Abstract
Objectives
The aim of this study was to explore sodium taurocholate co‐transporting polypeptide (NTCP) exerting its function with hepatitis B virus (HBV) and its targeted candidate compounds, in HBV therapy.
Materials and methods
Identification of NTCP as a novel HBV target for screening candidate small molecules, was used by phylogenetic analysis, network construction, molecular modelling, molecular docking and molecular dynamics (MD) simulation. In vitro virological examination, q‐PCR, western blotting and cytotoxicity studies were used for validating efficacy of the candidate compound.
Results
We used the phylogenetic analysis of NTCP and constructed its protein–protein network. Also, we screened compounds from Drugbank and ZINC, among which five were validated for their authentication in HepG 2.2.15 cells. Then, we selected compound N4 (azelastine hydrochloride) as the most potent of them. This showed good inhibitory activity against HBsAg (IC 50 = 7.5 μm) and HBeAg (IC 50 = 3.7 μm), as well as high SI value (SI = 4.68). Further MD simulation results supported good interaction between compound N4 and NTCP.
Conclusions
In silico analysis and experimental validation together demonstrated that compound N4 can target NTCP in HepG2.2.15 cells, which may shed light on exploring it as a potential anti‐HBV drug.
Introduction
Hepatitis B virus (HBV) is a member of the hepadnaviridae virus family, whose replication is not directly cytopathic 1. However, HBV infection, a global health problem, can lead to a wide spectrum of liver diseases ranging from acute to chronic viral hepatitis, which often develops into cirrhosis and hepatocellular carcinoma (HCC) 2. The China National Hepatitis Sero‐epidemiological Survey found that prevalence of HBsAg in the population aged 1–59 years was 9.8% in 1992. Based on this, 120 million people have been estimated to be carrying HBsAg, 20 million suffer from chronic hepatitis B and almost 300,000 die annually from chronic consequences of HBV infection 3.
Despite its enormous medical and social relevance, progress in HBV research has been impeded by lack of understanding of HBV cell‐entry by which the virus specifically infects human liver cells. HBV is an enveloped virus with a small genome −3.2 kb – of partially double‐stranded DNA encoding four overlapping reading frames. HBV has three envelope proteins, small (S), middle (M) and large (L), which are multiple transmembrane spanners sharing the same C‐terminal domain corresponding to the S protein, but differing at their N‐terminal domains 4, 5, 6. Pre‐S1 domain of the L protein is a key determinant for entry of HBV and can mediate viral interaction with hepatocyte receptors. HBV polymerase carries out primary enzymatic functions of viral replication, and it has been the main target of anti‐HBV drug development 7. So far, only a few agents have been approved for clinical treatment of HBV infection including two formulations of interferon (interferon α and pegylated interferon α‐2a) and five nucleos(t)ide analogues (lamivudine, adefovir, entecavir, telbivudine and tenofovir), all of which have apparent limits for treating HBV 8, 9, 10, 11, 12, 13, 14.
Although several HBV receptor candidates have been previously reported, none has been confirmed to be functional in supporting viral infection, until the inspiring finding of NTCP, which was reported for the first time as a new functional receptor for human HBV. Using a synthetic modified peptide as a probe as well as employing biochemical approaches and virological assays has confirmed NTCP to interact specifically with L proteins of HBV, functioning as a common receptor for the virus 15, 16. As NTCP plays a role in the HBV infection process, it was assumed that it could be a new target for HBV therapy, which, up to now to the best of our knowledge, has not been used in development of novel classes of anti‐HBV agents for chemotherapy of HBV infection.
Thus, in this study, we used in silico analysis and experimental validation of candidate compounds targeting sodium taurocholate co‐transporting polypeptide (NTCP) in HBV therapy. First, based on nucleotide sequence information, we established an evolutionary relationship of NTCP between human and other species under the guidance of phylogenetic analysis and sequences alignments. We also constructed the human protein–protein interaction (PPI) network and modified it into a NTCP‐related context. Subsequently, we screened five small molecule compounds (compound N1–compound N5) by molecular modelling, and performed several experiments including MTT and enzyme‐linked immunosorbent assay (ELISA) protocols to validate its authentication. Finally, we verified compound N4 (azelastine hydrochloride) as a new lead “best match” compound for targeting NTCP, which shed new light on HBV therapy with regard to the new target of NTCP.
Materials and methods
Phylogenetic analysis and sequence alignment
The corresponding phylogenetic tree was constructed with Molecular Evolutionary Genetics Analysis (MEGA5) software and neighbour‐joining method with default parameters 17. For sequence alignment, all reported sequences of NTCP from different species were collected from NCBI databases. These were then aligned using the Clustal W (version 2.1) program 18. Reliability of each branch was evaluated with the bootstrap method (1000 times repeat) and finally, the phylogenetic tree was visualized using TreeView program 19.
Data processing and network construction
To construct the global human PPI network, we collected diverse PPIs from 6 human PPI databases, Human Protein Reference Data base (HPRD) 20, Biomolecular Object Network Databank (BOND) 21, IntAct 22, HomoMINT 23, BioGRID 24 and Database of Interacting Proteins (DIP) 25. Protein interaction pairs from different sources were integrated to generate initial PPI networks 26. Subsequently, we built the NTCP PPI network according to the condition that there is at least one protein, by Gene Ontology (GO) consortium, which can interact with NTCP.
Modelling and molecular docking
Three‐dimensional structure of sodium taurocholate co‐transporting polypeptide (NTCP_HUMAN) was acquired by modelling, and then it was constructed using SWISS‐MODEL server with the structure of NTCP (PDB ID: 32OY) as template 27. Initial three‐dimensional geometric coordinates of the X‐ray crystal structure of NTCP was retrieved from the Protein Data Bank (http://www.pdb.org/) (PDB ID). Then, FDA‐approved small molecule compounds from the latest version of DrugBank (http://www.drugbank.ca/) and commercially available compounds from the latest version of ZINC database (http://zinc.docking.org/) were downloaded to construct the screening library for NTCP. Subsequently, all these candidate compounds were subjected to Open Babel tool box and ZINC database to launch structure editing 28. In addition, we used UCSF DOCK6.3 program with AMBER force‐field parameters to dock pre‐generated conformations of drugs to NTCP for virtually screening their inhibitors 29. We performed flexible‐ligand docking to a rigid receptor with grid‐based scoring, in which drugs were allowed to be flexible and structurally rearranged in response to NTCP. Subsequently, amber scoring function in DOCK6.3 was used to re‐rank the top 5 small molecule compounds (compounds N1–N5) from the previous grid‐based scoring. During amber score calculation, PDB2PQR server 30 was utilized to automate PDB file preparation and protonation state assignments of NTCP. In our docking processes, maximum number of orientations was set at 500.
Molecular dynamic (MD) simulations
MD simulations were performed with the GROMACS (version 4.5.5) software package 31 to monitor binding states between NTCP and the selected compounds. The top 1 scored compound from FDA‐approved drugs was subjected to PRODRG2 server 32 to generate ligand topologies. Topology of NTCP was edited by Amber force field 99SB and the small molecule was edited by Amber general force field. In this MD process, 200 ps simulations with a time step of 10 ns were performed, and resulting trajectory files were viewed and analysed using VMD software 33.
Cell culture
Cells of the HepG2.2.15 line were provided by Medical Sciences Center of West China, Sichuan University. They were cultured in DMEM with 10% foetal bovine serum (containing 100 U/ml penicillin, 100 U/ml streptomycin, 3% glutamine and 380 μg/ml G418) maintained at 37 °C with 5% CO2 in a humidified atmosphere.
Chemical agents
All small molecule compounds from Drugbank were commercially available, and purity of these compounds was assessed on the basis of analytical HPLC, with results >95%.
Analysis of cytotoxicity
Cytotoxicity of all five compounds was assessed by MTT assay. The compounds, dissolved in DMSO, were serially diluted in cell culture media then added to cell monolayers. The HepG2.2.15 cells were maintained in 96‐well tissue culture plates for 48 h before being treated with the compounds, and subsequently cells were incubated at 37 °C for 9 days. Cells with media alone were used as blank control, while adefovir was used as positive control. MTT reagents were added at 5 g/l, 4 h before cells were harvested and lysed with 10% sodium dodecyl sulphate (SDS) and 50% N, N‐dimethylformamide (DMF) at pH 7.2. OD values of cell lysates were read at 570 nm, and percentages of dead cells were determined 34.
Determination of HBsAg and HBeAg levels by ELISA
HBV antigen and HBV DNA inhibitory efficacies of these compounds were evaluated in the cultured HepG2.2.15 cells. Nine days after treatment with different doses of the compounds, cell culture supernatants were collected and HBV antigen activities were tested by ELISA 35.
qPCR and western blot
Cell supernatants were collected from 9 days culture after compounds had been added. HBV DNA present was quantified using fluorescence PCR with HBV DNA PCR kit (Shanghai Kehua, Shanghai, China). Briefly, 50 μl supernatant was added to extraction buffer, boiled for 10 min, centrifuged for 5 min, then aliquots were used for fluorescence PCR. PCR primers were: P1: 5′‐ATCCTGCTGCTATGCCTCATCTT‐3′, P2: 5′‐ACAGTGGGGAAAGCCCTACGAA‐3′, probe being 5′‐TGGCTAGTTTACTAGTGCCATTTTG‐3′. PCR reaction was run at MJ Research PTC‐200, and results were analysed using software OpticonMonitor v2.01 (Bio‐Rad Laboratories, Inc, Berkeley, CA, USA). Cells were treated with N4, adefovir and tenofovir. Both adherent and floating cells were collected, then western blot analysis was carried out by standard methods described previously.
Statistical analysis
Results are summarized from three independent experiments and presented as mean ± SD.
Results
Phylogenetic analysis of NTCP
To trace the evolutionary relationship of NTCP between humans and other species, we constructed a phylogenetic tree of mouse, rat, rabbit and cow, by MEGA5 software with the neighbour‐joining method as shown in Fig. 1. The phylogenetic tree might serve as framework to show genetic diversity and distance in different biological species, and other entities. We found that NTCP in human had a close relationship to that of rat and mouse, and their bootstrap was very high. Meanwhile, NTCP in humans can also be grouped together with NTCP2 in mouse/rat/crigr/rabbit/human, SOAT in rat/mouse/cow/human, NTCP4 in human/rat, NTCP5 in mouse/rat/human, P3 in human/mouse/cow. Besides, BMR1A in human has served as the root of this tree. To further characterize sequence information in one cluster which included NTCP, a variety of sequence alignments was utilized as shown in Fig. S1. Amino acid sequences of NTCP shared significant similarities, namely residues in mouse, rat and human, further demonstrating close relationships between these three species. Therefore, based on aforementioned studies, we considered NTCP to be shared by a limited number of species, showing the conserved sequence of NTCP.
Figure 1.
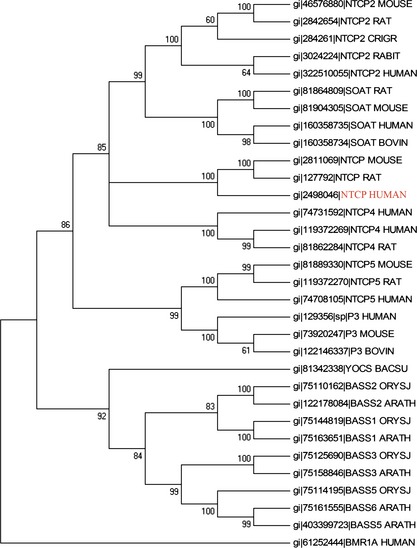
Phylogenetic analysis of NTCP and its relevant sequences.
Construction of the NTCP network
In this study, we computationally constructed the global human PPI network (in the order of 85,806 protein pairs) covering almost all PPIs, based on InAct, HPRD, Homo‐MINT, BOND, BioGRID and DIP (Table S1). To construct the set of true‐positive gene pairs, physical PPIs were derived from these human PPI databases that include 37,710 protein pairs (8982 proteins) from Bio‐GRID, 8044 protein pairs (4073 proteins) from BOND, 14892 protein pairs (6240 proteins) from HomoMINT, 39,044 protein pairs (9614 proteins) from HPRD, 34,935 protein pairs (8849 proteins) from IntAct and 12,809 protein pairs (4818 proteins) from DIP. These results show that a total number of unique 340 protein pairs were prepared in the NTCP‐related network (Fig. 2).
Figure 2.
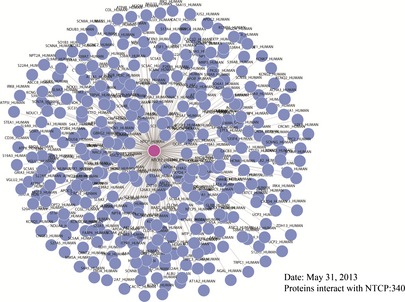
PPI network of NTCP .
Molecular docking of NTCP for screening candidate anti‐HBV small molecules
For further docking studies, various FDA‐approved small molecule drugs were subjected to virtual screening with molecular docking simulations, and 200 top‐scored drugs, first ranked by grid‐based scoring, were then selected for further re‐ranking by amber docking. After two phases of docking filtering, we identified 30 top‐scored drugs as initial hits (Table S2). Then, we illustrated candidate compounds as shown in Fig. 3. Similarly, we screened candidate compounds from ZINC and also achieved 30 top‐scored compounds as candidate drugs that bind to NTCP (Table S3). We also picked representative small molecules as shown in Fig. 4. Chemical structures, corresponding grid scores and amber scores of the newly identified 5 inhibitors, with lower scores indicating stronger binding interaction. To the best of our knowledge, these agents have not previously been used to target NTCP in HBV therapy.
Figure 3.
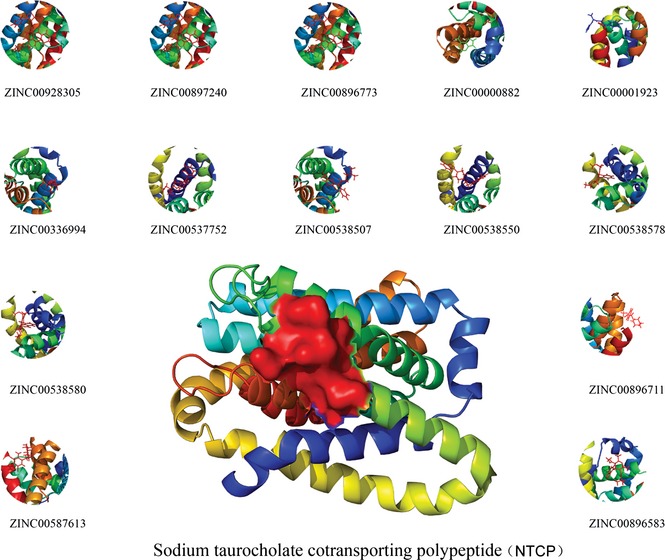
Top 14 candidate compounds targeting NTCP from Drugbank.
Figure 4.
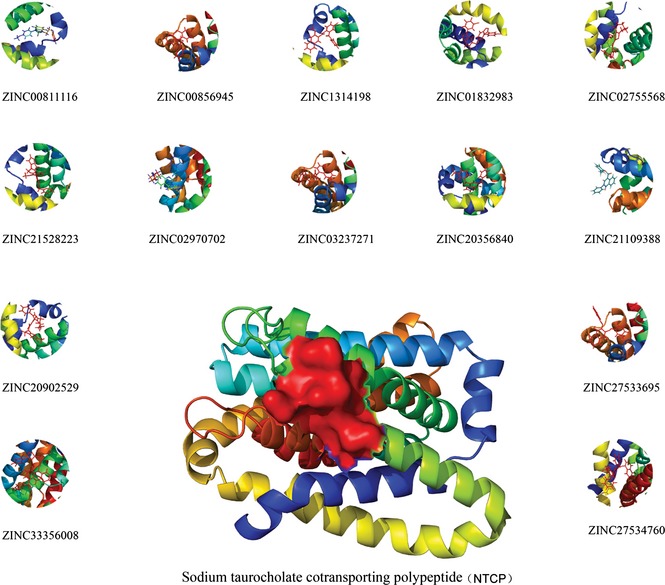
Top 14 candidate compounds targeting NTCP from ZINC .
In vitro virological examination
According to molecular docking of NTCP results, we selected five top‐scored compounds from the FDA database for in vitro virological examination. Potential anti‐HBV activity and cytotoxicity of these compounds were tested in the HepG2.2.15 cells, and adefovir was used as reference antiviral drug. Results are summarized in Table 1. Anti‐HBV activity of each compound was expressed as concentration of compound to achieve 50% inhibition (IC50) of HBsAg and HBeAg. Cytotoxicity of each compound was expressed as concentration of compound required to kill 50% (CC50) HepG 2.2.15 cells. Selectivity index (SI), an important pharmaceutical parameter to estimate possible future clinical development, was applied to determine ratio of CC50 value to IC50 value. Bioactivity of each compound was evaluated by combination of its IC50 values and SI.
Table 1.
Anti‐HBV activity and cytotoxicity of the targeted small molecule candidate compounds
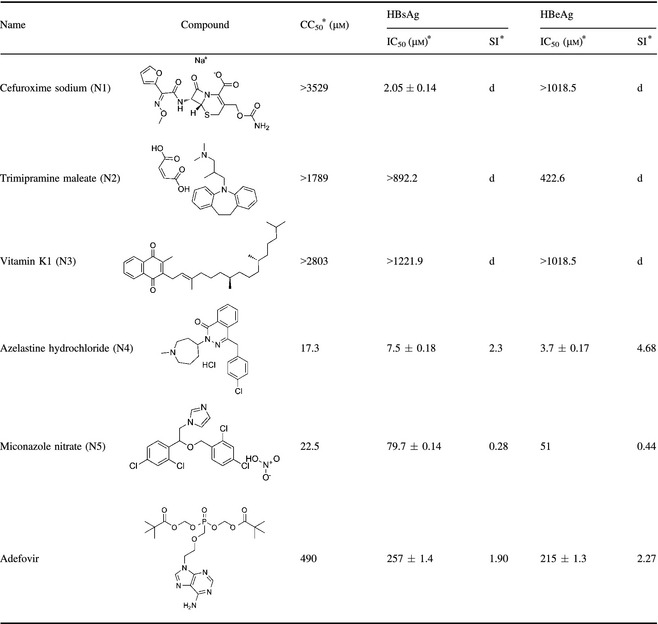
*CC50 is 50% cytotoxicity concentration in HepG2 2.2.15 cells. IC50 is 50% inhibitory concentration. Selectivity index (SI = CC50/IC50). No SI can be obtained.
In virological examination, all compounds N1‐N5, from the FDA database were identified as holding different structures. As shown in Table 1, these compounds generally exhibited moderate to good anti‐HBV potency. Compound N1 (cefuroxime sodium) was almost non‐toxic to the HepG2.2.15 cells (CC50 > 3529 μm), showing good inhibitory activity against HBsAg (IC50 = 2.05 μm), but inhibitory activity against HBeAg was almost zero (IC50 > 1018.5 μm). Compound N2 (trimipramine maleate) and compound N3 (vitamin K1) had poor inhibitory activity to both HBsAg and HBeAg. Compound N4 (azelastine hydrochloride) and compound N5 (Miconazole nitrate), showed excellent inhibitory activity to HBsAg and HBeAg, more potent than the referrence drug, adefovir. However, they were toxic with CC50 value of 17.3 and 22.5 μm, respectively. It is worth noting that compound N4 was the most potent of the five compounds with high SI value (SI = 4.68), suggesting that it would be a good starting point for further optimization (Table 1).
Molecular dynamic (MD) simulations of “best match” small molecules binding to NTCP
MD simulations of the compound azelastine hydrochloride (N4) and corresponding NTCP were carried out, to further confirm precise binding mechanisms and interaction stability. Root mean square deviation (RMSD) is an important parameter to evaluate system stability during MD simulations. As shown in Fig. 5, over 1100 ps dynamics, after rapid increase during the first 100 ps and 600 ps of NTCP and N4 respectively, and backbone RMSD and standard deviation of NTCP over the last 1100 ps is 12 Å, revealing that the NTCP‐N4 complex is stable after a period of dynamic variation. Based on results of MD simulations, compound N4 directly targetted the ATP‐binding site of NTCP and interacted with NTCP stability. Considering that NTCP is involved in HBV infection, it is reasonable to speculate that compound N4 targeting NTCP is a promising clinical agent for anti‐HBV drug discovery.
Figure 5.
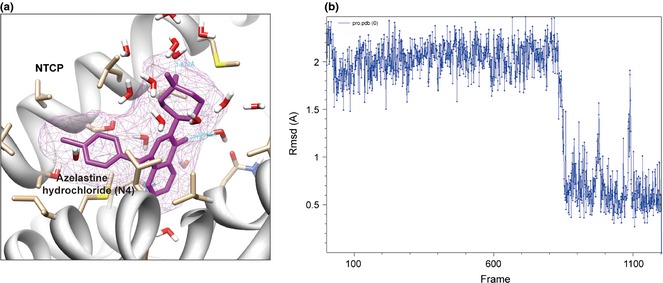
Molecular modelling and molecular dynamics ( MD ) simulation of azelastine hydrochloride (N4).
Anti‐HBV effects and NTCP regulation by azelastine hydrochloride (N4)
To further evaluate anti‐HBV activity of the drug, we detected expression level of HBV DNA in cells by using qPCR techniques. HBV DNA was extracted and analysed by qPCR using an HBV DNA PCR kit. Data are expressed as mean ± SD. As shown in Table 2, results demonstrated that titre of HBV DNA within cells was reduced markedly in those that had received N4. In addition, they demonstrated that N4 inhibited HBV DNA replication. An HBV ELISA kit was used to test concentration of HbsAg and HBeAg, results in shown in Table 3. When treating with N4, concentration of HbsAg and HbeAg was 355 ± 73 pg/ml and 9.2 ± 0.78 ng/ml. Compared to positive control, data pertaining to adefovir were 571 ± 47 pg/ml and 12.14 ± 1.33 ng/ml. In addition, we found that N4 inhibited expression of NTCP in the HepG2.2.15 cells, whereas adefovir and tenofovir did not affect it, suggesting that N4 could, indeed be a potential anti‐HBV drug to target NTCP (Fig. 6).
Table 2.
HBV DNA measured by qPCR
| Compound | HBV DNA level (AU) |
|---|---|
| Control | 6.42 ± 1.56 |
| N4 | 3.14 ± 1.74 |
| Adefovir | 4.12 ± 1.27 |
Table 3.
N4 and Adefovir by ELISA
| Compound | HBsAg (pg/nml) | HBeAg (ng/ml) |
|---|---|---|
| Control | 672 ± 46 | 15.12 ± 2.56 |
| N4 | 355 ± 73 | 9.2 ± 0.78 |
| Adefovir | 571 ± 47 | 12.14 ± 1.33 |
ELISA, enzyme‐linked immunosorbent assay.
Figure 6.
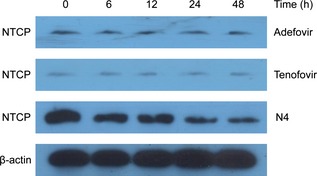
NTCP expression in N4‐treated, adefovir‐treated and tenofovir‐treated HepG2.2.15 cells.
Discussion
As the first functional receptor for HBV, NTCP can specifically interact with a key region in the pre‐S1 domain of HBV envelope L protein. In our study, through phylogenetic analysis and sequence alignment of NTCP, we found NTCP to be functionally conserved in mammals, but protein sequences of NTCP vary between species, which is likely to contribute to the narrow species tropism of viral infection. We also constructed the NTCP‐related PPI network. In a previous study by our group, we found the whole PPI network involved in many existing and potential HBx‐interacted proteins such as APOA1, APOA2 and APOA3. Apolipoprotein A‐I (APOA1), as the identified HBx‐binding partner, plays a significant inhibitory effect on HBV secretion, indicating the crucial role of the HBx–APOA‐1 axis in the HBV life cycle 36. Unlike HBx, which is well characterized as an important determinant mediating pathological effects of HBV by interacting with various cell proteins, the newly discovered functional receptor NTCP has a rather small number of interaction proteins and the network is not yet mature. However, we believe that with further development of study on the NTCP‐APOA1‐HBx axis, there will be more key proteins found in this network.
We also screened the top 30 small molecule compounds from Drugbank and ZINC, then picked 5 inhibitors (compound N1‐compound N5) with lowest scores, meaning stronger binding interactions. Under in vitro virological examination and in vitro cytotoxicity study of target compounds, we selected the most competent – compound N4 (azelastine hydrochloride). Compared to former FDA‐approved drugs for treating HBV (which only affect HBV polymerase due to relatively available few targets for antiviral development), azelastine hydrochloride was the direct target of NTCP in the anti‐HBV process. Previously, due to lack of specific targets, drugs usually provided little help in the HBV treatment 37.
Expression and subcellular distribution of NTCP are precisely regulated under physiological conditions, while NTCP expression is low and inversely correlated to degree of differentiation of cancer cells in human hepatocellular carcinoma, and severity of HBV‐related liver cirrhosis 38, 39, 40. The newly discovered role of NTCP as an entry receptor for HBV, raises interesting questions regarding its involvement in viral pathogenesis 41, 42. In silico analysis and experimental validation of this candidate compound N4, targeting functional receptor NTCP, can advance our understanding of how such small molecule compounds target NTCP in the host cell, which will lead to novel prevention and therapeutic strategies against HBV.
Supporting information
Fig. S1. Sequence alignment of NTCP from human, rat and mouse.
Table S1. NTCP and its interactors.
Table S2. Top 30 results of candidate compounds targeting NTCP from Drugbank.
Table S3. Top 30 results of candidate compounds targeting NTCP from ZINC.
Acknowledgements
We are grateful to Prof. Canhua Huang and Dr. Bo Liu (Sichuan University) for their critical reviews on this manuscript. This work was supported by grants from the Key Projects of the National Science and Technology Pillar Program (No. 2012BAI30B02), National Natural Science Foundation of China (Nos. U1170302, 81160543, 81260628, 81303270 and 81172374), and Shenyang Science and Technology Project (F12‐157‐9‐00).
References
- 1. Liu B, Wen X, Huang CH, Wei YQ (2013) Unraveling the complexity of hepatitis B virus: from molecular understanding to therapeutic strategy in 50 years. Int. J. Biochem. Cell Biol. 45, 1987–1996. [DOI] [PubMed] [Google Scholar]
- 2. Fattovich G, Bortolotti F, Donato F (2008) Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J. Hepatol. 48, 335–352. [DOI] [PubMed] [Google Scholar]
- 3. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F et al (2013) Reprint of: epidemiological serosurvey of Hepatitis B in China – declining HBV prevalence due to Hepatitis B vaccination. Vaccine 31, J21–J28. [DOI] [PubMed] [Google Scholar]
- 4. Zoulim F (2005) New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J. Hepatol. 42, 302–308. [DOI] [PubMed] [Google Scholar]
- 5. Liang TJ (2009) Hepatitis B: the virus and disease. Expert Rev. Anti. Infect. Ther. 7, 309–320.19344244 [Google Scholar]
- 6. Urban S, Schulze A, Dandri M, Petersen J (2010) The replication cycle of hepatitis B virus. J. Hepatol. 52, 282–284. [DOI] [PubMed] [Google Scholar]
- 7. Kao JH (2008) Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev. Gastroenterol. Hepatol. 2, 553–562. [DOI] [PubMed] [Google Scholar]
- 8. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G et al (2005) Peginterferon Alfa‐2a, lamivudine, and the combination for HBeAg‐positive chronic hepatitis B. N. Engl. J. Med. 352, 2682–2695. [DOI] [PubMed] [Google Scholar]
- 9. Schiff ER, Dienstag JL, Karayalcin S, Grimm IS, Perrillo RP, Husa P et al (2003) Lamivudine and 24 weeks of lamivudine/interferon combination therapy for hepatitis B e antigen‐positive chronic hepatitis B in interferon nonresponders. J. Hepatol. 38, 818–826. [DOI] [PubMed] [Google Scholar]
- 10. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M et al (2005) Long‐term therapy with adefovirdipivoxil for HBeAg‐negative chronic hepatitis B. N. Engl. J. Med. 352, 2673–2681. [DOI] [PubMed] [Google Scholar]
- 11. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC et al (2006) A comparison of entecavir and lamivudine for HBeAg‐positive chronic hepatitis B. N. Engl. J. Med. 354, 1001–1010. [DOI] [PubMed] [Google Scholar]
- 12. Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL et al (2009) 2‐Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 136, 486–495. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi K, Katano Y, Takeda Y, Honda T, Ishigami M, Itoh A et al (2007) Comparison of hepatitis B virus subgenotypes in patients with acute and chronic hepatitis B and absence of lamivudine‐resistant strains in acute hepatitis B in Japan. J. Med. Virol. 79, 366–373. [DOI] [PubMed] [Google Scholar]
- 14. Robert MF (2008) Clinical uses of interferons. Br. J. Clin. Pharmacol. 65, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulze A, Schieck A, Ni Y, Mier W, Urban S (2010) Fine mapping of pre‐S sequence requirements for hepatitis B virus large envelope protein‐mediated receptor interaction. J. Virol. 84, 1989–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z et al (2012) Sodium taurocholateco transporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1, e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins DG, Sharp PM (1988) CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73, 237–244. [DOI] [PubMed] [Google Scholar]
- 19. Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357–358. [DOI] [PubMed] [Google Scholar]
- 20. Mishra GR, Suresh M, Kumaran K, Kannabiran N, Suresh S, Bala P et al (2006) Human protein reference database–2006 update. Nucleic Acids Res. 34, D411–D414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alfarano C, Andrade CE, Anthony K, Bahroos N, Bajec M, Bant‐oft K et al (2005) The Biomolecular Interaction Network Data‐base and related tools 2005 update. Nucleic Acids Res. 33, D418–D424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerrien S, Alam‐Faruque Y, Aranda B, Bancarz I, Bridge A, De‐row C et al (2007) IntAct‐open source resource for molecular interaction data. Nucleic Acids Res. 35, D561–D565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Persico M, Ceol A, Gavrila C, Hoffmann R, Florio A, Cesareni G (2005) HomoMINT: an inferred human network based on orthology mapping of protein interactions discovered in model organisms. BMC Bioinformatics 6(Suppl. 4), S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winter AG, Wildenhain J, Tyers M (2011) BioGRID REST Service, BiogridPlugin2 and BioGRIDWebGraph: new tools for access to interaction data at BioGRID. Bioinformatics 27, 1043–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xenarios I, Rice DW, Salwinski L, Baron MK, Marcotte EM, Ei‐senberg D (2000) DIP: the database of interacting proteins. Nucleic Acids Res. 28, 289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang N, Xu HL, Zhao X, Wen X, Wang FT, Wang SY et al (2012) Network‐based identification of novel connections among apoptotic signaling pathways in cancer. Appl. Biochem. Biotechnol. 167, 621–631. [DOI] [PubMed] [Google Scholar]
- 27. Torsten S, Jürgen K, Nicolas G, Manuel CP (2003) SWISS‐MODEL: an automated protein homology‐modeling server. Nucleic Acids Res. 31, 3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu LL, Yang Y, Xu HL, Cheng Y, Wen X, Ouyang L et al (2013) Identification of novel caspase/autophagy‐related gene switch to cell fate decisions in breast cancers. Cell Prolif. 46, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lang PT, Brozell SR, Mukherjee S, Pettersen EF, Meng EC, Thomas V et al (2009) DOCK 6: combining techniques to model RNA‐small molecule complexes. RNA 15, 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G et al (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R et al (2013) GROMACS 4.5: a high‐throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schüttelkopf AW, van Aalten DM (2004) PRODRG: a tool for high‐throughput crystallography of protein‐ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363. [DOI] [PubMed] [Google Scholar]
- 33. Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–8, 27–28. [DOI] [PubMed] [Google Scholar]
- 34. Huang KL, Lai YK, Lin CC, Chang JM (2006) Inhibition of hepatitis B virus production by Boehmerianivea root extract in HepG2.2.15 cells. World J. Gastroenterol. 12, 5721–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu JM, Lin JS, Xie N, Liang KH (2003) Inhibition of hepatitis B virus by a novel L‐nucleoside, beta‐L‐D4A and related analogues. World J. Gastroenterol. 9, 1840–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang T, Xie N, He W, Liu R, Lei Y, Chen Y et al (2013) An integrated proteomics and bioinformatics analyses of hepatitis B virus X interacting proteins and identification of a novel interactorapoA‐I. J. Proteomics 84, 92–105. [DOI] [PubMed] [Google Scholar]
- 37. Hanazaki K (2004) Antiviral therapy for chronic hepatitis B: a review. Curr. Drug Targets Inflamm. Allergy 3, 63–70. [DOI] [PubMed] [Google Scholar]
- 38. Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K et al (2005) Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 25, 367–379. [DOI] [PubMed] [Google Scholar]
- 39. Kullak‐Ublick GA, Glasa J, Böker C, Oswald M, Grützner U, Hagenbuch B et al (1997) Chlorambucil‐taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology 113, 1295–1305. [DOI] [PubMed] [Google Scholar]
- 40. Lee S, Kim S (2007) Gene regulations in HBV‐related liver cirrhosis closely correlate with disease severity. J. Biochem. Mol. Biol. 40, 814–824. [DOI] [PubMed] [Google Scholar]
- 41. Watashi K, Urban S, Li W, Wakita T (2014) NTCP and beyond: opening the door to unveil hepatitis B virus entry. Int. J. Mol. Sci. 15, 2892–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li H, Zhuang Q, Wang Y, Zhang T, Zhao J, Zhang Y et al (2014) HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell. Mol. Immunol. 11, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Sequence alignment of NTCP from human, rat and mouse.
Table S1. NTCP and its interactors.
Table S2. Top 30 results of candidate compounds targeting NTCP from Drugbank.
Table S3. Top 30 results of candidate compounds targeting NTCP from ZINC.


