Abstract
Objectives
Physiochemical properties of biomaterials play critical roles in dictating types of cell behaviour. In this study, a series of poly(ε‐caprolactone) (PCL)‐derived polymers bearing different small chemical groups was employed as a platform to evaluate chondrogenesis of different cell types.
Materials and methods
Thin films were prepared by spin‐coating PCL derivatives. Rabbit articular chondrocytes (rACs) and rabbit bone marrow–derived mesenchymal stem cells (rMSCs) were seeded on to the films, and cell adhesion, proliferation, extracellular matrix production and gene expression were evaluated.
Results
The presence of hydrophilic groups (‐NH 2, ‐COOH, ‐OH and ‐C=O) promoted adhesion and proliferation of primary rACs and rMSCs. On these polymeric films, chondrogenesis of primary rACs depended on culture time. For passaged cells, re‐differentiation was induced on these films by chondrogenic induction, but less for cells of passage 5 compared to passage 3. While films with hydrophilic groups favoured chondrocytic gene expression of both types of passaged cells, production of glycosaminoglycans (GAG) was similar for those of passage 3 on all films, and PCL‐CH 3 film better supported GAG production for cells of passage 5. Under chondrogenic conditions, rMSCs were more efficient at GAG production on PCL and PCL‐NH 2 films.
Conclusions
This study demonstrates that different cells displayed distinct responses to substrate surface chemistry, implying that cell–biomaterial interactions can be developmental stage dependent. This provides a novel perspective for developing biomaterials for cartilage regeneration.
1. Introduction
Traumatic damage and osteoarthritic degeneration of articular cartilage are severe clinical issues due to its limited self‐repair capacity. Current surgical practices are unable to achieve a long‐term functional repair.1 Emerging technologies including cell therapy (i.e., autologous chondrocyte implantation, ACI) and tissue engineering hold great promise for cartilage regeneration.2 On one hand, ACI involves implantation of isolated chondrocytes to defects and necessitates ex vivo monolayer expansion of primary cells to obtain a sufficient quantity, which usually leads to dedifferentiation, namely downregulation in typical cartilage gene expression and extracellular matrix (ECM) production.3 On the other hand, tissue engineering aims at developing cartilage replacements in combination of biomaterials and cells such as chondrocytes and mesenchymal stem cells (MSCs).4 However, differentiation of MSCs towards articular chondrocytes remains challenging and current technologies often generate a hypertrophic, unstable cartilage phenotype.5, 6 It is of note that engineered cartilage with MSCs is still mechanically inferior to that with chondrocytes as well as native cartilage.7, 8
It has been recognized that in both ACI and tissue engineering, biomaterials play very important roles. For instance, the second generation of ACI (so‐called matrix‐induced ACI, MACI) exploits collagen as a cell delivery carrier to achieve better cell retention and function.1 As a matter of fact, cells in vivo reside in a microenvironment embodied with ECM, which plays important roles in regulating cellular fates. Hence, the design of biomaterial‐based tissue engineering scaffolds has been largely following the principle of mimicking native ECM, which are intended to provide a physicochemical microenvironment for cell adhesion, growth, migration and differentiation.9 It becomes very critical to gain understanding of cell–biomaterial interactions in developing optimal biomaterials to drive cells towards specific tissue formation, and recent studies have, in fact, documented that topography, mechanics and chemistry of biomaterials scaffolds are all significant in modulating cellular behaviours.10
In particular, intensive efforts have been devoted to obtaining biomaterials with appropriate chemistries that being both conductive and inductive to chondrogenesis. Incorporation of naturally derived molecules (e.g., collagen and chondroitin sulphate) either by chemical attachment or by physical entrapment in synthetic polymer substrates (e.g., poly[l‐lactic acid], PLLA) has been extensively studied to improve the chondrogenesis of both chondrocytes and MSCs.11, 12, 13, 14 Essentially, controlling surface chemical properties, such as electrostatic charge, wettability, and hydrophobicity of a substrate that recapitulates the basic characteristics of native ECM, represents another efficient route to biomaterials development. For example, chargeable molecules such as polydopamine and poly(acrylic acid) have been utilized to confer charges on a substrate to solicit cell adhesion and favourable chondrogenesis.15, 16 In a much simpler fashion, Ma et al. evaluated the effects of hydroxyl, carboxyl and amide groups on PLLA films on chondrocyte growth.17 In a three‐dimensional poly(ethylene glycol) (PEG) hydrogel, the carboxylic group chemically tethered on PEG network was found to support the best chondrogenic differentiation of human MSCs among a series of small chemical groups.18 Most recently, the design of surface chemistry to prevent cell adhesion and induce cell aggregation has been reported to promote chondrogenesis of MSCs.19, 20 For instance, by employing two polyelectrolytes with opposite charges, poly(l‐glutamic acid) and chitosan, a hydrophilic surface in three‐dimensional scaffolds was developed and aggregation of rabbit adipose‐derived MSCs was encouraged, showing improved chondrogenic differentiation.20 All these studies demonstrate that by designing surface chemistry, cellular behaviours can be modulated to obtain improved chondrogenesis. However, so far, a comparable evaluation of chondrogenesis of different cell types in a same biomaterials platform has not been performed yet.21
Previously, we had developed a novel series of poly(ε‐caprolactone) polymers bearing different pendent chemical groups (i.e. hydroxyl, methyl, carboxyl, amino and carbonyl) and successfully applied in studying the behaviours of MSCs.22 The objective of this study was to perform a comparative investigation on different chondrogenic cells by employing such a biomaterials platform. As illustrated in Fig. 1, MSCs and chondrocytes (primary and passaged cells) were seeded onto this series of polymeric films and chondrogenesis was evaluated. This study would deliver a comprehensive understanding of how biomaterials chemistry can influence chondrogenesis and offer a novel perspective to design of biomaterials scaffolds for cartilage regeneration.
Figure 1.
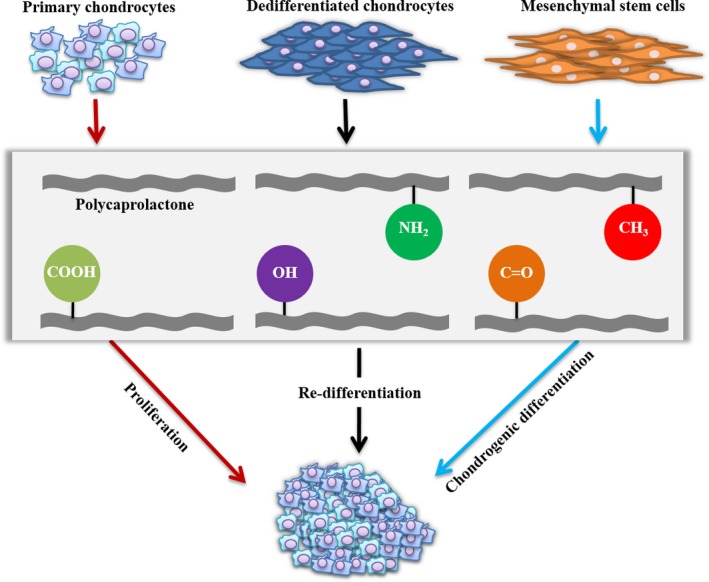
Experimental design
2. Materials and methods
2.1. PCL polymers and fabrication of thin films
The series of PCL polymers bearing different pendant functional groups were prepared through the ring‐opening co‐polymerization between ε‐caprolactone and distinct co‐monomers (feeding molar ratio: 9/1) using benzyl alcohol as initiator and Sn(Oct)2 as catalyst and characterized as described in our previous work.22 PCL polymers bearing ‐NH2, ‐CH3, ‐COOH, –OH and ‐C=O were designated as PCL‐NH2, PCL‐CH3, PCL‐COOH, PCL‐OH and PCL‐C=O in the following context, respectively. To prepare polymeric thin films, these polymers were dissolved in methylene chloride (10 mg/mL) and spin coated onto round glass coverslips (15 or 35 mm in diameter) for 15 seconds at a speed of 4000 rpm on a benchtop spin coater (Beijing JinSheng WeiNa, China). Residual solvent was removed in a vacuum oven at room temperature for 24 hours and stored in a desiccator until use.
2.2. Cell isolation
Rabbit ACs (rACs) and rabbit MSCs (rMSCs) were isolated as described in our previous work.23, 24, 25 All animal experiments were performed at Shanghai Laboratory Animal Center (Shanghai) in accordance with the institutional guidelines of animal care and use committee. New Zealand white rabbits aged 2–3 months were killed, and cartilage tissue samples were harvested from knee joints cartilage tissue and bone marrow from femoral and tibial bones. To isolate chondrocytes, cartilage slices were digested with 0.1% collagenase II (200 U/mg; Invitrogen Carlsbad, California, USA). Harvested cells were then either cultured in chondrocyte growth medium (GM1) consisting of Dulbecco's modified Eagle's medium (Gibco Invitrogen, Carlsbad, California, USA) supplemented with 10% foetal bovine serum (FBS; Hyclone Logan, Utah, USA), 0.1 mmol L−1 non‐essential amino acids, 0.4 mmol L−1 proline, 0.05 mg/mL vitamin C, 100 U/mL penicillin and 100 U/mL streptomycin at a cell density of 1.3 × 104 cells/cm2 for passaging or frozen down for future use. rMSCs were isolated from bone marrow using a density gradient method with Ficoll‐Paque Plus solution (density: 1.077 g/mL; GE Healthcare Pittsburgh, Pennsylvania, USA). Mononuclear cells collected were initially plated at 1×105 cells/cm2 in growth medium (GM2), consisting of α‐Minimum Essential Medium (α‐MEM; Gibco) supplemented with 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C and then subcultured at 5 × 103 cells/cm2. rMSCs were validated to be CD29+, CD44+, CD14− and CD45− and demonstrated the capability of differentiation into chondrocytes, osteoblasts and adipocytes (data not shown).
2.3. Cell culture on polymeric films
Prior to cell seeding, polymeric films fixed on coverslips were exposed to UV light for 30 minutes for sterilization and then incubated in sterile distilled water for 3 days. Then, after incubation in serum‐free growth medium for 10 hours, polymeric films were seeded with cells in either 24‐well plates (for films of 15 mm in diameter) or 6‐well plates (for films of 35 mm in diameter). For cell adhesion, rACs (passage 1, P1) and rMSCs (P5) were seeded at 1 × 104 cells/cm2 onto films in 24‐well plates in 1 mL of GM1 and GM2, respectively, and cultured for 24 hours. For cell growth, rACs (P1) and rMSCs (P5) were plated at 2.5 × 103 cells/cm2 onto films in 24‐well plates in 1 mL of GM1 and GM2 and maintained for 21 days and 16 days, respectively. Medium was refreshed twice a week. To induce chondrogenesis, passaged rACs (P3 and P5) and rMSCs (P5) were initially plated at 5×104 cells/cm2 onto films in 24‐well plates in GM1 and GM2 for 24 hours, respectively. Then, growth media were replaced with chondrogenic induction medium (CIM) consisting DMEM supplemented with 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenious acid, 1.25 mg/mL bovine serum albumin, 5.35 ng/mL linoleic acid, 100 μg/mL sodium pyruvate, 50 μg/mL l‐ascorbic acid‐2‐phosphate, 0.35 mmol L−1 l‐proline, 0.1 μmol L−1 dexamethasone and 10 ng/mL TGF‐β1. Culture was maintained for 14 days and medium was changed twice a week. As control, P3 and P5 rACs and P5 rMSCs were also cultured for a total of 15 days in GM1 and GM2, respectively. To ensure a sufficient cell number for gene analysis, films of 35 mm in diameter in six‐well plates were for cell culture.
2.4. F‐actin staining
To observe cell morphology on polymeric films, samples were rinsed with phosphate buffered saline (PBS), fixed with 4% paraformaldehyde and permeated with 0.1% Triton‐X 100 in PBS. After washing with PBS, samples were treated with Rhodamine‐phalloidin (70 nmol L−1; Invitrogen) in PBS containing 1% of BSA in dark for 20 minutes. Cell nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Fluorescence images were acquired on a fluorescence microscope (Eclipse Ti‐S; Nikon Chiyoda‐Ku, Tokyo, Japan). Cell spreading area per cell was determined using Image J software, and 4–5 images (200× magnification; 30–60 cells in total) were analysed for each group.
2.5. Biochemical assays
Cell number on polymeric films was assessed using cell counting kit‐8 (CCK‐8, Dojindo, Japan). Cell‐seeded polymeric films in 24‐well plates were rinsed with PBS; 0.5 mL of growth medium supplemented with 50 μL of CCK‐8 stock solution was added to each well and incubated for 1 hour at 37°C. Culture wells without cells were added with CCK‐8 solution and set as blank. The absorbance at 450 nm (optical density, OD450) was measured with a reference wavelength of 620 nm on a microplate reader (Bio‐Tek Instrument Winooski, Vermont, USA).
To quantify glycosaminoglycans (GAG), samples were treated with papain solution (125 μg/mL papain, 16–40 U/mg; Sigma St. Louis, Missouri, USA), 5 mmol L−1 l‐cysteine, 100 mmol L−1 Na2HPO4, 5 mmol L−1 EDTA and pH 6.2) at 60°C overnight. Contents of DNA and GAG were measured by using Hoechst 33258 dye and dimethyl methylene blue (DMMB) method as described,23 respectively, and GAG content was normalized to DNA content as GAG/DNA (μg/μg). All biochemical assays were performed in triplicates.
2.6. Histology
Deposition of GAG by cells on polymeric films was detected with both alcian blue and safranin O staining. Samples were rinsed with PBS, fixed with 4% paraformaldehyde in PBS, stained with 1% alcian blue (Sigma) in 3% (v/v) acetic acid for 30 minutes or 0.5% safranin O in distilled water for 5 minutes at room temperature, and finally rinsed with distilled water. Images were acquired under a phase contrast microscope.
2.7. Reverse transcription‐polymerase chain reaction (RT‐PCR)
Cells on polymeric films (35 mm in diameter) were lysed with Trizol reagent (Invitrogen), and total RNA was extracted following the manufacturer's protocol. cDNA was prepared with Superscript II Reverse Transcriptase (Invitrogen) using oligo(dT) and then amplified using PCR Master Mix (TaKaRa Kusatsu, Shiga, Japan) on a thermal cycler (TC‐XP‐A; Bioer, China Hangzhou). PCR reactions were performed as follows: 94°C for 10 minutes, 35 cycles of PCR (95°C for 15 seconds, 57°C for 45 seconds and 72°C for 45 seconds). PCR products were analysed by using 2% agarose gel electrophoresis and visualized under UV after ethidium bromide staining. Gapdh was used as a housekeeping gene and primer sequences were listed in Table S1.
2.8. Statistical analysis
Values were expressed as mean ± standard deviation. Statistical analysis was performed with the SPSS 19.0 software. Comparisons among groups were performed using one‐factor analysis of variance (ANOVA) and Tukey post hoc test. Statistical significance was set at P<.05.
3. Results
3.1. Polymeric films
Surface properties of the polymeric films were reported in our previous study.22 All films displayed flat, smooth surface under scanning electron microscope. However, the order of water contact angle of PCL (87.6°) > PCL‐CH3 (85.8°) > PCL‐C=O (80.5°) > PCL‐COOH (73.9°) ≈ PCL‐OH (73.9°) > PCL‐NH2 (72.8°), indicating different hydrophilicity. The total protein amount adsorbed on these films varied, with PCL, PCL‐CH3 and PCL‐C=O films having the highest protein contents and PCL‐OH film the lowest. These together implied the distinct surface chemistries of these polymeric films.
3.2. Cell adhesion on polymeric films
Adhesion of both P1 rACs and P5 rMSCs on this series of polymeric films within 24 hours was assessed with F‐actin staining and CCK‐8 assay. According to F‐actin staining, P1 rACs displayed a small rounded or polygonal shape on all films (Fig. 2a) and cell spreading area showed no significant difference among different films (Fig. 2b). Based on CCK‐8 assay, a significantly higher absorbance was on PCL tethered with small chemical groups than PCL film, indicating a higher cell number, and PCL‐C=O film further showed slightly higher absorbance than PCL‐NH2, PCL‐CH3 and PCL‐OH films (Fig. 2c).
Figure 2.
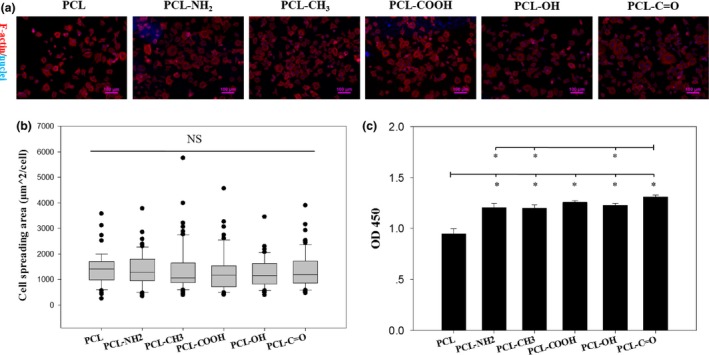
Adhesion of P1 rACs on polymeric films. P1 rACs were seeded at 1 × 104 cells/cm2 onto the films in GM1 and cultured for 24 h. (a) F‐actin staining, (b) cell‐spreading area (NS: not significant; n>30) and (c) CCK‐8 assay (*: P<.05; n=4)
For P5 rMSCs, according to F‐actin staining images after different time intervals, cells gradually adhered onto all the films within 24 hours, developed mature stress fibres and transformed from a small rounded shape into a large elongated spindle‐like one (Fig. 3a). Notably, rMSCs tended to be smaller on PCL and PCL‐CH3 films than others, which was confirmed by quantifying cell spreading area (Fig. 3b). The adhesion kinetics of rMSCs was determined based on CCK‐8 assay, displayed a gradually increasing trend and reached a plateau within 24 hours on all the films (Fig. 3c). It was also found that cell adhesion on PCL and PCL‐CH3 films was consistently lower than that on other films at all tested time points.
Figure 3.
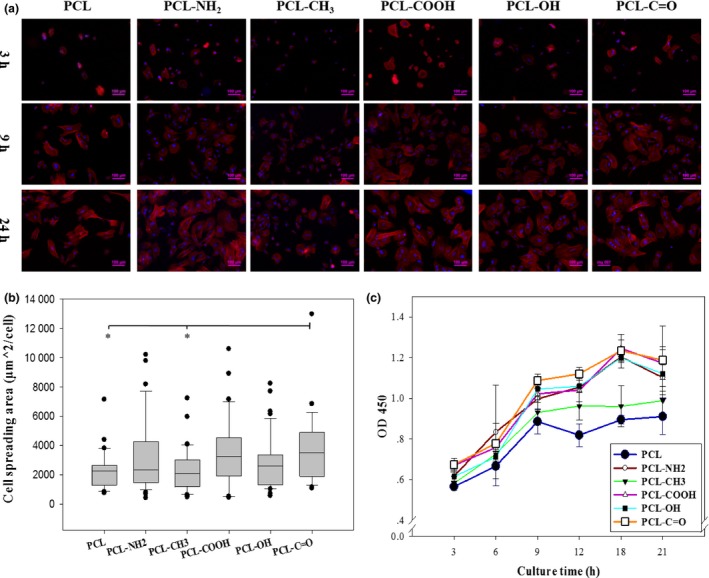
Adhesion of rMSCs on polymeric films. P5 rMSCs were seeded at 1 × 104 cells/cm2 onto the films and cultured for 24 h. (a) F‐actin staining at 3 h, 9 h and 24 h, (b) cell spread area at 24 h (*: P<.05; n>30) and (c) adhesion kinetics within 24 h
3.3. Cell growth on polymeric films
Growth of both P1 rACs and P5 rMSCs on PCL films was plotted against culture time as shown in Fig. 4. For these two types of cells, slight different curves were noticed. Although both cells gradually proliferated over time on all the films following a sigmoid curve, rACs displayed varied growth rates before reaching the plateau on day 18 and rMSCs grew all the way towards the maximum on day 12. Since P1 rACs and P5 rMSCs were seeded at a same cell density, considering a higher cell amount for rACs on day 12, P1 rACs grew much fast than P5 rMSCs on all polymeric films. Additionally, cell growth on PCL film was lower than that on the films of PCL derivatives bearing small chemical groups, especially for rMSCs.
Figure 4.
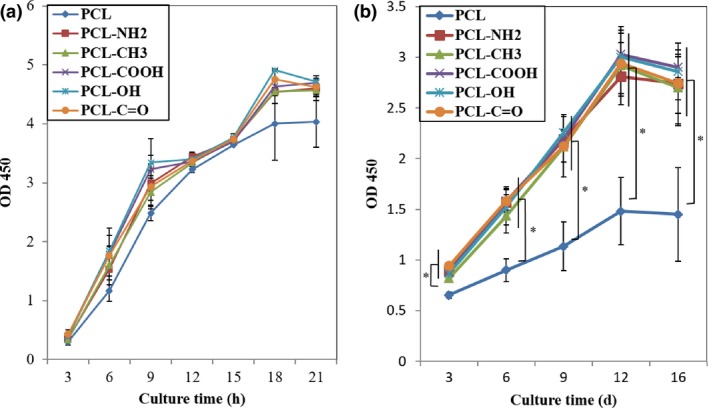
Cell growth kinetics on polymeric films. P1 rACs (a) and P5 rMSCs (b) were seeded at 2.5 × 103 cells/cm2 onto the films and cultured for 21 and 16 days in GM1 and GM2, respectively (*: P<.05; n=4).
3.4. Long‐term maintenance of P1 rACs on polymeric films
P1 rACs were cultured on these polymeric films for 3 weeks in GM1. Cell morphology was evaluated by F‐actin staining. While cells remained rounded on all the films on day 7, the spindle‐like cells could be apparently discerned on day 21 only on PCL and PCL‐CH3 films, based on the aligned organization of F‐actin (Fig. 5a). Safranin O and alcian blue staining were applied to detect the production of cartilaginous ECM component, GAG. As shown in Fig. 5a, the positive staining of safranin O was observed on all the films, but with different intensities, and the intensity on each film tended to vary over time. On day 21, less staining intensity was found on PCL and PCL‐CH3 films than that on others. Additionally, alcian blue staining followed the same trend as the safranin O staining (Fig. 5a).
Figure 5.
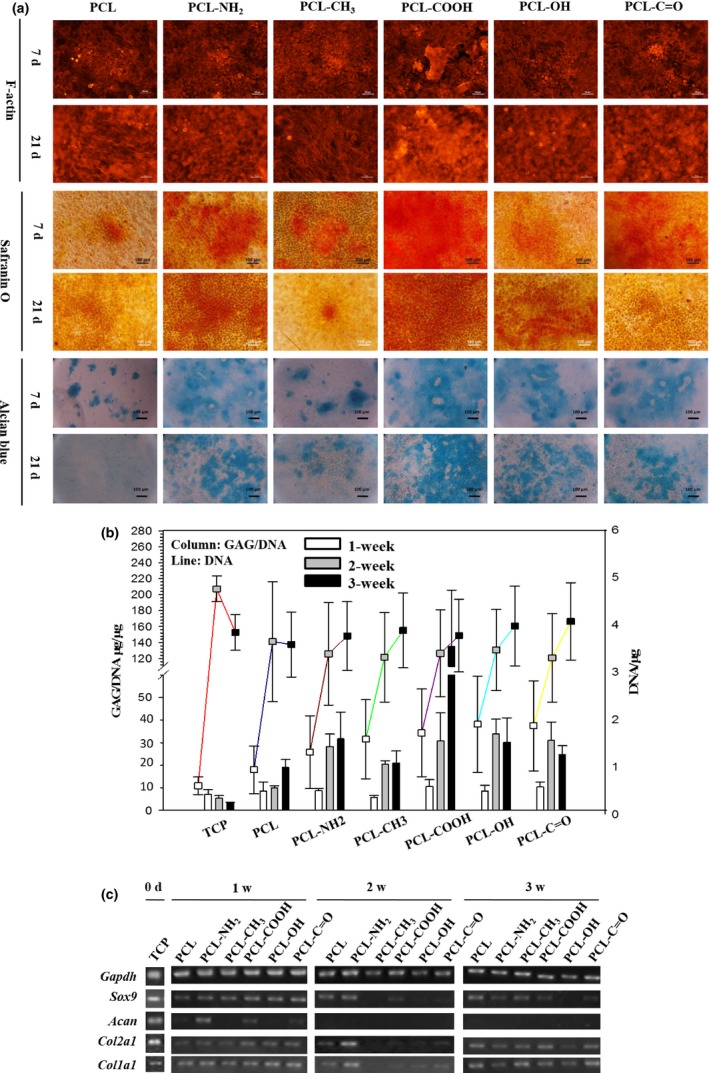
Phenotypic characterization and gene analysis of P1 rACs after a long‐term culture on polymeric films. P1 rACs were plated at 2.5×103 cells/cm2 onto the films and cultured in GM1 for 21 days. (a) F‐actin, safranin O and alcian blue staining, (b) GAG/DNA and DNA content (n=4) and (c) gene expression
Quantification of GAG/DNA was shown in Fig. 5b, and P1 rACs cultured on 24‐well tissue culture plates (TCP) for 3 weeks were included as a control. DNA contents on all the substrates were not significantly different on day 21, and slight reduction was noted after 2 weeks on both TCP and PCL film, which was in contrast to the gradual increasing trend on other films. GAG/DNA increased within 2 weeks on polymeric films and rose afterwards only on PCL and PCL‐COOH films, while remaining steady on others. However, GAG/DNA on PCL and PCL‐CH3 films was relatively lower than on other films with the highest value achieved on PCL‐COOH film. In contrast, GAG/DNA on TCP decreased over time and remained much lower than that on polymeric films.
Gene expression was evaluated and shown in Fig. 5c. On day 0, P1 rACs on TCP expressed hyaline cartilage‐specific genes, including Sox9, Acan and Col2a1, and fibrocartilage‐specific genes Col1a1. After 1 week on polymeric films, cells expressed Sox9, Col2a1 and Col1a1 on all the films and aggrecan was only detected on PCL‐NH2 and PCL‐COOH films. After 2 weeks, only on PCL and PCL‐NH2 films, Sox9, Col2a1 and Col1a1 were expressed and Acan became disappeared on all the films. However, after 3 weeks, expression of Sox9, Col2a1 and Col1a1 was upregulated on the films, except PCL‐NH2 and PCL‐C=O films, and expression of Acan still remained undetectable on all the films.
3.5. Re‐differentiation of passaged rACs on polymeric films
Both P3 and P5 rACs were cultured on polymeric films in CIM for 2 weeks, and these cells cultured in GM1 were included as controls. Safranin O and alcian blue staining were shown in Fig. 6a. In GM1, both P3 and P5 rACs displayed a fibroblast‐like shape on all the films and were barely stained by the two dyes. Conversely, in CIM, P3 rACs became rounded and were positively stained with both safranin O and alcian blue, and cellular aggregates could be noticed. For P5 rACs in CIM, some cells were induced to be round, and both dyes were noted on all the films, however, with less intensities compared with those for P3 rACs.
Figure 6.
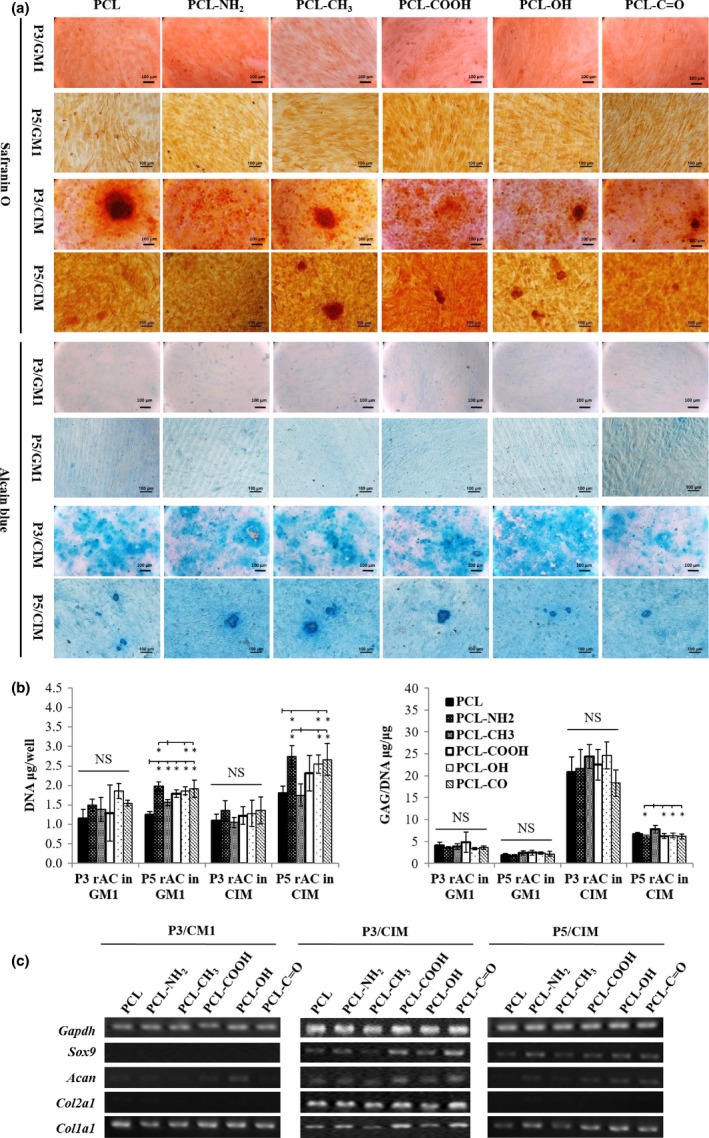
Re‐differentiation of passaged rACs on polymeric films. P3 and P5 rACs were seeded at 5 × 104 cells/cm2 onto the films in GM1 for 24 h and then cultured in CIM for 14 days. P3 and P5 rACs were also cultured in GM1 for 15 days as control. (a) Safranin O and alcian blue staining, (b) GAG, DNA and GAG/DNA (*: P<.05; n=4) and (C) gene expression
DNA content and GAG/DNA were quantified as shown in Fig. 6b. For P3 rACs, no significant difference in DNA content was found on all the films in both GM1 and CIM. For P5 rACs, compared with PCL and PCL‐CH3 films, slightly higher DNA content was obtained on other films in both media. In addition, P5 rACs in CIM had higher DNA contents than P5 rACs in GM1 and P3 rACs in both GM1 and CIM on all the films. For both P3 and P5 rACs, GAG/DNA was trivial in GM1 and significantly increased in CIM. In addition, GAG/DNA was much higher for P3 rACs in CIM than P5 rACs in CIM on all respective films. While GAG/DNA was found similar on all the films for P3 rACs in both GM1 and CIM, P5 rACs on PCL‐CH3 film had a significantly higher GAG/DNA value than those on PCL‐NH2, PCL‐COOH, PCL‐OH and PCL‐C=O films.
Gene expression was shown in Fig. 6c. While P3 rACs cultured in GM1 only expressed a high level of collagen I on all the films, P3 rACs were induced to express Sox9, Col2a1, Acan and Col1a1 in CIM, with slightly lower levels on PCL and PCL‐CH3 films. Expression of Sox9 and Acan could be detected for P5 rACs in CIM, also with slightly lower levels on PCL and PCL‐CH3 films.
3.6. Chondrogenic differentiation of rMSCs on polymeric films
P5 rMSCs were cultured in CIM for 2 weeks and were also cultured in GM2 for 15 days as a negative control. In Fig. 7a, safranin O and alcian blue staining demonstrated that cells formed aggregates in CIM, with GAG deposited on all the substrates including TCP, while cells in GM2 remained in monolayer with only trace staining. As shown in Fig. 7b, DNA contents were found much lower in CIM than those in GM2 on all substrates including TCP and followed a similar order among different substrates with the highest obtained on PCL‐COOH and PCL‐C=O films. In GM2, only trivial GAG/DNA was detected on all substrates. However, upon chondrogenic induction, significantly higher GAG/DNA values (15–30 μg/μg) were achieved on all the polymeric films, with the highest ones on PCL and PCL‐NH2 films, which were also comparable to those produced by P3 rACs in CIM (Fig. 6b), suggesting an efficient chondrogenic differentiation.
Figure 7.
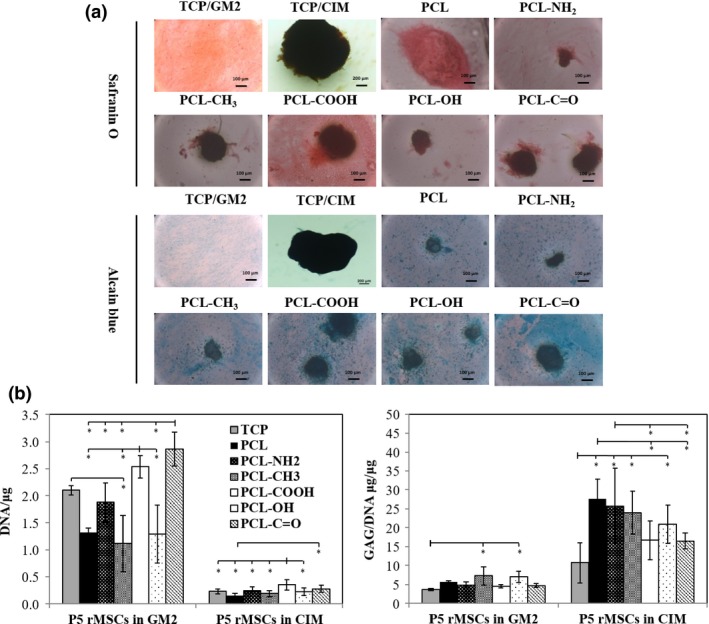
Chondrogenic differentiation of rMSCs on polymeric films. P5 rMSCs were seeded at 5 × 104 cells/cm2 onto the films and TCP in GM2 for 24 h and then cultured in CIM for 14 days. P5 rMSCs were also cultured in GM2 for 15 days as a control. (a) Safranin O and alcian blue staining and (b) GAG, DNA and GAG/DNA (*: P<.05; n=8)
4. Discussion
Comparison among different stem cells has been explored concerning chondrogenic potential previously.21 However, a systemic evaluation of different cells on a biomaterials platform would confer an insight perspective to biomaterials design for cartilage tissue engineering. In our previous study, a series of PCL polymer derivatives bearing different pendant chemical groups (i.e. ‐NH2, ‐CH3, ‐COOH, ‐OH and ‐C=O) were successfully synthesized and could be conveniently processed into thin, flat films for cell culture.22 Such a polymer series can serve as a potential platform for studying cell–biomaterial interactions.
In this study, an investigation was performed to uncover how substrate surface chemistry can influence chondrogenesis of different cell types by exploiting such a series of polymeric films. For P1 rACs, while all the films supported both adhesion and proliferation, PCL films bearing hydrophilic groups (‐NH2, ‐COOH, ‐OH and ‐C=O), especially those with charges (‐NH2 and ‐COOH), favoured cartilaginous ECM production (i.e. GAG). However, in general, gene expression displayed a dynamic variation on polymeric films within 3 weeks. Within 1 week, all the films supported expression of Sox9 and Col2a1, and Acan only expressed on charged films (PCL‐NH2 and PCL‐COOH). Significant downregulation of chondrocytic genes was observed after 2 weeks, with only Sox9 and Col2a1 detected on PCL and PCL‐NH2 films. However, a slight upregulation of Sox9 and Col2a1 was present after 3 weeks, especially on PCL, PCL‐CH3 and PCL‐COOH films. For passaged rACs, re‐differentiation could be induced on these films upon chondrogenic induction for both P3 and P5 cells, but to different extents, with P3 cells having much higher GAG production and chondrocytic gene expression. P3 rACs had similar GAG/DNA on all the films and also slightly higher gene expression on hydrophilic films, especially PCL‐COOH and PCL‐C=O films. For P5 rACs, polymeric films bearing hydrophilic groups, especially PCL‐NH2, promoted growth and upregulation of Sox9 and Acan in chondrogenic condition and PCL‐CH3 film supported better GAG production. For rMSCs, cell adhesion was found least on both PCL and PCL‐CH3 films, and all the films bearing pendant chemical groups were superior in supporting growth compared with PCL film. In chondrogenic condition, rMSCs showed slightly more efficient chondrogenic differentiation on both PCL and PCL‐NH2 films. Collectively, these results suggested that different cells displayed distinct behaviours on this series of polymeric substrates.
To apply chondrocytes for therapeutic applications, in vitro expansion to obtain a sufficient amount is necessary, which causes dedifferentiation inevitably, and thus, a variety of strategies, including growth factor supplementation, three‐dimensional and/or dynamic culture and biomaterials design, have been explored to promote the chondrocytic phenotype during in vitro culture.23, 26, 27, 28, 29, 30 It has been generally considered that expansion of chondrocytes beyond 4 passages leads to irreversible dedifferentiation.29 Hence, in this study, P1, P3 and P5 rACs were all evaluated on this series of polymeric films to investigate both maintenance and induction of re‐differentiation of chondrocytes. For P1 rACs in GM1, it might be possible that more hydrophilic films, especially charged ones, were beneficial to the accumulation of GAG, which potentially favoured chondrocytic gene expression at the early stage, and more hydrophobic films tended to favour gene expression at the late stage in culture, suggesting the potential in maintaining chondrocytic phenotype in long‐term in vitro culture. Several studies have shown that an enhancement in wettability of a hydrophobic substrate, such as PCL, poly(l‐lactic acid) and polystyrene, is beneficial for growth and GAG production of early passages of chondrocytes, which is in consistent with our observation with P1 rACs.11, 16, 17, 31, 32 However, while Ma et al.17 demonstrated that hydroxyl (‐OH) and amide (‐CONH2) on poly(l‐lactic acid) substrates were more favourable to chondrocyte growth than ‐COOH, the best GAG production was found on PCL‐COOH film among the series in this study.
So far, few studies have evaluated the effects of substrate surface properties on re‐differentiation of passaged chondrocytes. In this study, for P3 and P5 rACs in CIM, induction of re‐differentiation was possible on all the films, but to a less extent for P5 cells. It appeared that more hydrophilic films supported better chondrocytic gene expression for both cells. But, less impact was observed for surface chemistry on GAG production, which was possibly due to the short culture period (14 days). In addition, presentation of ‐NH2 on the hydrophobic PCL substratum seemed to support better expression of Sox9 than other hydrophilic groups for inducing re‐differentiation of P5 rACs. However, this is in contrast to the study by Yang et al.30 wherein neutral or low negatively charged surfaces induced more efficient spontaneous re‐differentiation of P7 human ACs on a hydrophilic gel than positively charged one.
Chondrogenesis of MSCs has also been recognized to be affected by substrate surface chemistry.15, 18, 19, 20, 33, 34, 35 In this study, while rMSCs formed aggregates on all the films, both positively charged (PCL‐NH2) and hydrophobic (PCL) surfaces seemed to be favourable for chondrogenesis‐based GAG quantification. This finding is partially in agreement with the study by Guo et al.15 wherein polystyrene surfaces modified with polyallylamine (containing ‐NH2) supported better chondrogenic differentiation than that with poly(acrylic acid) (with ‐COOH), which instead promoted growth of MSCs. In contrast, in several other studies, ‐COOH was claimed to be beneficial for chondrogenesis rather than ‐NH2.18, 33, 34 It is possible that different substrates applied (hydrophilic glass16, 17 and poly(ethylene glycol)18 versus hydrophobic polystyrene15 and PCL in this study might have caused such variation. Notwithstanding, Phillips et al.36 found no difference in chondrogenesis of human MSCs on self‐assembled monolayers bearing different terminal chemical groups.
Based on the above analysis, one important finding is that substrate surface chemistry had differential effects on chondrogenesis of P1, P3, P5 rACs and P5 rMSCs. Moreover, P1 rACs displayed the stage‐specific dependence on surface chemistry during the long‐term culture. Since these cells represent distinct differentiation status, these observations imply a possibly developmental stage‐dependent mechanism for cell–biomaterial interactions concerning chondrogenesis, which is significant in devising strategies for cartilage regeneration. There is a view that dedifferentiated chondrocytes upon in vitro serial expansion might resemble certain biological characteristics of MSCs.37 Hence, both passaged rACs and rMSCs tended to form cellular aggregates upon chondrogenic induction on all the polymeric films, which mimics the condensation event during early cartilage development.38 In essence, the balance between cell–cell and cell–substrate interactions becomes critical, with a strong adhesion on substratum being unfavourable in conducting chondrogenesis.30, 39 However, this balance can be sensitive to the differentiation status of the cells, which further varies dynamically during culture process. For example, a dynamic control over cell adhesion in a hydrogel has been demonstrated to promote the chondrogenic differentiation of human MSCs.40 This might explain why different cells displayed different behaviours on this series of polymeric films. Specifically, a weak adhesion on substratum not only favours the formation of cellular aggregates but also maintains cells in a rounded morphology, which intrinsically stimulates chondrogenesis.41 This is consistent with the fact that PCL and PCL‐CH3 films, which were less cell adhesive compared to more hydrophilic films, were better in inducing chondrocytic gene expression for P1 rACs at the late stage and ECM deposition of both P5 rACs and rMSCs. However, besides from the balanced hydrophobicity/hydrophilicity, surface charges might represent an additional instructive cue for cells to undergo chondrogenesis, by recapitulating the essential characteristics of native cartilage tissue matrix (i.e. GAG).18, 33 This is evidenced by the fact that PCL‐NH2 or PCL‐COOH promoted either ECM production of P1 rACs or chondrocytic gene expression of P5 rACs. Collectively, this may suggest the compounding mechanisms that mediate cell–substrate interactions during chondrogenesis.
5. Conclusions
In this study, a PCL‐based polymer series bearing distinct pendant chemical groups was employed to study the effects of surface chemistry on chondrogenesis of different cell types. It was found that surface chemistry is influential on chondrogenesis in vitro. Importantly, different cell types demonstrated distinct behaviours on such a biomaterials platform concerning chondrogenesis, implicating a developmental dependency on surface chemistry. These findings confer an insight perspective to designing biomaterials for cartilage tissue regeneration.
Supporting information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31000424 and 31170951) and the Basic Research Project of Shanghai Science and Technology Commission (12JC1403101 and 15JC1401402).
References
- 1. Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. [DOI] [PubMed] [Google Scholar]
- 2. Madeira C, Santhagunam A, Salgueiro JB, Cabral JM. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015;33:35–42. [DOI] [PubMed] [Google Scholar]
- 3. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2014;16:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellingman CA, Koevoet W, van Osch GJ. Can one generate stable hyaline cartilage from adult mesenchymal stem cells? A developmental approach. J Tissue Eng Regen Med. 2012;6:e1–e11. [DOI] [PubMed] [Google Scholar]
- 6. Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. [DOI] [PubMed] [Google Scholar]
- 7. Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure‐function relationships in mesenchymal stem cell‐ and chondrocyte‐seeded hydrogels. Tissue Eng Part A. 2009;15:1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang AH, Yeger‐McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte‐ and MSC‐laden hydrogels. Osteoarthr Cartil. 2008;16:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21:3255–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keung AJ, Kumar S, Schaffer DV. Presentation counts: microenvironmental regulation of stem cells by biophysical and material cues. Annu Rev Cell Dev Biol. 2010;26:533–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui YL, Qi AD, Liu WG, et al. Biomimetic surface modification of poly(L‐lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials. 2003;24:3859–3868. [DOI] [PubMed] [Google Scholar]
- 12. Hsu SH, Chang SH, Yen HJ, Whu SW, Tsai CL, Chen DC. Evaluation of biodegradable polyesters modified by type II collagen and Arg‐Gly‐Asp as tissue engineering scaffolding materials for cartilage regeneration. Artif Organs. 2006;30:42–55. [DOI] [PubMed] [Google Scholar]
- 13. Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253–1259. [DOI] [PubMed] [Google Scholar]
- 14. Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. [DOI] [PubMed] [Google Scholar]
- 15. Guo L, Kawazoe N, Fan Y, et al. Chondrogenic differentiation of human mesenchymal stem cells on photoreactive polymer‐modified surfaces. Biomaterials. 2008;29:23–32. [DOI] [PubMed] [Google Scholar]
- 16. Tsai WB, Chen WT, Chien HW, Kuo WH, Wang MJ. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011;7:4187–4194. [DOI] [PubMed] [Google Scholar]
- 17. Ma Z, Gao C, Gong Y, Shen J. Chondrocyte behaviors on poly‐L‐lactic acid (PLLA) membranes containing hydroxyl, amide or carboxyl groups. Biomaterials. 2003;24:3725–3730. [DOI] [PubMed] [Google Scholar]
- 18. Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel‐encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldshmid R, Cohen S, Shachaf Y, et al. Steric interference of adhesion supports in‐vitro chondrogenesis of mesenchymal stem cells on hydrogels for cartilage repair. Sci Rep. 2015;5:12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang K, Yan S, Li G, Cui L, Yin J. In‐situ birth of MSCs multicellular spheroids in poly(L‐glutamic acid)/chitosan scaffold for hyaline‐like cartilage regeneration. Biomaterials. 2015;71:24–34. [DOI] [PubMed] [Google Scholar]
- 21. Seda Tigli R, Ghosh S, Laha MM, et al. Comparative chondrogenesis of human cell sources in 3D scaffolds. J Tissue Eng Regen Med. 2009;3:348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen M, Zhang Y, Zhou Y, et al. Pendant small functional groups on poly(‐caprolactone) substrate modulate adhesion, proliferation and differentiation of human mesenchymal stem cells. Colloids Surf B Biointerfaces. 2015;134:322–331. [DOI] [PubMed] [Google Scholar]
- 23. Xu F, Xu L, Wang Q, Ye Z, Zhou Y, Tan WS. 3D dynamic culture of rabbit articular chondrocytes encapsulated in alginate gel beads using spinner flasks for cartilage tissue regeneration. Biomed Res Int. 2014;2014:539789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu FY, Xu L, Wang Q, Zhou Y, Ye ZY, Tan WS. A three‐dimensional dynamic coculture system enabling facile cell separation for chondrogenesis of mesenchymal stem cells. Biochem Eng J. 2015;103:68–76. [Google Scholar]
- 25. Xu L, Wang Q, Xu F, Ye Z, Zhou Y, Tan WS. Mesenchymal stem cells downregulate articular chondrocyte differentiation in noncontact coculture systems: implications in cartilage tissue regeneration. Stem Cells Dev. 2013;22:1657–1669. [DOI] [PubMed] [Google Scholar]
- 26. Jakob M, Demarteau O, Schafer D, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. [DOI] [PubMed] [Google Scholar]
- 27. Lee TJ, Bhang SH, La WG, et al. Spinner‐flask culture induces redifferentiation of de‐differentiated chondrocytes. Biotechnol Lett. 2011;33:829–836. [DOI] [PubMed] [Google Scholar]
- 28. Schrobback K, Klein TJ, Schuetz M, Upton Z, Leavesley DI, Malda J. Adult human articular chondrocytes in a microcarrier‐based culture system: expansion and redifferentiation. J Orthop Res. 2011;29:539–546. [DOI] [PubMed] [Google Scholar]
- 29. Schulze‐Tanzil G, de Souza P, Villegas Castrejon H, et al. Redifferentiation of dedifferentiated human chondrocytes in high‐density cultures. Cell Tissue Res. 2002;308:371–379. [DOI] [PubMed] [Google Scholar]
- 30. Yang JJ, Chen YM, Liu JF, Kurokawa T, Gong JP. Spontaneous redifferentiation of dedifferentiated human articular chondrocytes on hydrogel surfaces. Tissue Eng Part A. 2010;16:2529–2540. [DOI] [PubMed] [Google Scholar]
- 31. Ma Z, Gao C, Shen J. Surface modification of poly‐L‐lactic acid (PLLA) membrane by grafting acrylamide: an effective way to improve cytocompatibility for chondrocytes. J Biomater Sci Polym Ed. 2003;14:13–25. [DOI] [PubMed] [Google Scholar]
- 32. Tsai WB, Wei TC, Lin MC, Wang JY, Chen CH. The effect of radio‐frequency glow discharge treatment of polystyrene on the behavior of porcine chondrocytes in vitro. J Biomater Sci Polym Ed. 2005;16:699–714. [DOI] [PubMed] [Google Scholar]
- 33. Curran JM, Chen R, Hunt JA. Controlling the phenotype and function of mesenchymal stem cells in vitro by adhesion to silane‐modified clean glass surfaces. Biomaterials. 2005;26:7057–7067. [DOI] [PubMed] [Google Scholar]
- 34. Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–4793. [DOI] [PubMed] [Google Scholar]
- 35. Gross‐Aviv T, Vago R. The role of aragonite matrix surface chemistry on the chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2009;30:770–779. [DOI] [PubMed] [Google Scholar]
- 36. Phillips JE, Petrie TA, Creighton FP, Garcia AJ. Human mesenchymal stem cell differentiation on self‐assembled monolayers presenting different surface chemistries. Acta Biomater. 2010;6:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tallheden T, Dennis JE, Lennon DP, Sjogren‐Jansson E, Caplan AI, Lindahl A. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;85‐A(suppl 2):93–100. [DOI] [PubMed] [Google Scholar]
- 38. Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351–371. [DOI] [PubMed] [Google Scholar]
- 39. Woodfield TB, Miot S, Martin I, van Blitterswijk CA, Riesle J. The regulation of expanded human nasal chondrocyte re‐differentiation capacity by substrate composition and gas plasma surface modification. Biomaterials. 2006;27:1043–1053. [DOI] [PubMed] [Google Scholar]
- 40. Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rottmar M, Mhanna R, Guimond‐Lischer S, Vogel V, Zenobi‐Wong M, Maniura‐Weber K. Interference with the contractile machinery of the fibroblastic chondrocyte cytoskeleton induces re‐expression of the cartilage phenotype through involvement of PI3K, PKC and MAPKs. Exp Cell Res. 2014;320:175–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


