agarwal ML (2007) A novel role for p73 in the regulation of Akt‐Foxo1a‐Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 67, 5617–5621. [DOI] [PubMed] [Google Scholar]
- 26. Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria‐mediated ROS‐p38‐p53 pathway. Cancer Lett. 275, 54–60. [DOI] [PubMed] [Google Scholar]
- 27. Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY et al (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis and autophagy via blocking Ras‐Raf and PI3K‐Akt signaling pathways. Biochimie 92, 1934–1938. [DOI] [PubMed] [Google Scholar]
- 28. Lyu SY, Choi SH, Park WB (2002) Korean mistletoe lectin‐induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharm. Res. 25, 93–101. [DOI] [PubMed] [Google Scholar]
- 29. Pae HO, Oh GS, Kim NY, Shin MK, Lee HS, Yun YG et al (2001) Roles of extracellular signal‐regulated kinase and p38 mitogen‐activated protein kinase in apoptosis of human monoblastic leukemia U937 cells by lectin‐II isolated from Korean mistletoe. In Vitr. Mol. Toxicol. 14, 99–106. [DOI] [PubMed] [Google Scholar]
- 30. De Mejia EG, Dia VP (2010) The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev. 29, 511–528. [DOI] [PubMed] [Google Scholar]
- 31. Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 45, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu LL, Cheng Y, Liu B (2013) Beclin‐1: autophagic regulator and therapeutic target in cancer. Int. J. Biochem. Cell Biol. 45, 921–924. [DOI] [PubMed] [Google Scholar]
- 33. Chang CP, Yang MC, Liu HS, Lin YS, Lei HY (2007) Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma mode. Hepatology 45, 286–296. [DOI] [PubMed] [Google Scholar]
- 34. Lei HY, Chang CP (2009) Lectin of Concanavalin A as an anti‐hepatoma therapeutic agent. J. Biomed. Sci. 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pratt J, Roy R, Annabi B (2012) Concanavalin‐A‐induced autophagy biomarkers requires membrane type‐1 matrix metalloproteinase intracellular signaling in glioblastoma cells. Glycobiology 22, 1245–1255. [DOI] [PubMed] [Google Scholar]
- 36. Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK (2011) Plant lectins: targeting programmed cell death pathways as anti‐tumor agents. Int. J. Biochem. Cell Biol. 43, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 37. Schaffrath B, Mengs U, Schwarz T, Hilgers RD, Beuth J, Möckel B et al (2001) Anticancer activity of rViscumin (recombinant mistletoe lectin) in tumor colonization models with immunocompetent mice. Anticancer Res. 21, 3981–3987. [PubMed] [Google Scholar]
- 38. Choi JH, Lyu SY, Lee HJ, Jung J, Park WB, Kim GJ (2012) Korean mistletoe lectin regulates self‐renewal of placenta‐derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif. 45, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu B, Min MW, Bao JK (2009) Induction of apoptosis by concanavalin A and its molecular mechanisms in cancer cells. Autophagy 5, 432–433. [DOI] [PubMed] [Google Scholar]
- 40. Kienle GS, Berrino F, Büssing A, Portalupi E, Rosenzweig S, Kiene H (2003) Mistletoe in cancer – a systematic review on controlled clinical trials. Eur. J. Med. Res. 8, 109–119. [PubMed] [Google Scholar]
- 41. Fu LL, Zhao X, Xu HL, Wen X, Wang SY, Liu B et al (2012) Identification of microRNA‐regulated autophagic pathways in plant lectin‐induced cancer cell death. Cell Prolif. 45, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu QJ, Li ZY, Yao S, Ming M, Wang SY, Liu B et al (2011) In silico analysis of molecular mechanisms of GNA‐related lectin‐induced cancer cell death from carbohydrate‐binding motif evolution hypothesis. Appl. Biochem. Biotechnol. 165, 1037–1046. [DOI] [PubMed] [Google Scholar]
- 43. Gupta G, Surolia A, Sampathkumar SG (2010) Lectin microarrays for glycomic analysis. OMICS 14, 419–436. [DOI] [PubMed] [Google Scholar]
- 44. De Mejía EG, Prisecaru VI (2005) Lectins as bioactive plant proteins: a potential in cancer treatment. Crit. Rev. Food Sci. Nutr. 45, 425–445. [DOI] [PubMed] [Google Scholar]
- 45. Maiese K, Chong ZZ, Shang YC, Wang S (2012) Targeting disease through novel pathways of apoptosis and autophagy. Expert. Opin. Ther. Targets. 16, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]


