Abstract
The spleen is the largest secondary lymphoid organ in the body and as such hosts a wide range of immunological functions alongside its roles in hematopoiesis and red blood cell clearance. The physical organization of the spleen allows it to filter blood of pathogens and abnormal cells and facilitate low probability interactions between antigen presenting cells (APCs) and cognate lymphocytes. APCs unique to the spleen regulate the T and B cell response to these antigenic targets in the blood. This review will focus on cell types, cell organization and immunologic functions specific to the spleen, and how these impact initiation of adaptive immunity to systemic blood-borne antigens. Potential differences in structure and function between murine and human spleen will also be discussed.
One sentence Summary
This review focuses on the cell types, cell organization and immunologic functions specific to the spleen.
Splenic Architecture
The spleen is divided by function and structure into the red and white pulp; in between these two regions is the marginal zone (MZ) in rodents and the perifollicular zone in humans (1, 2) (Fig. 1). The white pulp (WP) is the primary immunologic region of the spleen in both species; however, the WP makes up less than a quarter of splenic tissue. The red pulp (RP) makes up the majority of the tissue and has an immune function distinct from that of the WP. Unlike lymph nodes (LNs), the spleen lacks afferent lymphatic vessels and therefore all cells and antigen enter the spleen via the blood.
Figure 1. Mouse and human splenic immune cellular architecture at steady state.
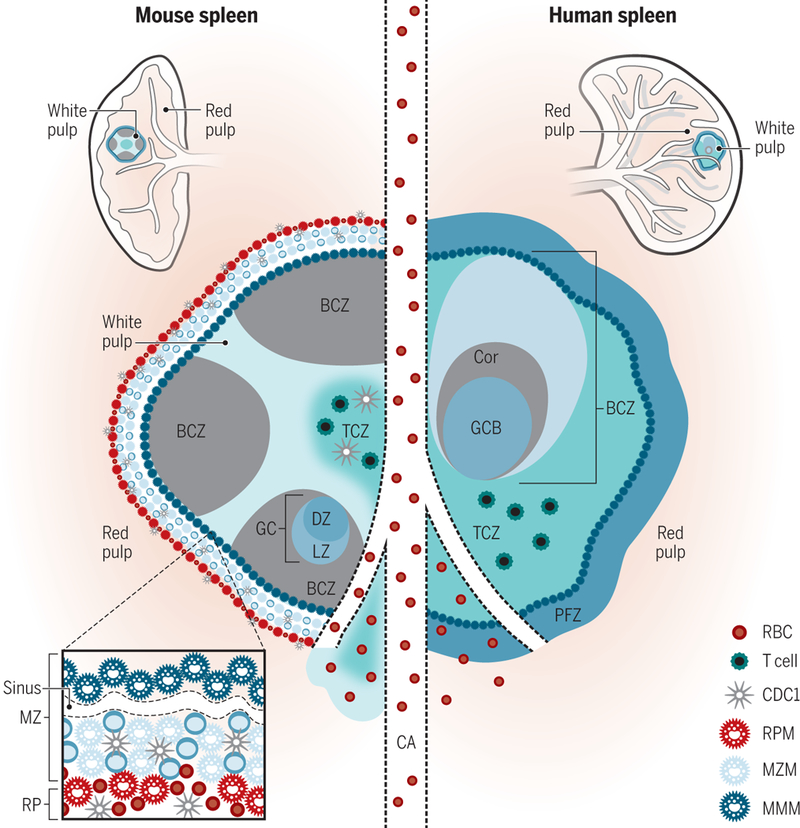
There are structural differences between the murine (left) and human (right) splenic immune system, most notably, the organization of T cell zone (TCZ, turquoise; also known as PALS) and B cell zone (BCZ) follicles (gray and shades of blue, shown with light zone, LZ, and dark zone, DZ, organization in mouse spleen) within the WP and the border between the WP and RP, the MZ (marginal zone) in mouse or perifollicular zone (PFZ) in human (dark blue outer ring). Because applications of advanced imaging techniques to the human spleen have been limited, the extent to which the mouse MZ and human PFZ are analogous remains unknown. For example, the precise layering and composition of macrophage subsets in the MZ is known for mice (see bottom left box)—CD169+ MMMs (dark blue) form a concentric ring around the WP with MZMs (light blue) and MZB cells (darker blue)—but not for humans. In humans, MZB cells surround activated B cells, containing a GC (light blue in the human spleen on the right) and Corona (gray, “Cor”). The homeostatic location of dendritic cell (DC) subsets in mice is shown (with cDC2s in the bridging channel, BC, and cDC1s in the TCZ, MZ and RP, red pulp). Release of blood into the MZ of the WP from a central arteriole (CA) is shown.
Red Pulp
The splenic RP extracts aged, dead or opsonized cells from the circulation, while simultaneously surveying for pathogens and tissue damage. Blood is delivered to the MZ by terminal arterioles, which release their contents into an open blood system without traditional endothelial linings. The RP filters out aged red blood cells (RBCs), which must traverse tortuous venous sinusoids in order to re-enter the circulation. Aged, infected or dysfunctional RBCs that cannot adequately deform, have lost the “don’t eat me” signal, CD47, or that are opsonized by antibody or complement are removed from the circulation by RP macrophages and their iron is reclaimed for systemic use. After percolating through the RP cords, blood is re-collected in sinuses to form the venous sinusoidal system and ultimately enters the efferent vein for return to the circulatory system.
Although adaptive immune responses to systemic antigens are initiated in the WP, immune effector function often takes place in the RP. Many leukocytes with innate functions reside in the RP, including neutrophils, monocytes, dendritic cells (DCs), gamma delta (γδ) T cells and macrophages (3). These myeloid populations can change dynamically both in location and number during an inflammatory response to respond quickly to an insult and shape the adaptive immune response. Plasmablasts migrate from the WP to the RP following gradients of CXCL12 (which is higher in the RP) to produce antibodies that are carried throughout the circulatory system (3). Effector CD8+ T cells emigrate to the RP to clear bacteria (4). Extramedullary hematopoiesis and storage of cellular reserves (monocytes, platelets, RBCs, etc.) are other important functions of the splenic RP, but beyond the scope of this review (see (1) for review).
White Pulp
The spleen can be considered a peripheral tissue of the circulatory system embedded with multiple LN-like structures (the WP) (5), with two important distinctions from LNs. The WP in both mouse and human does not contain a capsule to separate it from the parenchyma of the spleen (the RP). Instead, a cellular border primarily made up of innate immune cells demarcates the boundaries of the WP; this is well defined in mouse and only partially defined in humans (Fig. 1). Despite the lack of a capsule, antigen larger than 60kDa does not freely flow into the WP and is instead carried in by cells from the MZ (6). Second, afferent lymphatics do not drain into the WP to deliver cells and antigens. Despite the widely held notion in murine studies that the spleen is the draining secondary lymphoid organ (SLO) for the peritoneum, antigen injected intraperitoneally actually tracks to the mediastinal LNs (7), although eventually some antigen reaches the spleen. Instead, the WP of the spleen functions as the SLO of the circulatory system, similar to how LNs drain and monitor antigens from tissues. In the WP, splenic naïve and central memory T cells are activated in response to cognate antigen and T-dependent B cell germinal center (GC) reactions resulting in antibody production (8).
Inside the WP, compartmentalization separates the T and B cell areas into distinct zones, analogous to those found in LNs (Fig. 1). The T cell zone (TCZ) is also called the periarteriolar lymphoid sheath (PALS) as it forms around the central arteriole that runs through the WP on its way to the RP-WP border. From mouse studies we know that CCR7 and its ligands CCL19 and CCL21 are crucial for this concentration of T cells; loss of CCR7 results in a scattering of T cells throughout the spleen (5, 9). The B cell zones (BCZ) are the follicles (and GCs during an active immune response), which contain a mixture of cells important for the activation and survival of B cells. Non-hematopoietic follicular dendritic cells (FDCs) present antigen to follicular B cells, while also producing CXCL13 to help organize the B cell follicle (10).
This basic structure, which is slightly different between mouse and human (Fig. 1), is enforced by regions of chemoattractant produced primarily by non-hematopoietic cells (10). Integrins (αLβ2, α4β1) are required for T and B cells to enter the splenic WP (5), then fibroblast channels possibly originating in the MZ help direct T cell movement into the WP through the BC (11), consistent with older work demonstrating a conduit for chemokine-directed cellular trafficking through the splenic WP (6). Fibroblast reticular cells are lined by the CCR7 ligand CCL21, which acts to direct T cells through the TCZ and interfollicular zone, where this network terminates. Similarly, B cells migrate along the same network, but switch to an FDC network in follicles lined by the CXCR5 ligand CXCL13.
Marginal Zone
Part of the blood released by terminal arterioles drains into the MZ (mouse) or perifollicular zone (humans). A unique type of innate-like B cell, marginal zone B cells (MZBs), resides here at steady state in mice anchored by integrins LFA-1 and α4β7 binding to ICAM-1 and VACM-1 along with chemoattract signals from by S1P (12, 13). At this RP-WP interface, specialized leukocytes including DCs and MZBs capture and transport blood-borne antigens to the WP for surveillance by T and B cells (9, 14, 15). Two populations of macrophages populate the murine MZ, retained by CCL21 chemokine signals (5): marginal metallophilic macrophages (MMMs) and marginal zone macrophages (MZMs). Together, these populations form the MZ borders (Fig. 1) and help filter the blood as it is released into the MZ (16). The MZ is also where lymphocytes are released from the circulation to enter the WP.
Bridging Channel
A conduit exists between the red and white pulp – the bridging zone/channel (BC) (Fig. 1). This direct extension of the TCZ into the RP contains many T cells but also antibody-producing cells. The BC, as will be discussed in the section on DC subsets, is also home to a particular population of splenic DCs with defined functions in CD4+ T cell activation. It is widely believed that naïve and activated lymphocytes enter and exit the WP via the BC (11), migrate through the MZ to the RP, ultimately re-joining the circulation (4, 5). Follicular B cells might have additional escape routes through the MZ (14). However, some work has proposed, but remains to be validated using newer imaging techniques, that efferent lymphatics exist in the spleen, originating in the WP and carrying a small fraction of lymphocytes out of the spleen via lymph (1, 17)
Pulp fiction: perceived differences and similarities between mouse and human splenic structure
While the function of specific immune cell types and regions of the spleen are largely similar between mouse and human, some fundamental differences in splenic structure and cell types are nevertheless thought to exist between mouse and human spleen (18). The lack of detailed cellular characterizations of the human spleen further complicates a proper comparison (19). Most comparisons between the two species use non-specific cellular architecture based on H&E staining or single antigenic characterization with immunohistochemistry of human spleen sections compared with multi-color immunofluorescence and multiparameter flow cytometry in mice. Some differences do arise. From early human studies, we know that the TCZ and BCZ within the human WP are organized like grapes on a vine rather than layers of follicles surrounding a central TCZ as in mouse. However, the most striking difference between mouse and human spleen is not the cells contained in or the organization of either the RP or WP, but rather the border between the two. What is called the marginal zone (MZ) in rodents is composed of multiple cellular layers. A border of MMMs surrounds the WP and a marginal sinus is formed by non-hematopoietic sinus-lining cells (5). Then a ring of MZM and DCs concentrically form around the WP (16). An innate-like B cell population exists in this area of the mouse spleen, called MZB cells, with unique functions such as circulatory migration patterns into and out of follicles for antigen delivery to follicular B cells and production of rapid, T-independent IgM during immunization (20, 21). In contrast, the region referred to as the MZ in humans is defined by H&E staining of secondary follicles but is not the RP-WP border; it contains a potentially heterogenous memory IgM+ B cell population called MZB cells that is embedded within the BCZ and is not clearly flanked by the macrophage populations observed in the mouse MZ (22). Unlike their mouse counterparts, human MZB cells show evidence of somatic hypermutation and systemic recirculation (23). Therefore, mouse and human MZB cells resemble each other in name only and might represent very different B cell populations. Future work will be needed to determine whether murine-equivalent innate-like MZB (iMZB) cells exist in humans and whether human-equivalent memory, affinity matured MZB cells adjacent to GCs exist in mice.
In humans, but not mice, the perifollicular zone is the border between the WP and RP (2); however, the immune cellular constituents of this region have not been well characterized. It is reported that MMMs do not exist in the perifollicular zone, although CD169+ macrophages have been identified in an isolated study using immunohistochemistry (24). In this region, humans have sheathed capillaries rather than a marginal sinus, as assessed by light microscopy. However, a human marginal sinus was identified using electron microscopy (25). Therefore, newer imaging and cellular characterization techniques need to be applied to the human spleen in order to reconcile these ambiguities; in particular, the function of different cells and regions of the spleen in textbooks of human anatomy rely almost completely upon work done with the mouse or other rodent spleens. Whether these perceived or real differences in cellular organization alter the initiation or execution of an immune response in the murine versus human spleen is not known. Clarifying MZ similarities and differences between mouse and human will aid in discussions of the applicability of mouse immunology research to human biology and disease.
The cellular initiators and effectors of the adaptive splenic immune response
In this section, cellular functions and organization specific to the spleen will be discussed. It should be noted that most of the work identifying cell subsets, location and function has been performed in mice and how these compare to humans remains to be clarified.
Innate immune cell function and organization
Recognition of infection or host damage in the spleen activates a plethora of pattern recognition receptors (PRRs) on myeloid cells, which in turn induce requisite T cell activation signals on antigen presenting cells (APCs), cytokine secretion, and pathogen clearance in phagocytes. PRRs include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like RNA helicases and C-type lectin receptors (CLRs). TLRs recognize extracellular or phagocytosed pathogen-associated molecular patterns (PAMPs). A number of cytosolic NLRs sense both microbial products that gain access to the cell interior (e.g., intracellular flagellin) as well as host molecules released during states of cell stress or damage (e.g., extracellular ATP) called damage-associated molecular patterns (DAMPs). A number of CLRs are used as molecular markers for specific DC subsets in the spleen, such as Langerin and DC-SIGN, and help these particular subsets recognize carbohydrate moieties and internalize pathogens such as fungi and the exoskeleton of insects. PRRs are selectively expressed on different cell types in particular regions of the spleen, helping tailor the nature of both the early innate and subsequent adaptive immune response (5, 16, 20, 26).
Splenic DC subsets
In part due to dynamic and overlapping marker expression by different DC subsets, it has historically been challenging to segregate DCs into clear lineages. Further complicating categorization is the expression of classic T cell markers by DC subsets, which has led to one of the most widely used classification systems of splenic murine DCs as CD4+, CD8+ or double negative (CD4-CD8-) DCs (27). Multiple types of myeloid cells also express CD11c without acting as classical or conventional DCs (cDCs). Discovery of particular transcription factors that define the ontogeny of murine DC and monocytic lineages has recently allowed for a clearer separation of both monocytes from DCs and of DC subsets from each other (28, 29). All DCs are derived from the bone marrow (BM) and as such must arrive at the various tissues via the blood stream; further, they have a high turnover rate (hours to a few weeks). cDCs, which make up about 3% of all CD45+ cells in the spleen, are derived from a common dendritic cell progenitor (CDP), express the transcription factor ZBTB46, and act almost exclusively as “professional” APCs (30).
cDCs can be divided into two major subsets: cDC1s and cDC2s (Table 1). All splenic cDC1s express XCR1 and most express CD8αα. Within this subset, some reside at steady state in the WP and express the lectin receptor DEC205 (31). The other cDC1s reside in the MZ and RP at steady state and express CD103 and some express Langerin (15, 27). After immunization, all cDC1s co-localize with and preferentially activate CD8+ T cells in the WP. In part, this is due to the selective ability of murine cDC1s to cross-present antigen. In contrast, all cDC2s reside in the BC at steady state and express SIRPα and most express CD11b. The chemotactic receptor EBI2, which recognizes oxysterol ligands, and S1P are important cues that localize and help maintain cDC2s at the BC (12, 32–34). Two major sub-populations of cDC2s exist in the spleen. One requires NOTCH2 and RBPJ signaling for development and expresses the adhesion molecule ESAM (Endothelial cell-selective adhesion molecule) (35–37). The ESAMhi subset also expresses CD11b, CD4 and the C-type lectin receptor DC-inhibitor receptor 2 (DCIR2) stained by 33D1, but are negative for CX3CR1 (32). Lymphotoxin B receptor (LTBR), activated by B cell-derived lymphotoxin, retinoic acid (RA), a vitamin A derivative, and the transcriptional regulators IRF4 and KLF4 play crucial roles in the development and homeostasis of this cDC2 subset (36–39). The CD4+ ESAMhi cDC2 subset excels in priming naïve CD4+ T cells. In contrast, ESAMlo cDC2s express CD11b, CX3CR1 and 33D1, but less CD4, and probably constitute the majority of double negative DCs as defined in the older classification system (30, 40, 41). Functionally, this NOTCH2- and IRF4-independent cDC2 subset seems to be more proficient at producing inflammatory cytokines such as TNFα and IL-12 (36).
Table 1. Innate immune cells and markers unique to the spleen.
Selected innate immune cells adapted to the murine spleen are shown with key markers, developmental pathways and location in the spleen at steady state. Markers in italics are expressed in subsets of the population. CSF1R (Colony stimulating factor 1 receptor)/CD115/MCSFR (Macrophage colony-stimulating factor receptor); CCR2 (Chemokine, cc motif receptor 2)/MCP-1R (Monocyte chemoattractant protein 1 receptor); XCR1 (X-C motif chemokine receptor 1); CLEC9A (C-type lectin domain containing 9A)/DNGR1 (DC NK lectin group receptor 1); DEC205/CD205; SIRPα (Signal regulatory protein alpha)/CD172α; DCIR2 (DC immunoreceptor 2)/33D1 staining/CLEC4A4 (C-type lectin domain family 4, member 4a); CX3CR1(C-X3-C motif chemokine receptor 1); (CD317/BST2 (BM stromal cell antigen 2)/PDCA1 (plasmacytoid DC Ag-1); SIGLECH (Sialic acid binding Ig-like lectin H); B220/PTPRC (Protein tyrosine phosphatase receptor type c)/LY5.
| Markers | Origin | Splenic Location |
Key transcription factors |
Function | |
|---|---|---|---|---|---|
| RP macrophage (16, 26) | CD11b+ F4/80+ CD68+ CD206+ Dectin-2+ VCAM+ | BM monocyte and fetal monocytes from yolk sac | RP | SPIC | Clearance of aged or foreign RBCs, iron recycling, heme breakdown |
| MZ macrophage (MZM) (16, 26) | CD209b/SIGN-R1+ MARCO+ SR-A+ ER-TR9+ CD68+ Dectin-2+ Tim4+ | Bone marrow monocyte | MZ | LXRα | Recognition of pathogens, activation of MZB cells |
| MZ metallophilic macrophage (MMM) (16, 26) | CD169/Siglec-1+/MOMA1 staining CD68+ | Bone marrow monocyte | At the interface of the WP and MZ | LXRα | Recognition of pathogens, speculated transfer of antigen from blood to WP |
| Tingible body macrophages (16) | MERTK+ TIM4+ F4/80−CD11b− CD68+MFG-E8+ | ? | GC DZ in B cell follicle | ? | Removal of apoptotic B cells |
| MZB cells (mouse) (20) (21) | IgM+ IgDlo CD21+CD23- CD1d+ B220lo | Transitional B cells | MZ | Aiolos | Rapid production of IgM antibodies and antigen trafficking to follicle |
| Inflammatory monocytes/TIP-DC (59) | CCR2+ CD115/CSF1R+ Ly6C+ Ly6G- F4/80+ CD11b+ MAC3+ CD11c+ | Bone marrow | Recruited to spleen from bone marrow via CCR2 | Mafb | TNF and NO production, bacterial clearance |
| cDC1(28, 30) | XCR1+ CD11c+ CD8αα+ CLEC9A/DNGR1+ DEC205+ CD207+ CD24+ CD103+ | Bone marrow CDP (common DC precursor) | RP/MZ and PALS of WP | BATF3, IRF8 | Cross-presentation, CD8+ T cell activation |
| cDC2(28, 30) | CD11c+ CD11b+ DCIR2/33D1 staining SIRPα/CD172+ ESAM+ CD4+ CX3CR1+ | Bone marrow CDP | Bridging channel | Notch2, IRF4, KLF4 | MHC II antigen processing, CD4+ T cell activation |
A major non-conventional DC subset in the spleen is plasmacytoid DCs (pDCs), which are derived from both CDPs and CLPs (common lymphoid progenitor), in particular IL-7R+ lymphoid progenitors (42). Murine pDCs are identified by expression of B220 and PDCA-1 along with lower levels of CD11c. pDCs express TLR7 and TLR9, which enables them to recognize viruses. After activation, pDCs rapidly secrete large amounts of type I interferons, IL-12 and IL-18, leading to increased Natural killer T (NKT) cell activity, apoptosis of virally infected cells, and enhanced cross-priming (for review see (43)).
Although fewer analyses of human splenic DCs exist, grossly equivalent DC subsets exist to the mouse, albeit expressing some markers unique to humans (44). cDC1s express BDCA3 (blood DC antigen 3) also known as CD141 or Thrombomodulin in addition to the overlapping XCR1 and CLEC9A/DNGR-1 markers. cDC2s express BDCA1 (blood DC antigen 1) also known as CD1c in addition to the common SIRPα expression. pDCs express BDCA2 (blood DC antigen 2) also known as CD303 along with CD304 and CD123. Differences between mouse and human DC specialization do exist, however, similar transcription factors have been identified in the mouse-equivalent DC populations and the human DC subsets have overlapping functions to their murine counterparts including cytokine production predilection (45).
Migratory and Resident Splenic DC Subsets
The functional specialization of cDC1s in CD8+ T cell priming and cDC2s in CD4+ T cell priming can only be partially explained by differences in innate immune receptors, phagocytosis pathways, and MHC class I vs. II machinery (reviewed in (46)). In vitro or even ex vivo, all DC subsets can activate CD4+ or CD8+ T cells; however, loss in vivo of cDC1s through BATF3- or IRF8-deficiency impairs primarily CD8+, but not CD4+ T cell responses, and conversely, loss of cDC2s typically impairs CD4+ but not CD8+ T cell responses (9, 28, 32, 33, 35, 47). Accumulating data from a variety of studies suggest that DC subset specificity for activating particular T cell lineages is driven by the spatiotemporal cellular organization of T cells and DC subsets (see (30) for review).
DCs collect antigen in peripheral tissues and once they receive a licensing signal, primarily from PRR activation, modulate their chemokine receptor expression and migrate through the lymphatics to LNs where they activate naïve T cells. For systemic blood-borne antigens such as transfused RBCs or bacteremia this entire process must occur in the spleen, which, unlike all other secondary lymphoid structures, does not contain afferent lymphatics (1). Because splenic cDC1s reside in the T cell zone at steady state and the architecture of the spleen juxtaposes antigen-exposed tissue (MZ and RP) directly with the lymphoid compartment (WP) it was assumed that movement of DCs within the spleen was not essential for T cell priming. Yet splenic DC migration does occur, and we are only just beginning to understand how this process is regulated and how it impacts immunity. As in the periphery, DC migration within the spleen is initiated by PRR activation (9, 48–50). Using models to disrupt the ability of splenic DCs to reposition within the spleen, we and others recently demonstrated that DC migration from the bridging channel or MZ/RP into the T cell zone of the WP is required for CD4+ T cell priming (9, 12). Again, similar to migratory DCs in the periphery, this re-localization to the WP is CCR7-dependent (9, 32). Therefore, splenic DCs mirror DCs in peripheral tissues: resident (LN/WP) and migratory (tissue/RP) DCs exist at steady state in different locations and then, upon activation, undergo chemokine-directed re-localization, which brings both resident and migratory DCs into segregated regions of the T cell zone, in this case the splenic PALS.
Macrophages
Macrophages are tissue-resident phagocytes that maintain homeostasis by clearing cell debris and regulating the function of neighboring cells, but during infection or tissue damage can respond with inflammatory cytokine responses that activate other leukocytes. Multiple subsets of macrophages exist in the spleen, each scanning distinct anatomical regions and expressing particular sets of PRRs and scavenger receptors. The four most studied subsets include the MZM, MMM, red pulp macrophages (RPM) and tingible body macrophages (see (26) for review) (Table 1). Two macrophage populations reside in the MZ at steady state, MZM and MMM, both of which clear debris including apoptotic cells via specialized sets of phagocytic receptors, share BM monocyte ontogeny and are important for the induction of tolerance to self-antigens (16). MZMs and MMMs are BM-derived and M-CSF- and LXRα-dependent cells (16); they can rapidly turn over during inflammatory states, replenished by BM-derived monocytes. MZM express MARCO and SIGN-R1 and can interact with MZB cells. In contrast, MMM express SIGLEC1/CD169 and MHC II, have processes that can penetrate into the WP from the MZ and can share antigen with DCs in the MZ (26, 51). RPMs reside throughout the RP and filter the blood of bacteria, apoptotic or fragmented cells, and other debris as well as “groom” RBCs of inappropriate inclusions such as denatured hemoglobin. RPMs are long-lived, self-renewing cells derived from a yolk sac progenitor and are M-CSF independent; however, during states of inflammation, some can also be replenished by BM-derived monocytes (52). Tingible body macrophages are B cell follicle-resident macrophages responsible for removing apoptotic B cell debris that accumulates during B cell selection (affinity maturation and class switching) in the light zone of the germinal center (26). The ontogeny of tingible body macrophages requires further investigation.
NK Cells
NK cells localize in the RP but can contribute to T cell polarization by migrating into the WP after infection and by producing IFNγ, which can also affect the early innate response by promoting TIP-DC differentiation (53, 54). Most studies into NK cell function have focused on their role in defending against viral infection (using PRRs), tumors, or damaged self-cells (using NKG2D). However, their role in regulating splenic adaptive immunity is still an area of active research (reviewed in (55)).
Monocytes
Monocytes are present in the BM, blood, and spleen, and can differentiate into multiple specialized myeloid subsets (56). Two major monocyte populations in the blood have been identified, Ly6Clo and Ly6Chi, that perform different functions– one is circulating and mobilizes to tissues when needed and the other is the patrolling monocyte whose job is to monitor the vasculature. These blood-derived monocytes, after encountering bacteria in the blood, can exit into the MZ and induce T cell-independent MZB cell responses (48). Monocytes in the spleen can also help maintain self-tolerance through the clearance of apoptotic bodies and production of immunosuppressive factors such as IDO, TGF-β and IL-10 (1). During an inflammatory response, CCR2 triggered by MCP-1 and MCP-3 (presumably provided by macrophages in the MZ) (57) also recruits Ly6Chi monocytes from the BM to the spleen. There, they can differentiate into multiple myeloid cell types, including certain types of non-conventional DCs and macrophages (58). A particular type of monocyte develops in the red pulp from these precursors, analogous to inflammatory monocytes in other tissues, CD11c+ “TIP (TNF-iNOS)-DCs”. TIP-DCs are highly inflammatory, producing TNFα and nitric oxide (NO), but are not proficient APCs for naive T cells (59); however, these inflammatory monocytes are capable of reactivating effector or memory T and NK cells (60). The spleen also acts as a reservoir for undifferentiated monocytes that can be mobilized to other organs under inflammatory conditions likely to augment the monocytes released from the BM. These reservoir monocytes are marked by CX3CR1 along with variable amounts of Ly6C and are concentrated in the subcapsular red pulp (1).
Adaptive immune cell function and organization
T and B cells, the key effectors of the adaptive immune system, are present throughout the spleen. Their localization changes with activation state and is organized by expression of cell surface receptors and chemotactic gradients. B-2, follicular B cells are the canonical T-dependent antibody-producing B cells in the spleen (61). Naïve follicular B cells reside in WP follicles, but once activated, B cells rapidly cycle between the light and dark zone of the GC. CXCR5 drives B cells to the light zone, where they receive T cell help, whereas CXCR4 drives them into the dark zone, where they undergo rapid proliferation, class switching, and somatic hypermutation (8). Epstein-Barr virus-induced gene 2 (EBI2; GPR183) is another important receptor that organizes B cells within the BCZ based on oxysterol chemoattractants (8). Following this GC reaction, some B cells depart into the BC and RP to become antibody-secreting cells. Some also become long-lived plasma cells in the RP.
Recently it was shown that CD4+ and CD8+ T cells segregate within the PALS of the murine WP at steady state and after systemic infection (9, 12, 62); whether a similar pattern exists in the human spleen is unknown. CD4+ T cells are concentrated in the outer border of the PALS, adjacent to B cell follicles. CD4+ T cells, in particular T follicular helper (Tfh) cells, are found in the splenic TCZ/PALS and provide help to B cells in the follicle for the production of high-affinity antibodies through cytokine production (e.g., IL-21) and direct co-stimulation (e.g., ICOS-ICOS ligand) (63). During an active immune response, Tfh cells upregulate CXCR5 to reach the T-B border while B cells upregulate CCR7 to move from the BCZ to the T-B border (reviewed in (30)). Oxysterols (EBI2 ligands) are also expressed at the T-B border, which recruits Tfh cells, cDCs and B cells.
In contrast, naïve CD8+ T cells reside in the central PALS of the WP, awaiting antigen presentation by APCs (64). Following priming, CD8+ T cell responses evolve during the course of splenic infection and different fates are compartmentalized (4). Once primed, activated cytotoxic T lymphocytes (CTLs) leave the WP through the BC to the MZ and RP, ultimately helping to clear the infection in the “periphery” of the spleen (RP) (4). Later, some memory CD8+ T cells return to the PALS of the WP (4, 65) while CD62L-CXCR3+ memory CD8+ T cells remain in the RP (64, 66).
Hybrid Cells: Innate-like lymphocytes
NKT cells & Gamma Delta (γδ) T cells
A specialized type of innate lymphocyte called the NKT cell expresses an invariant TCR and recognizes glycolipid antigens on the MHC class I-like molecule, CD1d. CD1d is highly expressed by MZB cells, which can activate NKT cells. Activated NKT cells produce inflammatory cytokines and can license splenic DCs for cross-priming of CD8+ T cells (67, 68). γδ T cells also exhibit innate-like function due to their lack of MHC restriction, rapid cytokine secretion, expression of PRRs, and ability to directly lyse infected cells (68, 69). γδ T cells are relatively rare in the spleen but are important for the early response to infection. NKT and γδ T cells in the spleen promote Th1 polarization and CD8+ T cell activation by producing cytokines such as IFNγ, TNFα and IL-12, which also help to activate cDC1s (70).
Innate lymphoid cells (ILCs)
ILCs are defined by their common lymphoid progenitor ontogeny and lack of RAG or T cell receptor expression; they are further sub-divided by their cytokine secretion profile (71). A vital function of ILCs is to rapidly produce cytokines in response to infection or tissue injury. Th2-like cytokines, IL-5, −9 and −13, are produced by ILC2s found in the spleen (72). ILC3s can activate MZB cells and neutrophils by producing TNFα, lymphotoxin and GM-CSF and can provide co-stimulation to splenic CD4+ T cells through production of IL-2, IL-6 and MIP1α. The role of ILCs in direct antigen presentation remains controversial (73).
Marginal zone B cells
Murine MZB cells are an innate-like B cell subset unique to the spleen, characterized by both location and function. They exhibit higher expression of PRRs than other B cells, have polyreactive B cell receptors, and rapidly produce low-affinity T cell-independent antibodies, primarily IgM (20). MZB cells originate from T1 B cells in the BM and differentiate in the spleen after receiving survival signals through BAFF (22). They are NOTCH2, RBP-J, AIOLOS, DOCK8, PI3K and CD19-dependent (21). In the absence of any of these molecules, MZB cells are significantly reduced in the spleen, while follicular B cell development remains intact (22). MZB cells capture antigen via complement receptors and migrate into the WP, both into the follicles to deliver opsonized antigen to B cells (14) and into the TCZ where they have been suggested to activate CD4+ T cells (20). This shuttling of antigen into the WP is followed by return of MZB cells to the MZ and depends on cyclical expression of S1P receptors and CXCR5 (74). Therefore, MZB cells are important in both T-dependent and T-independent antibody production. The extent to which human MZB cells map onto the above description of mouse MZB cells remains unclear.
B-1 cells
B-1 cells are another innate-like B cell; this population is most abundant in the peritoneum, but a small population also exists in the spleen. Like MZB cells, B-1 cells produce IgM antibodies, and additionally contribute to circulating “natural” antibodies (those produced without antecedent exposure to the antigen) (75). B-1 cells can be further divided into B-1a cells (CD5+, produce natural antibodies) and B-1b cells (CD5-, less common, produce T-independent antibodies and participate in memory responses). The difference in function of their produced antibodies inspired the “division of labor hypothesis” to describe the observed phenotypic differences between B-1a and B-1b cells (76). Some antibodies produced by B-1a cells are self-reactive, for example, IgM antibodies that recognize oxidized lipids and aid in removal of apoptotic cells (75). Whether B-1 cell counterparts exist in the human spleen is still under debate.
Innate control of adaptive immunity to insults in the spleen
The functional importance of the spleen in controlling systemic infections is evidenced by splenectomized patients, who show decreased clearance of malaria-parasitized RBCs and are at greater risk of meningitis and sepsis after infection with Streptococcus pneumoniae, Neisseria meningitidis, and Hemophilus influenza type B (1). This section will review how foreign pathogens or cells in the blood lead to coordinated splenic innate and adaptive immune responses, and how pathogens target immune cells to prevent linkage of the two immune arms.
The spleen in bacterial infection
Listeria monocytogenes is a Gram-positive, food-borne bacterium that, when injected intravenously in mice, provides a model pathogen for understanding splenic filtering of bacteria from blood. During physiologic infection, the pathogen is ingested and travels from the gastrointestinal tract to the liver and spleen. Since Listeria is a facultative intracellular pathogen, antibody-mediated immunity is not particularly effective for clearance. Instead, the innate branch of the immune system is crucial for controlling early infection, in particular monocyte-derived TIP-DCs, neutrophils and multiple macrophage populations (59, 77, 78). While depletion of macrophages impairs the early response to infection, it does not impair activation of T cells, which is accomplished by DCs (79). However, macrophages can facilitate the process of live Listeria delivery into DCs through transinfection of CD8+ T cells by MMMs (51). As will be discussed, this can potentiate CD8+ T cell priming, but paradoxically helps propagate the bacterial infection.
At later stages of infection, primed CD8+ cytotoxic T cells promote phagocyte clearance of bacteria, directly lyse infected cells, and prevent re-infection (80). These cytotoxic T cells are primed by cDC1s; however, cDC1s also act as a “Trojan Horse” to propagate Listeria infection in the spleen (80, 81) (Fig. 2). cDC1s harboring Listeria migrate to the TCZ, where few phagocytes or neutrophils reside at steady state, enabling the pathogen to temporarily avoid immune clearance (82, 83) although ultimately, NK cells and TIP-DCs cluster in the WP to help contain the infection (54). Thus, by co-opting cDC1s’ natural ability to hold onto intact antigens in non-acidified phagosomes and migrate to the WP, Listeria effectively utilizes the immune system itself to briefly escape the innate immune response. The importance of this mechanism in infection is highlighted by cDC1 depletion in BATF3-deficient mice, which actually confers resistance to Listeria (81).
Figure 2. The cellular organization of the murine WP is dynamic.
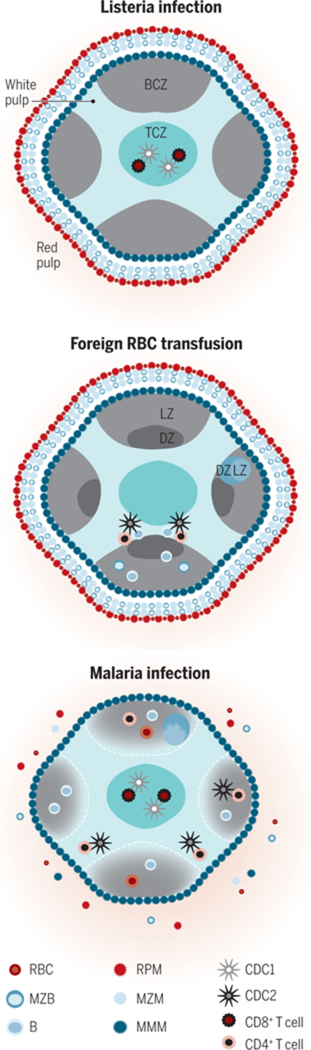
The cells of the TCZ (turquoise) are organized into CD8+ T cells (maroon) in the center and CD4+ T cells (orange) in the outer TCZ. This organization enables rapid formation of necessary cellular contacts, after migration within the WP, for the response to an immunologic insult. Shown are selected, dominant immune reactions in the spleen relevant to each insult. (A) Listeria induces rapid migration of cDC1s (silver) into the central TCZ where they present antigen and prime CD8+ T cells. (B) Transfusion of xenogeneic or allogeneic RBCs induces migration of cDC2s (black) to the outer TCZ, for CD4+ T cell priming and effective B cell antibody production (with the LZ, light zone, and DZ, dark zone, of a germinal center, GC, shown). (C) Malaria-infected RBCs induce a poorly coordinated and slow humoral immune response that includes disintegration of WP-RP border, MZ disruption and GC disorganization. After repeated infections, protective antibodies are ultimately produced.
Several innate immune mechanisms have evolved for Listeria recognition in different cellular locations. Prior to entering the cytosol, Listeria is sensed by TLR5 (for flagellin), and TLR2 (for cell wall lipoproteins) in a MyD88-dependent pathway leading to NF-kB-driven production of pro-IL-1β and TNFα (84). The presence of Listeria peptidoglycan in the cytosol activates NOD1 and NOD2 to amplify the activation signals from TLRs via RIP2, resulting in IL-6 and TNFα production (84). Other cytosolic receptors, like cGAS-STING and RIG-I, are required for sensing Listeria after escape from endosomes (85, 86). Multiple aspects of Listeria or its effect on host cells are detected by inflammasomes, which induce caspase-1 activity and IL-1β and IL-18 production (84). Loss of any one of the above innate immune pathway typically has little impact on initiation of adaptive immunity, likely because of this redundancy; however, these pathways are particularly important for innate immune cell activity including recruitment and effector function of TIP-DCs (59). The study of Listeria in the spleen highlights both the myriad of innate and adaptive effector cells in the spleen that cooperate to control a bacterial pathogen and how the pathogen circumvents many of these attempts to replicate in an SLO.
The splenic response to foreign red blood cells
Another immune response uniquely regulated by the spleen is to transfusion of foreign RBCs. Blood transfusion is a life-saving therapy, but due to the myriad of RBC non-ABO antigen polymorphisms between individuals, some patients generate a deleterious antibody response to alloantigens on non-self RBCs. The fundamental rules that govern when someone will generate an antibody response to transfused RBCs remain unclear. Identifying such rules from human studies has been challenging, and mice have a poorly understood system of blood group antigens (Ea1-Ea10); further, mice do not express the same minor antigens as human RBCs. Therefore, transgenic mice expressing well-defined foreign antigens on RBCs such as the human KEL glycoprotein, glycophorin A, or model protein antigens have recently enabled the study of alloimmunization in murine models (87). The humoral alloantigen response to transfused leukoreduced RBCs requires an intact spleen and, in some models, requires T cell help (47, 87, 88); however, certain antigens have been recently shown to induce a T-independent MZB cell antibody response (88). Although RBCs are consumed in both the spleen and liver, T cell activation does not occur in the liver (or lymph nodes) in these models (89). The spleen is the main SLO regulating this humoral response to RBCs and accordingly, splenectomized mice generally fail to generate alloantibodies following transfusion (87). Both splenic DCs and macrophages phagocytose allogeneic murine RBCs (90, 91) and because splenic macrophages clear endogenous aged RBCs, it had been assumed that macrophages also regulate the adaptive immune response (92). However, more recent studies have concluded that DCs are the primary APC for initiating the allogeneic response to RBCs (47, 93).
Historically, murine models have utilized transfusion of xenogeneic sheep RBCs rather than allogeneic RBCs to study GC responses. In contrast to the allogeneic RBC immune response in which transfused RBCs circulate for weeks, sheep RBCs are cleared within hours (32, 47). Both forms of RBCs induce DC activation and migration to the WP (32, 47, 50, 94). Interestingly, antibody generation to both types of RBCs requires the same subset of conventional DCs. Impairment of 33D1+ bridging channel cDC2s in EBI2-, IRF4-, CD47- or DOCK8-deficient mice results in impaired T-dependent antibodies to sheep RBCs or murine allogeneic RBCs (32, 33, 47, 50). More specifically, a subset of cDC2s regulated by NOTCH2 signaling likely regulates this Tfh cell-dependent antibody response (35). As either cDC1 or cDC2 subsets can activate CD4+ or CD8+ T cells in vitro (9) we searched for a mechanism that could explain this restricted in vivo DC subset requirement. We found that CD4+ T cells segregated within the WP with cDC2s and that CD8+ T cells co-localized with cDC1s (Fig. 2); disrupting this pairing selectively blocked naïve CD4+ or CD8+ T cell activation to multiple antigenic targets including sheep and murine RBCs (9, 12). The GC reaction to sheep RBCs requires EBI2 expression on both Tfh cells and cDC2s, recognizing 7α,25-dihydroxycholesterol for proper positioning, further supporting a model in which chemoattractant-driven co-localization is necessary for adaptive immune induction (12). In summary, the response to transfused RBCs demonstrates the importance of spatiotemporal cellular organization of splenic T cell and DC subsets in regulating the humoral immune response to systemic antigens.
cDC2s recognize sheep RBCs as foreign through species differences in the “don’t eat me” signal CD47, an integrin-associated protein that binds SIRPα and inhibits phagocytosis (50). DC priming of Tfh cells after recognition of CD47-deficient sheep RBCs is dependent on the integrin-signaling adapter protein, Talin-1 (95). However, the mechanism by which cDC2s sense allogeneic RBCs as foreign remains unknown. Processing and storage of RBCs for transfusion results in biochemical and morphological modifications known as the “storage lesion.” Whether this confers immunogenicity to stored RBCs (e.g., DAMP production) is controversial and requires further study (96). Nonetheless, transfusion of stored but not fresh murine RBCs induces innate cytokine production, including IL-6, which is relevant for the subsequent alloantibody response and DC activation (94). Murine RBCs lack inhibitory ligands for the B cell lectin CD22 and therefore, if they additionally contain an antigen recognized by the B cell receptor, they can directly activate B cell responses (97). Hemoglobin-derived hemin has been proposed to stimulate human monocytes to promote a regulatory T cell response to damaged RBCs (98). In contrast, heme released from lysed RBCs has been shown to induce NLRP3 activation in macrophages and pro-inflammatory cytokine production (99). However, NLRP3, caspase-1 or caspase-11 inflammasomes are not required for alloantibody production to one tested strain of transgenic RBCs (94). Although CD47 declines with aging of RBCs in vivo, it is not clear that the same is true of processed and stored RBCs (91); therefore, how transfused allogeneic RBCs trigger requisite innate immunity is a mystery that remains to be solved.
The spleen in parasitic infections
Plasmodium falciparum is a parasite that infects RBCs, causing malaria, a disease of significant morbidity and mortality (100). Both protective and harmful inflammatory responses to malaria are coordinated in the spleen that contribute to cellular and humoral parasite clearance, but also to inflammation-induced host damage (reviewed in (19)). The malaria parasite enters the human host through a mosquito vector and reproduces in the liver for 7–10 days (101). After this stage, the parasite infects and replicates inside RBCs and triggers innate and adaptive immune responses in the spleen. Although CD8+ T cell-mediated immunity can restrict infection during the non-RBC phase, infected RBCs can evade CD8+ T cell-mediated killing, because they mostly lack MHC expression. CTL control of infected reticulocytes (immature RBCs) might partially help control parasite burden (102), but antibody-mediated immunity is most efficacious at clearing parasitized RBCs (103). Mice with T cell-specific BCL6 deficiency or ICOS blockade, and therefore loss of Tfh cell differentiation, have impaired T-dependent antibody production and are unable to clear malaria infection, showing that Tfh cells are required for the protective humoral response (104, 105). Reduced antibody production in IL-12-deficient mice suggests that these CD4+ T cells are IFNγ-producing type 1 Tfh cells (106). Type 1 immunity characterizes the first erythrocytic stage of infection with elevated IL-6, IL-12, IFNγ, NO and TNFα production by both NK cells and Th1 cells (reviewed in (107)). However, induction of Th1 cells in fact might worsen symptoms during infection rather than enabling parasite clearance (108). Originally it was thought that the nature of the cellular response evolves over the course of infection to a type 2-skewed Th2-dominated response. New evidence suggests that mixed CD4+ T effector cell fates along with regulatory T cell subsets better characterize this phase of immunity (104).
The coordinated immune response to malaria involves multiple splenic myeloid populations. Work using CD11c-DTR (diphtheria toxin receptor) mice demonstrated that CD11c+ cells selectively phagocytose infected RBCs and are required for presenting parasite antigens to CD4+ T cells (109). Using methods to target subsets of DCs, it was found that the relevant APC shifts during the two stages of infection. During the early blood stage, cDC1s are required for Th1 cell priming (110). During the late blood stage, cDC2s support antigen-specific CD4+ T cell responses and T-dependent antibody production; cDC1s appear to be more sensitive to apoptosis during this later stage (111). Early in the immune response, monocytes are also recruited from the BM to the spleen in both mice and humans, where they play an important role in controlling parasite burden, but less of a role in T cell priming (112).
Multiple aspects of the malaria parasite are sensed by the splenic innate immune system. Once inside host RBCs, the pathogen uses proteases to digest hemoglobin, converting it into an inert crystal, hemozoin (100, 113). Mouse and human monocytes respond to this DAMP through the NLRP3 inflammasome, resulting in production of the fever-promoting cytokine IL-1β (107). P. falciparum DNA engages multiple PRRs including TLR9 that drives TNFα and IL-12 production by DCs and IFNγ production by NK cells through MyD88 (107), as well as cGAS and STING (114, 115). TLR2, TLR4 and TLR7 also sense different aspects of the parasite or its effect on RBCs, including glycosylphosphatidylinositols (GPIs) in the parasite membrane, heme from parasitized RBCs, and RNA (116–118). pDC activation by these PRRs induces type 1 IFN production, which facilitates cDC function (119). Possibly because numerous innate immune pathways are triggered by this parasite, deletion of individual pathways does not significantly impair the T cell response (114, 120). However, many of the cytokines important for promoting adaptive immunity or the symptoms of malaria are induced by particular PRRs or their downstream pathways and have been directly linked to APC activation (119). It is worth noting that different groups utilize different Plasmodium strains and this might favor different mechanisms of innate sensing or responses from particular sub-populations of cells (121).
Unlike many infections, malaria requires repeated or prolonged infection before protective humoral immunity is established (122). This may be due to the severe disruption of the splenic architecture induced by Plasmodium infection, including loss of the MZ, blurring of the RP-WP distinction, and disrupted GCs (114, 123) (Fig. 2). Parasitized RBCs also impair DC activation, antigen presentation and survival, possibly through the production of hemozoin, resulting in reduced T cell priming (19). However, other studies have argued that in vivo DC activation and antigen presentation is intact (124). The nature of DCs used, the methods of study, along with different Plasmodium species, may account for some of these differences. Regardless, Tfh cell induction and GC formation appear to be inhibited by Plasmodium in part through modulation of DC and other innate cell function. One proposed mechanism for blunted Tfh cell priming is type 1 IFN stimulation of DCs (105), but likely multiple pathways result in the delayed humoral response, including direct B cell effects (120). Further, CXCL10 production by inflammatory monocytes and parasite-induced IFNγ and TNFα has been associated with impaired Tfh cell differentiation, GC formation, antibody production, and an increase in parasite burden (125). Altogether these effects diminish the potentially protective humoral adaptive immune response.
The response to malaria highlights the delicate balance between redundant immune sensing and activation mechanisms in controlling systemic infection and the detrimental effect of excessive cytokine production in causing tissue damage and inefficient adaptive responses.
Conclusions and future perspectives
The intricate positioning of immune cells within the spleen and the ways in which their migration is orchestrated allows specific tailoring of the immune response to match the insult. However, we are only just beginning to identify the mechanisms controlling the location of cells, both at steady state and upon immune insult. Intravital imaging has revolutionized our understanding of how immune cells interact; however, a challenge in applying this technology to the spleen is the inability to penetrate deeply into the WP by 2-photon microscopy. Some limited glimpses of the outer border of the WP, including pDC-T cell and DC-apoptotic cell interactions in the MZ, T cell entry from the blood, as well as follicular B cell-Tfh cell interactions have been visualized (11, 14). Early neutrophil and myeloid cell responses to bacterial infection in the RP have also been visualized (77). Use of fluorescent protein-expressing Plasmodium parasites has provided insights into the three-dimensional structural changes in the spleen resulting from infection (19). Two-photon imaging ex vivo has been used to show infection of DCs by Listeria and trafficking into the CD8+ T cell dense PALS (83). However, we have not yet achieved a complete picture of how lymphocytes throughout the WP in vivo interact with each other and with other cells of the innate immune system. Despite these limitations, application of this technology holds promise for understanding single cell dynamics governing innate cellular regulation of adaptive immunity in the spleen. Finally, a concerted effort must be made to understand how different immune cell subsets are similarly or distinctly organized in the human spleen as compared to mouse.
References and notes
- 1.Bronte V, Pittet MJ, The spleen in local and systemic regulation of immunity. Immunity 39, 806–818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Krieken JH, te Velde J, Normal histology of the human spleen. Am J Surg Pathol 12, 777–785 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Nolte MA, Hoen EN, van Stijn A, Kraal G, Mebius RE, Isolation of the intact white pulp. Quantitative and qualitative analysis of the cellular composition of the splenic compartments. European journal of immunology 30, 626–634 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Khanna KM, Lefrancois L, Geography and plumbing control the T cell response to infection. Immunology and cell biology 86, 416–422 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mebius RE, Kraal G, Structure and function of the spleen. Nature reviews. Immunology 5, 606–616 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Nolte MA et al. , A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. The Journal of experimental medicine 198, 505–512 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kool M et al. , Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. Journal of immunology 181, 3755–3759 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Pereira JP, Kelly LM, Cyster JG, Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. International immunology 22, 413–419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabro S et al. , Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep 16, 2472–2485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SN, Germain RN, Stromal cell contributions to the homeostasis and functionality of the immune system. Nature reviews. Immunology 9, 618–629 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajenoff M, Glaichenhaus N, Germain RN, Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. Journal of immunology 181, 3947–3954 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu E, Dang EV, McDonald JG, Cyster JG, Distinct oxysterol requirements for positioning naive and activated dendritic cells in the spleen. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinamon G et al. , Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nature immunology 5, 713–720 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Arnon TI, Horton RM, Grigorova IL, Cyster JG, Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature 493, 684–688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu CH et al. , Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. Journal of immunology 182, 4127–4136 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Davies LC, Jenkins SJ, Allen JE, Taylor PR, Tissue-resident macrophages. Nature immunology 14, 986–995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godart SJ, Hamilton WF, Lymphatic drainage of the spleen. Am J Physiol 204, 1107–1114 (1963). [DOI] [PubMed] [Google Scholar]
- 18.Steiniger BS, Human spleen microanatomy: why mice do not suffice. Immunology 145, 334–346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrer M et al. , Imaging of the spleen in malaria. Parasitol Int 63, 195–205 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Cerutti A, Cols M, Puga I, Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature reviews. Immunology 13, 118–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillai S, Cariappa A, Moran ST, Marginal zone B cells. Annual review of immunology 23, 161–196 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Pillai S, Cariappa A, The follicular versus marginal zone B lymphocyte cell fate decision. Nature reviews. Immunology 9, 767–777 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Weller S et al. , Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104, 3647–3654 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR, The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology 92, 307–316 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt EE, MacDonald IC, Groom AC, Changes in splenic microcirculatory pathways in chronic idiopathic thrombocytopenic purpura. Blood 78, 1485–1489 (1991). [PubMed] [Google Scholar]
- 26.den Haan JM, Kraal G, Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun 4, 437–445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shortman K, Heath WR, The CD8+ dendritic cell subset. Immunol Rev 234, 18–31 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Satpathy AT, Wu X, Albring JC, Murphy KM, Re(de)fining the dendritic cell lineage. Nature immunology 13, 1145–1154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilliams M et al. , Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature reviews. Immunology 14, 571–578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenbarth SC, Dendritic cell subsets in T cell programming: location dictates function. Nature reviews. Immunology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudziak D et al. , Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Yi T, Cyster JG, EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife 2, e00757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatto D et al. , The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nature immunology 14, 446–453 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Czeloth N et al. , Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. Journal of immunology 179, 5855–5863 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Briseno CG et al. , Notch2-dependent DC2s mediate splenic germinal center responses. Proceedings of the National Academy of Sciences of the United States of America 115, 10726–10731 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis KL et al. , Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 35, 780–791 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satpathy AT et al. , Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nature immunology 14, 937–948 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tussiwand R et al. , Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 42, 916–928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beijer MR et al. , A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. European journal of immunology 43, 1608–1616 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Vander Lugt B. et al. , Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nature immunology 15, 161–167 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Thacker RI, Janssen EM, Cross-presentation of cell-associated antigens by mouse splenic dendritic cell populations. Front Immunol 3, 41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues PF et al. , Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nature immunology 19, 711–722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swiecki M, Colonna M, The multifaceted biology of plasmacytoid dendritic cells. Nature reviews. Immunology 15, 471–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittag D et al. , Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. Journal of immunology 186, 6207–6217 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Reynolds G, Haniffa M, Human and Mouse Mononuclear Phagocyte Networks: A Tale of Two Species? Front Immunol 6, 330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merad M, Sathe P, Helft J, Miller J, Mortha A, The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology 31, 563–604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calabro S et al. , Bridging channel dendritic cells induce immunity to transfused red blood cells. The Journal of experimental medicine 213, 887–896 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balazs M, Martin F, Zhou T, Kearney J, Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM, Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proceedings of the National Academy of Sciences of the United States of America 106, 1524–1529 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi T et al. , Splenic Dendritic Cells Survey Red Blood Cells for Missing Self-CD47 to Trigger Adaptive Immune Responses. Immunity, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez OA et al. , CD169(+) macrophages orchestrate innate immune responses by regulating bacterial localization in the spleen. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto D et al. , Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekiaris V et al. , Ly49H+ NK cells migrate to and protect splenic white pulp stroma from murine cytomegalovirus infection. Journal of immunology 180, 6768–6776 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Kang SJ, Liang HE, Reizis B, Locksley RM, Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity 29, 819–833 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun JC, Lanier LL, NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nature reviews. Immunology 11, 645–657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geissmann F et al. , Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia T et al. , Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. Journal of immunology 180, 6846–6853 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leon B, Lopez-Bravo M, Ardavin C, Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Serbina NV, Shi C, Pamer EG, Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Adv Immunol 113, 119–134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soudja SM, Ruiz AL, Marie JC, Lauvau G, Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allman D, Pillai S, Peripheral B cell subsets. Current opinion in immunology 20, 149–157 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eickhoff S et al. , Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell 162, 1322–1337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi H, T follicular helper cells in space-time. Nature reviews. Immunology 16, 612–625 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Sharma N, Benechet AP, Lefrancois L, Khanna KM, CD8 T Cells Enter the Splenic T Cell Zones Independently of CCR7, but the Subsequent Expansion and Trafficking Patterns of Effector T Cells after Infection Are Dysregulated in the Absence of CCR7 Migratory Cues. Journal of immunology 195, 5227–5236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM, Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. Journal of immunology 185, 5315–5325 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu JK, Kagari T, Clingan JM, Matloubian M, Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proceedings of the National Academy of Sciences of the United States of America 108, E118–127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semmling V et al. , Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nature immunology 11, 313–320 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB, The burgeoning family of unconventional T cells. Nature immunology 16, 1114–1123 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Vantourout P, Hayday A, Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature reviews. Immunology 13, 88–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leslie DS et al. , CD1-mediated gamma/delta T cell maturation of dendritic cells. The Journal of experimental medicine 196, 1575–1584 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eberl G, Colonna M, Di Santo JP, McKenzie AN, Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker JA, Barlow JL, McKenzie AN, Innate lymphoid cells--how did we miss them? Nature reviews. Immunology 13, 75–87 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Bar-Ephraim YE, Mebius RE, Innate lymphoid cells in secondary lymphoid organs. Immunol Rev 271, 185–199 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Cinamon G, Zachariah MA, Lam OM, Foss FW Jr., Cyster JG, Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature immunology 9, 54–62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baumgarth N, The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology 11, 34–46 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Haas KM, Poe JC, Steeber DA, Tedder TF, B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7–18 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Waite JC et al. , Dynamic imaging of the effector immune response to listeria infection in vivo. PLoS Pathog 7, e1001326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Witter AR, Okunnu BM, Berg RE, The Essential Role of Neutrophils during Infection with the Intracellular Bacterial Pathogen Listeria monocytogenes. Journal of immunology 197, 1557–1565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aichele P et al. , Macrophages of the Splenic Marginal Zone Are Essential for Trapping of Blood-Borne Particulate Antigen but Dispensable for Induction of Specific T Cell Responses. The Journal of Immunology 171, 1148–1155 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Qiu Z, Khairallah C, Sheridan BS, Listeria Monocytogenes: A Model Pathogen Continues to Refine Our Knowledge of the CD8 T Cell Response. Pathogens 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edelson BT et al. , CD8alpha(+) dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity 35, 236–248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neuenhahn M et al. , CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25, 619–630 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Aoshi T et al. , Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity 29, 476–486 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Eitel J, Suttorp N, Opitz B, Innate immune recognition and inflammasome activation in listeria monocytogenes infection. Front Microbiol 1, 149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdullah Z et al. , RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. The EMBO journal 31, 4153–4164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansen K et al. , Listeria monocytogenes induces IFNbeta expression through an IFI16-, cGAS- and STING-dependent pathway. The EMBO journal 33, 1654–1666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hendrickson JE, Eisenbarth SC, Tormey CA, Red blood cell alloimmunization: new findings at the bench and new recommendations for the bedside. Current opinion in hematology 23, 543–549 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Patel SRGD, Girard-Pierce K, et al. , Marginal Zone B Cells Induce Alloantibody Formation Following RBC Transfusion. Front. Immunol. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hendrickson JE et al. , The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion 49, 1678–1684 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC, Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood 110, 2736–2743 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Larsson A, Hult A, Nilsson A, Olsson M, Oldenborg PA, Red blood cells with elevated cytoplasmic Ca(2+) are primarily taken up by splenic marginal zone macrophages and CD207+ dendritic cells. Transfusion 56, 1834–1844 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Swierkosz JE, Rock K, Marrack P, Kappler JW, The role of H-2 linked genes in helper T-cell function. II. Isolation on antigen-pulsed macrophages of two separate populations of F1 helper T cells each specific for antigen and one set of parental H-2 products. The Journal of experimental medicine 147, 554–570 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richards AL, Sheldon K, Wu X, Gruber DR, Hudson KE, The Role of the Immunological Synapse in Differential Effects of APC Subsets in Alloimmunization to Fresh, Non-stored RBCs. Front Immunol 9, 2200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gibb DR et al. , The Nlrp3 Inflammasome Does Not Regulate Alloimmunization to Transfused Red Blood Cells in Mice. EBioMedicine 9, 77–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J, Wu H, An J, Ballantyne CM, Cyster JG, Critical role of integrin CD11c in splenic dendritic cell capture of missing-self CD47 cells to induce adaptive immunity. Proceedings of the National Academy of Sciences of the United States of America 115, 6786–6791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belpulsi D, Spitalnik SL, Hod EA, The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus 15, 112–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spiller F, Nycholat CM, Kikuchi C, Paulson JC, Macauley MS, Murine Red Blood Cells Lack Ligands for B Cell Siglecs, Allowing Strong Activation by Erythrocyte Surface Antigens. Journal of immunology 200, 949–956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong H, Bao W, Friedman D, Yazdanbakhsh K, Hemin controls T cell polarization in sickle cell alloimmunization. Journal of immunology 193, 102–110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutra FF et al. , Hemolysis-induced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of America 111, E4110–4118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller LH, Ackerman HC, Su XZ, Wellems TE, Malaria biology and disease pathogenesis: insights for new treatments. Nature medicine 19, 156–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT, The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell host & microbe 22, 232–245 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Junqueira C et al. , Cytotoxic CD8+ T cells recognize and kill Plasmodium vivax–infected reticulocytes. Nature medicine 24, 1330–1336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teo A, Feng G, Brown GV, Beeson JG, Rogerson SJ, Functional Antibodies and Protection against Blood-stage Malaria. Trends Parasitol 32, 887–898 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Perez-Mazliah D, Langhorne J, CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front Immunol 5, 671 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sebina I et al. , IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection. PLoS Pathog 12, e1005999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su Z, Stevenson MM, IL-12 Is Required for Antibody-Mediated Protective Immunity Against Blood-Stage Plasmodium chabaudi AS Malaria Infection in Mice. The Journal of Immunology 168, 1348–1355 (2002). [DOI] [PubMed] [Google Scholar]
- 107.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT, Innate sensing of malaria parasites. Nature reviews. Immunology 14, 744–757 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Franklin BS et al. , MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes and infection / Institut Pasteur 9, 881–890 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Borges da Silva H. et al. , In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 11, e1004598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Draheim M et al. , Profiling MHC II immunopeptidome of blood‐stage malaria reveals that cDC1 control the functionality of parasite‐specific CD4 T cells. EMBO molecular medicine 9, 1605–1621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sponaas AM et al. , Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. The Journal of experimental medicine 203, 1427–1433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Antonelli LR et al. , The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog 10, e1004393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olivier M, Van Den Ham K., Shio MT, Kassa FA, Fougeray S, Malarial pigment hemozoin and the innate inflammatory response. Front Immunol 5, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hahn WO et al. , cGAS-mediated control of blood-stage malaria promotes Plasmodium-specific germinal center responses. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharma S et al. , Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35, 194–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baccarella A, Fontana MF, Chen EC, Kim CC, Toll-like receptor 7 mediates early innate immune responses to malaria. Infection and immunity 81, 4431–4442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Figueiredo RT et al. , Characterization of heme as activator of Toll-like receptor 4. The Journal of biological chemistry 282, 20221–20229 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Durai P, Govindaraj RG, Choi S, Structure and dynamic behavior of Toll-like receptor 2 subfamily triggered by malarial glycosylphosphatidylinositols of Plasmodium falciparum. FEBS J 280, 6196–6212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu X et al. , Cross-Regulation of Two Type I Interferon Signaling Pathways in Plasmacytoid Dendritic Cells Controls Anti-malaria Immunity and Host Mortality. Immunity 45, 1093–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.James KR et al. , IFN Regulatory Factor 3 Balances Th1 and T Follicular Helper Immunity during Nonlethal Blood-Stage Plasmodium Infection. Journal of immunology 200, 1443–1456 (2018). [DOI] [PubMed] [Google Scholar]
- 121.Wu J et al. , Strain-specific innate immune signaling pathways determine malaria parasitemia dynamics and host mortality. Proceedings of the National Academy of Sciences 111, E511–E520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weiss GE et al. , The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog 6, e1000912 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carvalho LJ, Ferreira-da-Cruz MF, Daniel-Ribeiro CT, Pelajo-Machado M, Lenzi HL, Germinal center architecture disturbance during Plasmodium berghei ANKA infection in CBA mice. Malar J 6, 59 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ing R, Segura M, Thawani N, Tam M, Stevenson MM, Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. Journal of immunology 176, 441–450 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Obeng-Adjei N et al. , Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep 13, 425–439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


