Abstract
Objectives: Curative properties of medicinal plants such as Psidium guajava L. (Myrtaceae) have often been indicated by epidemiological studies on populations in which these fruits are consumed daily. However, complete characterization of the active principles responsible for this ability has never been performed. Here, we have characterized P. guajava’s anti‐cancer potential and identified the parts of the fruit involved in its anti‐neoplastic action.
Materials and methods: We studied morphology of our cells, cell cycle characteristics and apoptosis and performed immunostaining, differentiation and western blot analyses.
Results: We report that the P. guajava extract exerted anti‐cancer control on both haematological and solid neoplasias. P. guajava extract’s anti‐tumour properties were found to be tightly bound to induction of apoptosis and differentiation. Use of ex vivo myeloid leukaemia blasts corroborated that P. guajava was able to induce cell death but did not exhibit anti‐cancer effects on all malignant cells investigated, indicating selective activity against certain types of tumour. Analyses of P. guajava pulp, peel and seeds identified the pulp as being the most relevant component for causing cell cycle arrest and apoptosis, whereas peel was responsible for causing cell differentiation. P. guajava itself and its pulp‐derived extract were found to induce apoptosis accompanied by caspase activation and p16, p21, Fas ligand (FASL TNF super‐family, member 6), Bcl‐2‐associated agonist of cell death (BAD) and tumour necrosis factor receptor super‐family, member 10b (DR5), overexpression.
Conclusions: Our findings showed that P. guajava L. extract was able to exert anti‐cancer activity on cultures in vitro and ex vivo, supporting the hypothesis of its anti malignant pro‐apoptotic modulation.
Introduction
Medicinal use of natural products – compounds derived from natural sources such as plants, animals and micro‐organisms – precedes recorded human history, probably by thousands of years. However, only over the last number of decades have natural products been adopted for roles in drug discovery and development, as it appears that emerging scientific fields such as molecular biology and combinatorial chemistry have not been able to totally satisfy needs of the pharmaceutical industry. In concrete terms, rational design of chemical compounds to target specific molecules often draws upon what already exists in nature. Thus, improving and understanding biological effects of natural compounds represent a significant option for drug discovery in the biomedical sciences.
Natural compounds, and particularly plant derivatives, are the subject of an increasing numbers of studies and growing interest is being directed to plants that are already well known for their medicinal properties. Among these, Psidium guajava L. (Myrtaceae) has a long history of medicinal use in many tropical American and Southeast Asian populations (1); being considered native to Mexico (2), it is widespread throughout South America, Europe, Africa and Asia although reported in indigenous systems of medicine in the Americas more than elsewhere. Based on archaeological evidence, this plant has been known and used widely in Peru since pre‐Columbian times. It grows in all tropical and subtropical areas and adapts to different climatic conditions, although dry climates are preferred (3). P. guajava’s main traditional use is as an anti‐diarrhoeal although other reported uses include treatment of gastroenteritis, dysentery, stomach ailments and intestinal disorders due to pathogenic infections of the intestine (4). In form, P. guajava is a tree around 10 m in height with thin, smooth, patchy, peeling bark. Leaves are apposite and short‐petiolate, 5–15 cm in length with oval blades and prominent pinnate veins. Flowers are somewhat showy, with whitish petals up to 2‐cm long and numerous stamens (3). Fruit of P. guajava are fleshy, yellow and globose to ovoid berries roughly 5 cm in diameter, with edible, pink mesocarp containing numerous small, hard, white seeds. From a phytochemical point of view, P. guajava fruits contain a whole range of phenolic compounds (for example, gallic and ferulic acids), flavonoids (quercetin, kaempferol, guajaverin), carotenoids, triterpenes, tannins and quinones. The heterogeneity of P. guajava fruit constituents might explain the immense potential of this plant in reported treatment of such diverse conditions as diarrhoea (5), gastroenteritis and rotavirus enteritis (6), wounds, acne, dental plaque, malaria, allergies, coughs, diabetes, cardiovascular disorders, degenerative muscular diseases (7, 8), inflammatory ailments (including rheumatism), menstrual pain, liver diseases and more (9). Not surprisingly, P. guajava also exhibits antioxidant (10) and anti‐inflammatory effects, as oxidative injury underlies many of these diseases (11). Recently, anti‐cancer activity has been reported for it and some other types of Myrtaceae fruit (12, 13), supported by epidemiological studies on populations in which they are regularly consumed. However, thorough analysis and characterization of the active principles responsible for this capability has not previously been performed.
Here, we have characterized anti‐cancer potential of P. guajava L. and identified components of the fruit involved in its anti‐malignant properties.
Materials and methods
Plant material
Red guavas of variety IAC‐4 (P. guajava L.) were obtained in April 2007 from the Central Market of São Paulo, Brazil (CEAGESP). The fruits were of excellent quality and maturity and ripeness preferred by consumers, for eating them fresh. Precise species identification was carried out by Prof. Adriana Basile, section of Plant Biology, Department of Biological Sciences, Federico II University of Naples, IT and a voucher specimen (NAP # 96‐147) was deposited in the Herbarium Neapolitanum (NAP), Department of Biological Sciences, University “Federico II” of Naples (Italy).
Method of extraction
The P. guajava fruits, fresh or after storage at −20 °C, were treated with Triton X‐100 0.8% water solution to remove any epiphytic commensals normally found on the surface. After extensive washing in TAP buffer (Tris–HCl pH 7.0 50 mm, NaCl 180 mm, NP‐40 0.15%, glycerol 10%, MgCl2 1.5 mm, NaMO4 1 mm, NaF 0.5 mm, with protease inhibitors (Sigma, Sigma Aldrich, MI, Italy), 1 mm DTT and 0.2 mm PMSF) and distilled water, fruits were dried on filter paper. Four different acetone extracts were then prepared. For the first, 826 g of whole P. guajava fruit were blended, freeze‐dried then extracted with acetone for 3 days, at room temperature (3 × 5 l). Following filtration, the solvent was evaporated at low pressure and moderate temperature (35 °C) to provide a gum (48 g). The same protocol was applied for preparation of the other three extracts (of peel, pulp and seed) after isolating each proposed part of the fruit (14). In brief, 364 g of fresh P. guajava peel provided 6 g of dry acetone extract, 1220 g fresh P. guajava pulp resulted in 50 g of dry acetone extract and 31 g of seeds produced 300 mg of dry acetone extract. All four extracts were freeze‐dried and successively assayed for their anti‐neoplastic potential.
Cell lines, primary cells and culture conditions
NB4 cells were provided by M. Lanotte (INSERM U‐496, Centre G. Hayem Hôpital Saint‐Louis Paris, France). All other cell lines were purchased from ATCC and were routinely cultured. NB4 and U937 cells were grown at 37 °C in 5% CO2 atmosphere in RPMI‐1640 medium (Gibco, NY, USA), supplemented with 10% heat‐inactivated foetal bovine serum (FBS), 1%l‐glutamine, 1% ampicillin/streptomycin and 0.1% gentamicin. U2OS osteosarcoma cells were grown at 37 °C in air and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 5% FBS (Gibco), 1% L‐glutamine, 1% ampicillin/streptomycin and 0.1% gentamicin. MDA‐MB231 breast cancer cells were grown at 37 °C in air and 5% CO2 in DMEM (Gibco) supplemented with 5% FBS (Gibco), 1%l‐glutamine, 1% ampicillin/streptomycin and 0.1% gentamicin. Acute myeloid leukaemia (AML) samples’ purification and cultures were carried out as previously described (15). This study was approved by the Ethical Committee of the Second University of Naples. SAHA (kindly provided by Merck) and MS275 (Alexis, Vinci‐Biochem SRL, FI, Italy) were resuspended in DMSO and used at final concentration of 5 μm. All‐trans retinoic acid (ATRA; Sigma) was resuspended in 100% ethanol and used at a final concentration of 1 μm.
Cell cycle analysis
Samples were applied to a FACS‐Calibur flow cytometer and analysed using standard procedures using Cell Quest software (Becton Dickinson, Mi, Italy) and ModFit LT version 3 Software (Verity Sotfware House, Topsham Me, USA), as previously reported (16). Cell lines analysed were NB4, U937, U2OS.
FACS analysis of apoptosis
Apoptosis was measured using annexin V/propidium iodide (PI) double staining detection (Roche, Basel, Switzerland and Sigma‐Aldrich, Mi, respectively) as recommended by the suppliers; samples were analysed by FACS with Cell Quest software (Becton Dickinson) as previously reported (15, 17). The apoptotic fraction was calculated by measuring annexin V positive/PI negative cells. As second assays, caspases 8, 9 and 7‐3 detection (B‐Bridge) were performed as recommended by the suppliers and quantified by FACS (Becton Dickinson) analysis.
Granulocytic differentiation assay
Granulocytic differentiation analysis was carried out as previously described (15, 18). Briefly, NB4 cells were harvested and resuspended in 10 μl phycoerythrine‐conjugated CD11c (CD11c‐PE) or 10 μl fluorescein isothiocyanate‐conjugated CD14 (CD14‐FITC) (Pharmingen, San Diego, California, US). Control samples were treated with 10 μl PE or FITC conjugated mouse IgG1, incubated for 30 min at 4 °C in the dark, washed in phosphate‐buffered saline (PBS) and resuspended in 500 μl PBS containing PI (0.25 μg/ml). Samples were analysed by FACS with Cell Quest software (Becton Dickinson). PI positive cells were excluded from the procedure.
Western blot analysis
Forty micrograms total protein extract was separated on 15% polyacrylamide gel and blotted as previously described (19). Western blots were performed of p21 (dilution 1:500; Transduction Laboratories, BD group, MI, Italy) and p16 (dilution 1:500; Santa Cruz, S.C., California, US) and total mitogen‐activated protein kinases (ERKs) (dilution 1:1000; Santa Cruz) were used for equal loading. To quantify TRAIL, 100 μg total protein extract was separated on 10% polyacrylamide gel and blotted. Western blots were performed of TNF‐related apoptosis inducing ligand (TRAIL) (dilution 1:200; Abcam), and ERKs (dilution 1:1000; Santa Cruz) were used for equal loading and the same protocol was applied for DR5 (dilution 1:1000; Millipore, Billerica, MA). To determine CASP8 and FADD‐like apoptosis regulator (c‐FLIP) levels, 35 μg of total protein extract was separated on 12% polyacrylamide gel and blotted. Western blots were performed of FLIP (dilution 1:500; Alexis); total ERKs (dilution 1:1000; Santa Cruz) were used for equal loading control. To determine BAD levels, 35 μg of total protein extract was separated on 12% polyacrylamide gel and blotted and the same was performed for FASL (dilution 1:500; ProSci Incorporated, Poway, California, US). Total ERKs (dilution 1:1000; Santa Cruz) were used for equal loading.
Cell morphology
NB4 cells were spun on to glass slides using a cytospin centrifuge. Cell morphology was analysed after May–Grunwald Giemsa staining (Sigma).
Results
Anti‐proliferative activity in NB4 cells of Total acetone Psidium guajava L. fruit extract
Psidium guajava L. exerted anti‐proliferative activity and this property was initially confirmed by using total acetone P. guajava fruit extract, at different concentrations for 48 h on NB4 promyelocitic leukaemia cells. Trypan blue staining carried out on these cells treated with total acetone fruit extract at different concentrations indicated its strong anti‐proliferative effect. Interestingly, the effect of P. guajava total acetone extract was linear and dose‐dependent (Fig. 1a,b). This was confirmed by two different time‐length treatments (3 and 5 days) that displayed greatest effect and over a concentration range of 5–1.5 mg/ml. When cell cycle effects were analysed, treatment with total acetone P. guajava extract (over the range of its biologically active dose) induced G1 cell cycle block (Fig. 1c) of around 80% at concentrations of 1.5 and 3 mg/ml.
Figure 1.
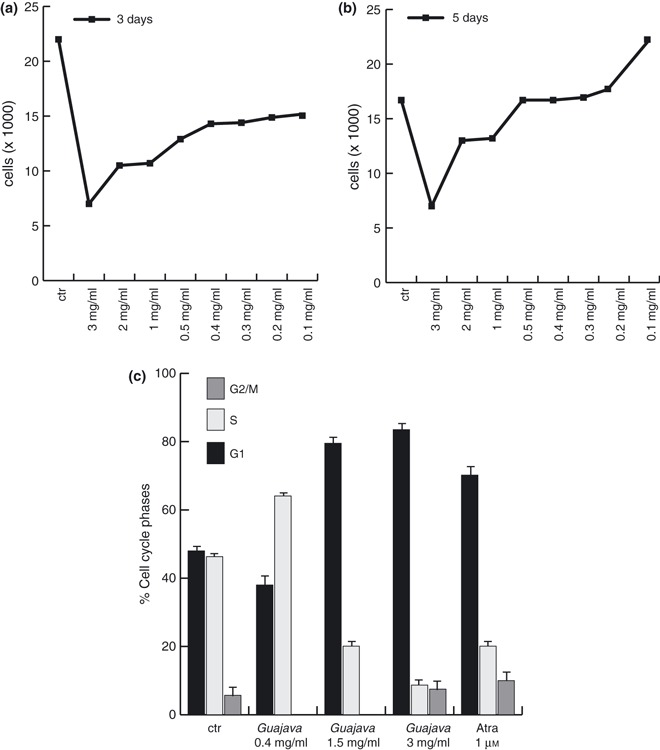
Total acetone Psidium guajava L. fruit extract exerted anti‐proliferative action on leukaemia NB4 cells. (a) Proliferation curve by trypan blue assay on NB4 cells at day 3 after treatment with acetone P. guajava L. extract at reported concentrations. (b) Proliferation curve by trypan blue assay on NB4 cells at day 5 after treatment with acetone P. guajava extract at reported concentrations. Results represent average of triplicates. (c) Cell cycle analysis of NB4 cells after treatment with selected crude P. guajava extracts for 48 h in comparison with ATRA (all‐trans retinoic acid).
Anti malignancy activity of Total acetone Psidium guajava L. fruit extract
Annexin V/PI double staining was carried out to discern apoptosis of NB4 cells after treatment with total acetone P. guajava extract. Percentage of apoptotic cells increased during treatment and the effect was measurable even at low‐dosage (in the order of 0.4 mg/ml) (Fig. 2a). When total acetone P. guajava fruit extract was administered for 48 h ex vivo to primary acute myeloid leukaemia blasts (AML #106), induction of apoptosis was confirmed, indicating that not only leukaemia cell lines but also primary AML cells responded to it by apoptotic cell death (Fig. 2b). As positive controls for induction of apoptosis, two different histone deacetylase inhibitors (HDACis), SAHA (Vorinostat, Merck, MI, Italy) and MS275 were used.
Figure 2.
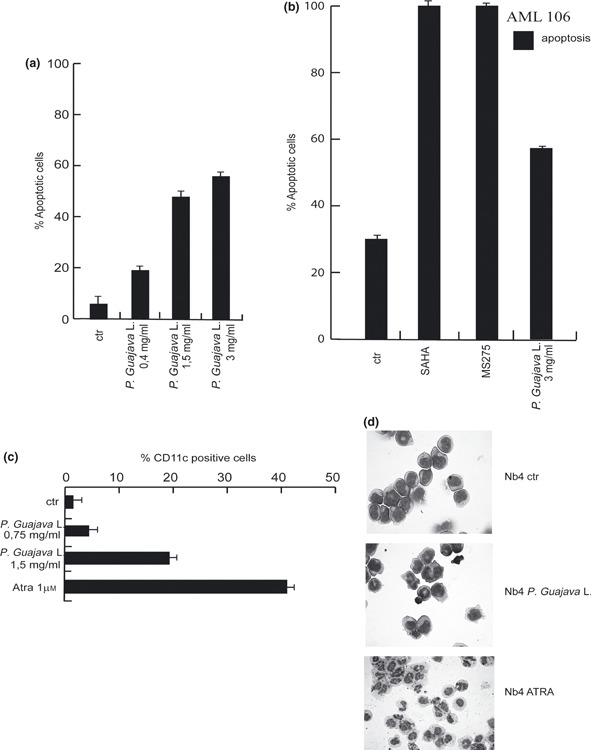
Total acetone Psidium guajava L. fruit extract exerted anti‐malignant and differentiation activity. (a) Percentage of apoptotic cells after 24 h treatment with indicated crude P. guajava extracts on NB4 cells. (b) Apoptosis evaluation carried out by FACS analysis on primary AML blasts after 24 h treatment with indicated dose of total P. guajava extract. MS275 and SAHA were used as positive controls. (c) CD11c expression levels measured by FACS after 48 h treatment with indicated amounts of P. guajava extract on NB4 cells. Note that PI positive cells were excluded from the analysis. ATRA was used as positive pro‐differentiation compound. (d) Morphological analysis of granulocytic differentiation induction of NB4 cells after treatment with P. guajava extract at day 4 in comparison to ATRA induction.
To establish whether P. guajava fruit extract was also able to induce cell differentiation, presence of CD11c, a specific marker for granulocytic differentiation, was evaluated in NB4 cells, after 48 h treatment. CD11c expression increased even at 0.75 mg/ml concentration, and reached its maximum level at 1.5 mg/ml (Fig. 2c). Nevertheless, the differentiation effect was lower than that of all‐trans retinoic acid (ATRA), a well‐known anti‐tumour and pro‐differentiation agent (17, 18, 20). In agreement with these data, immunohistochemical staining revealed that after 4 days treatment with P. guajava total acetone extract, NB4 cells assumed characteristic granulocyte morphology with lobed nuclei and presence of characteristic granules, as shown in Fig. 2d. Interestingly, total acetone P. guajava extract exerted both anti‐proliferative effects and induction of cell death in MDA‐MB 231 breast cancer cells (Fig. 2e), thus suggesting that its anti‐neoplastic activity was present in at least this solid tumour models as well.
Psidium guajava L. pulp extract was essential for apoptosis
To understand better the biological action of P. guajava extract, we fractioned the fruit parts, aiming to identify which component(s) (peel, pulp and seeds) were actively responsible for its anti‐neoplastic activity. As shown in Fig. 3a, after indicated treatments, cell cycle progression was mainly caused to alter by the pulp’s components, whereas peel and seeds only marginally affected the cell cycle. Furthermore, the pulp also proved to be mainly responsible for induction of apoptosis, with a value of 76.9% ± 1.6SD (Fig. 3b). Also, extract from the peel weakly induced apoptosis, indicating partial contribution of this to the observed cell death. When cell differentiation was measured by CD11c detection, peel components were shown to be as active as total extract. Morphology of NB4 cells examined after May–Grunwald Giemsa staining further confirmed these data (Fig. 3c,d). Thus, pulp components mainly influenced apoptosis, whereas peel extract was responsible for cellular differentiation.
Figure 3.
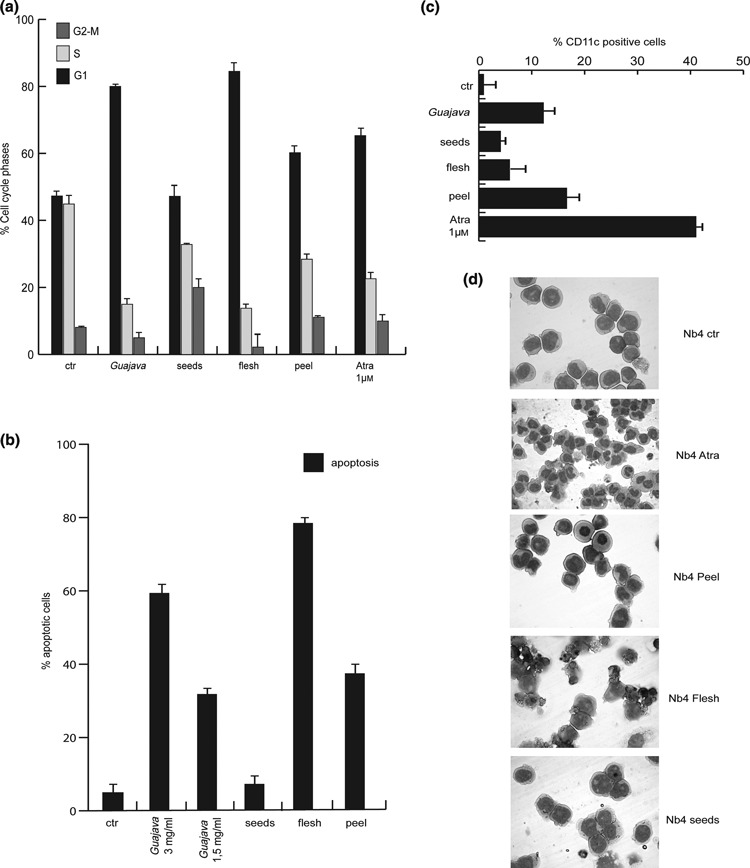
Pulp Psidium guajava L. component was essential for apoptosis, peel for differentiation. (a) Cell cycle analysis on NB4 cells after stimulation with total P. guajava extract and fractioned parts of P. guajava fruit, for 24 h and at 1.5 mg/ml concentration. (b) Apoptosis carried out by FACS analysis on NB4 cells after 24 h induction with the same total and fractioned P. guajava extracts. (c) FACS evaluation of CD11c of NB4 cells. Data show average of triplicates and ATRA was used as positive control. (d) Morphological analysis of granulocyte induction after treatment with fractioned P. guajava components at day 4, of NB4 cells.
To understand whether apoptotic activity may apply to different cancer models, we tested total and component‐specific P. guajava extracts on breast, bone and leukaemia cancer cells.
As shown in Fig. 4a, both total acetone P. guajava and pulp extract exerted anti‐proliferative effects and induced cell death in MDA‐MB 231 breast cancer cells. However, U937 AML cells and U2OS osteosarcoma cells displayed neither cell cycle variation nor apoptosis over time and concentrations tested, suggesting that only some cancer models are sensitive to P. guajava treatment (Fig. 4b,c).
Figure 4.
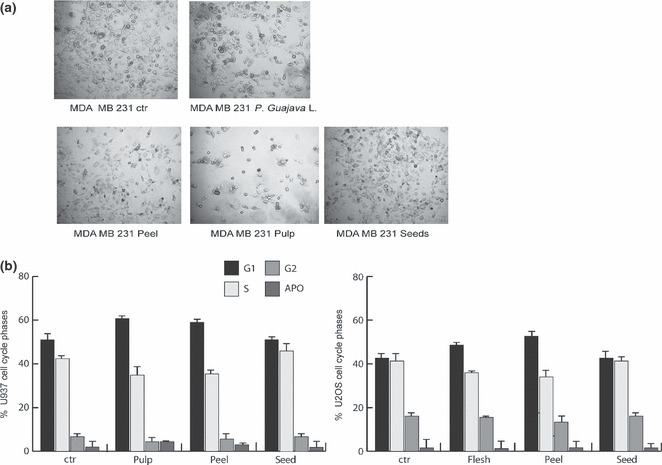
Psidium guajava L. extract and pulp extract exerted anti‐proliferative effects on different cancer cell models. (a) Morphological analysis of MDA‐MB231 cells after 4 days treatment with total P. guajava, peel, pulp and seed extracts at 1.5 mg/ml concentration (b) Cell cycle analysis of U937 and U2OS cells after induction with pulp, seed and peel extracts for a period of 24 h and at 1.5 mg/ml concentration. Data show average of triplicates.
Caspase activation and apoptotic molecular events mediated by Psiduim guajava L. pulp extract
To investigate molecular events underlying P. guajava‐induced cell death, caspase assays (caspase 8, 9 and caspase 3/7) were performed. As shown in Fig. 5a, caspase 8 and 9 were mainly activated by pulp extracts, thus suggesting that cell death was due to apoptosis and confirming that the pulp component was the active anti‐neoplastic portion of the fruit. To evaluate which molecular players might be involved in this anti‐cancer action, expression levels of different known key factors in cell cycle progression and apoptosis were analysed by western blot analysis, in NB4 cells. As shown in Fig. 5b, after 48 h of induction, total acetone P. guajava, peel, pulp and seed extracts induced expression of p21 and p16, known cell cycle inhibitors. Taken together, these data indicate that the most active fraction was the pulp, confirming observations on cell cycle blocking (1, 5). Under similar experimental conditions, expression levels of pro‐ and anti‐apoptotic proteins were analysed. Interestingly, DR‐5, FASL and BAD appeared to be induced after treatment with total P. guajava and pulp extracts, validating the pro‐apoptotic activity of both. In particular, only treatment with pulp fraction led to significant reduction in level of protein FLIP‐L (strong inhibitor of caspase 8‐mediated apoptosis), although expression level of protein FLIP‐S remained unchanged. (Fig. 5b).
Figure 5.
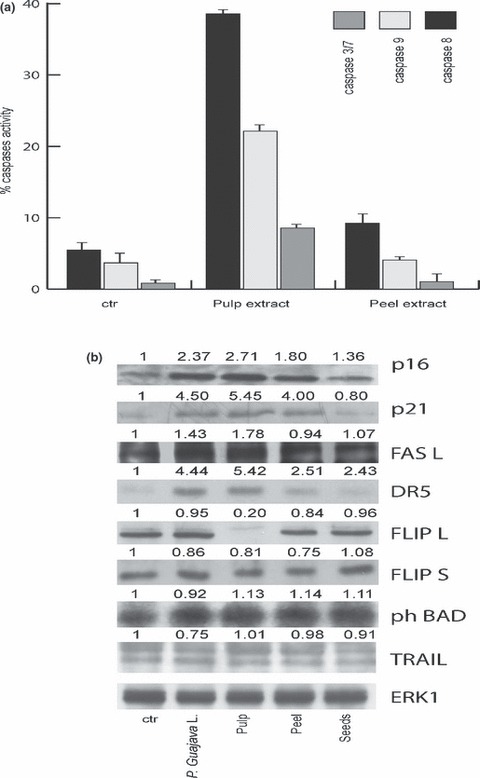
Pulp Psidium guajava L. extract mediated caspase activation and apoptotic molecular events. (a) FACS analysis of caspase 8, 3/7 and 9 of NB4 cells after 2 days incubation with selected extracts at 1.5 mg/ml concentration. Data represent average of duplicates. (b) Western blot analysis carried out on NB4 cells treated with total P. guajava, and peel, pulp and seed extracts for 24 h at 1.5 mg/ml concentration. ERKs signalling was used for equal loading. Band intensity was quantified using Image. Values indicate relative levels determined after normalization to ERKs.
Discussion
Plants have long served as a useful natural sources of therapeutic agents. It is generally recognized that consumption of a variety of local herbs and vegetables significantly contributes to improvement of human health, in terms of prevention and treatment of disease. At present in many developing countries, around 80% of available drugs are derived from medicinal plants. Frequently, in industrialized countries, raw materials used to synthesize pure chemical derivatives have had plant origins (12).
Psidium guajava L. belongs to the family of Myrtaceae (21). It is a tropical plant widely grown in Taiwan, Hawaii, Thailand, the Philippines and Malaysia, where its fruits, leaves, and bark have been traditionally used in herbal medicines, and they exhibit many therapeutic effects including amebicide, analgesic, vermifuge, anti‐malarial, anti‐bacterial, colic‐relief, anti‐spasmodic, astringent, anti‐ulcerous, hypotensive, anti‐inflammatory, anti‐hyperglycaemic and anti‐diarrhoeal properties, as well as acting as a gastrototonic cough suppressant and combating some psychic diseases.
Anti‐malignant activity of P. guajava has been investigated in our study and data concerning active anti‐neoplastic components of the fruit and its constituents are presented here. Active factors of P. guajava fruits involve ursolic acid, oleanolic acid, arjunolic acid and glucuronic acid (22), saponin combined with oleandolic acid: morin‐3‐O‐α‐L‐lyxopyranoside and morin‐3‐O‐α‐Larabinopyranoside; pentane‐2‐thiol (23, 24, 25, 26, 27); and flavonoids: guaijavarin and quercetin (28, 29, 30, 31). In comparison, huge amounts of β‐sitosterol glucoside, brahmic acid, and polyphenolics (165.61 ± 10.39 mg/g dried crude extract) including gallic acid, ferulic acid, and quercetin (32, 33); and flavonoids (82.85 ± 0.22 mg/g dried crude extract) (33, 34, 35, 36, 37), and triterpenoids (38), exist in guava leaves (32, 33, 38). Thus, it is clear that P. guajava contains many components reported to display efficacy against cancer. Although a variety of constituents is present in the fruit extracts, the main ones are bioflavonoids. Experimental evidence that crude acetone extract was able to induce cell cycle arrest and cell death in NB4 cells confirmed our hypothesis that some of its components would have anti‐cancer properties. When acetone extracts were prepared from P. guajava peel, pulp and seeds, the active parts were shown to be peel and pulp, capable of inducing CD11c expression and apoptosis in acute promyelocytic leukaemia cells respectively. Differential sensitivity shown by different cancer models, such as breast cancer and osteosarcomas, suggests both some cell‐selectivity and very low toxic effect of the extract.
This hypothesis was also confirmed by evidence that cell death, occurring after treatment, was caspase‐mediated and able to activate both cell cycle and molecular death programmes. Not only caspase 8 but also FAS, DR‐5 and BID were regulated, mainly by total and by pulp extracts (Fig. 5), strongly suggesting involvement of death receptor‐mediated pathways in modulation of apoptosis. Although not fully understood, abundance of flavonoids in P. guajava pulp extract indicates that they may play a key role in anti‐malignant activity of the fruit.
Taken together, these data provide a new perspective for analysis and use of natural products in the treatment of human pathologies, and indicate that plant components can exert anti‐cancer activity. Given the need for new ‘smart’ anti‐cancer drugs, in‐depth investigation of the properties of natural products with no more than minor side effects, and targeted action, may represent a new approach to future development of cancer‐selective drugs.
Acknowledgements
This study was supported by EU (‘Aposys’ contract no 200767), by AIRC (Associazione Italiana per la Ricerca sul Cancro project no 4625) and by MIUR (PRIN no 2007RZWFBZ_004). We acknowledge Dr Catherine Fisher for kindly revising the manuscript. The authors declare that they have no conflict of interests.
References
- 1. Deguchi Y, Miyazaki K (2010) Anti‐hyperglycemic and anti‐hyperlipidemic effects of guava leaf extract. Nutr. Metab. (Lond) 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rios CD, Salazar CR, Cardona C, Victoria K, Torres M (1977). Guayaba. Frutales. Manual de Asistencia Tecnica 4, 221–248. [Google Scholar]
- 3. Stone B (1970) The flora of Guam. Micronesica 6, 454–455. [Google Scholar]
- 4. Gutierrez RMP, Mitchell S, Solis RV (2008) Psidium guajava.: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 117, 1–27. [DOI] [PubMed] [Google Scholar]
- 5. Birdi T, Daswani P, Brijesh S, Tetali P, Natu A, Antia N (2010) Newer insights into the mechanism of action of Psidium guajava L. leaves in infectious diarrhoea. BMC Complement Altern. Med. 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yusuf S, Agunu A, Katung NV, Umana UE (2010) Ethanolic leaf extract of Psidium Guajava L. [Myrtaceae] protects the stomach against ischemia‐reperfusion induced gastric mucosal injury. Asian J. Med. Sci. 1, 1–3. [Google Scholar]
- 7. Belemtougri RG, Constantin B, Cognard C, Raymond G, Sawadogo L (2006) Effects of two medicinal plants Psidium guajava L. (Myrtaceae) and Diospyros mespiliformis L. (Ebenaceae) leaf extracts on rat skeletal muscle cells in primary culture. J. Zhejiang Univ. Sci. B 7, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ieven M, Vanden Berghe DA, Mertens F, Vlietinck A, Lammens E (1979) Screening of higher plants for biological activities. I. Antimicrobial activity. Planta Med. 36, 311–321. [DOI] [PubMed] [Google Scholar]
- 9. Arima H, Danno G (2002) Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biosci. Biotechnol. Biochem. 66, 1727–1730. [DOI] [PubMed] [Google Scholar]
- 10. Ogunlana OE, Ogunlana OO (2008) In vitro Assessment of the Free Radical Scavenging Activity of Psidium Guajava . Res. J. Agric. Biol. Sci. 4, 666–671. [Google Scholar]
- 11. Abreu PRC, Almeida MC, Bernardo RM, Bernardo LC, Brito LC, Garcia EAC et al. (2006) Guava extract (Psidium guajava) alters the labelling of blood constituents with technetium‐99m. J. Zhejiang Univ. Sci. B 7, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bontempo P, Mita L, Miceli M, Doto A, Nebbioso A, De Bellis F et al. (2007) Feijoa sellowiana derived natural Flavone exerts anti‐cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 39, 1902–1914. [DOI] [PubMed] [Google Scholar]
- 13. Nirmal J, Babu CS, Harisudhan T, Ramanathan M (2008) Evaluation of behavioural and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern. Med. 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basile A, Vuotto ML, Violante U, Sorbo S, Martone G, Castaldo‐Cobianchi R (1997) Antibacterial activity in Actinidia chinensis, Feijoa sellowiana and Aberia caffra . Int. J. Antimicrob. Agents 8, 199–203. [DOI] [PubMed] [Google Scholar]
- 15. Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P et al. (2005) Tumor‐selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 11, 77–84. [DOI] [PubMed] [Google Scholar]
- 16. De Luca A, Baldi A, Russo P, Todisco A, Altucci L, Giardullo N et al. (2003) Coexpression of Helicobacter pylori’s proteins CagA and HspB induces cell proliferation in AGS gastric epithelial cells, independently from the bacterial infection. Cancer Res. 63, 6350–6356. [PubMed] [Google Scholar]
- 17. Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H (2001) Retinoic acid‐induced apoptosis in leukemia cells is mediated by paracrine action of tumor‐selective death ligand TRAIL. Nat. Med. 7, 680–686. [DOI] [PubMed] [Google Scholar]
- 18. Altucci L, Gronemeyer H (2001) The promise of retinoids to fight against cancer. Nat. Rev. Cancer 1, 181–193. [DOI] [PubMed] [Google Scholar]
- 19. Mai A, Massa S, Rotili D, Simeoni S, Ragno R, Botta G et al. (2006) Synthesis and biological properties of novel, uracil‐containing histone deacetylase inhibitors. J. Med. Chem. 49, 6046–6056. [DOI] [PubMed] [Google Scholar]
- 20. Altucci L, Wilhelm E, Gronemeyer H (2004) Leukemia: beneficial actions of retinoids and rexinoids. Int. J. Biochem. Cell Biol. 36, 178–182. [DOI] [PubMed] [Google Scholar]
- 21. Chen KC, Peng CC, Chiu WT, Cheng YT, Huang GT, Hsieh CL et al. (2010) Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr. Cancer 62, 260–270. [DOI] [PubMed] [Google Scholar]
- 22. Chang WS (1982). Studies on Active Principles of Hypoglycemic Effect from Psidium Guajava (I). Master Thesis, The Graduate Institute of Pharmacy, Taipei Medical College; [Google Scholar]
- 23. Bassols F, Demole EP (1984) The occurrence of pentane‐2‐thiol in guava fruit. J. Essential Oil Res. 6, 481–483. [Google Scholar]
- 24. Hernandez DF (1971). Plants of the Philippines, M&L Licudine Enterprises. First Printing. Philippines: University of the Philippines, 678–680. [Google Scholar]
- 25. Nadkarni KM, Nadkarni AK (1999) Indian Materia Medica with Ayurvedic, Unani‐Tibbi, Siddha, allophatic, homeopathic, naturopathic and home remedies. Popular Prakashan Private Ltd, Bombay, India 1, 142–149. [Google Scholar]
- 26. Paniandy JC, Chane MJ, Pieribattesti JC (2000) Chemical composition of the essential oil and headspace solid‐phase microextraction of the guava fruit (Psidium guajava L.). J. Essent. Oil Res. 12, 153–158. [Google Scholar]
- 27. Jordan MJ, Margaria CA, Shaw PE, Goodner KL (2003) Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC–MS and multidimensional GC/GC‐O. J. Agric. Food Chem. 51, 1421–1426. [DOI] [PubMed] [Google Scholar]
- 28. Dweck AC (2003). A review of guava (Psidium guajava). Dweck Data. FLS FRSC FRSH
- 29. Dweck AC (2001) A review of Psidium guajava . Malayan Journal of Medical Science (M.M.A) 8, 27–30. [Google Scholar]
- 30. Hwang JS, Yen YP, Chang MC, Liu CY (2002) Extraction and identification of volatile components of guava fruits and their attraction to Oriental fruit fly, Bactrocera dorsalis (Hendel). Plant Protection Bull. 44, 279–302. [Google Scholar]
- 31. Matsuzaki K, Ishii R, Kobiyama K, Kitanaka S (2010) New benzophenone and quercetin galloyl glycosides from Psidium guajava L. J. Nat. Med. 64, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee SH, (1986). Studies on Active Principles of Hypoglycemic Effect from Psidium guajava (II). Master Thesis, The Graduate Institute of Pharmacy, Taipei Medical College; [Google Scholar]
- 33. Peng RY, Hsieh CL (2006) Review on the medicinal uses of Psidium guajava L. Recent Progress in Medicinal Plants 20, 215–248. [Google Scholar]
- 34. Medina ML, Pagano FG (2003) Characterization of Psidium guajava pulp “criolla roja. Revista de la Facultad de Agronom′ıa de La Universidad del Zulia, (LUZ) 20, 72–76. [Google Scholar]
- 35. Conway P (2002). Tree Medicine: A Comprehensive Guide to the Healing Power of Over 170 Trees. London: Judy Piatkus (Publishers) Ltd, 2173–2177. [Google Scholar]
- 36. Iwu MM (1993). Handbook of African Medicinal Plants. BOCA RATON: CRC Press, 786–789. [Google Scholar]
- 37. Fujita T, Massaharu K, Tamotsu K, Kenji Y, Kejichi O, Kiyoshi S (1985) Nutrient contents in fruit and leaves of guava and in leaves of Japanese persimmon. Seikatsu Eisei. 29, 206–209. [Google Scholar]
- 38. Hsieh CL, Lin YC, Ko WS, Peng CH, Huang CN, Peng RY (2005) Inhibitory effect of some selected nutraceutic herbs on LDL glycation induced by glucose and glyoxal. J. Ethnopharmacol. 102, 131–312. [DOI] [PubMed] [Google Scholar]


