Abstract
Objectives
Expression of dishevelled‐1 (DVL1) has recently been linked to cancer progression, however, its role in resistance to cancer therapy is unclear. In this study, we aimed to explore the function of DVL1 in paclitaxel‐resistant human ovarian cancer cells.
Materials and methods
The MTT assay was used to assess effects of DVL1 silencing on sensitivity of cells that were otherwise resistant to paclitaxel (Taxol). Western blotting and immunofluorescence staining were used to examine effects of DVL1 on AKT/GSK‐3β/β‐catenin signalling.
Results
Dishevelled‐1 was found to be over‐expressed in a paclitaxel‐resistant cell line derived from human ovarian cancer cell line A2780 (A2780/Taxol line) as well as parental A2780 cells. Down‐regulation of DVL1 (using the inhibitor 3289‐8625 or siRNA (siDVL1) against DVL1) sensitized A2780/Taxol cells to paclitaxel. Over‐expression of DVL1 in A2780 cells increased protein levels of P‐gp, BCRP and Bcl‐2, which are known targets of β‐catenin. Silencing DVL1 in A2780/Taxol cells also reduced levels of these proteins, and led to accumulation of β‐catenin. In addition, DVL1 aberrantly activated AKT/GSK‐3β/β‐catenin signalling. Inactivation of AKT signalling attenuated DVL1‐mediated inhibition of GSK‐3β and accumulation of β‐catenin, in both A2780 and A2780/Taxol cells.
Conclusions
Taken together, these results suggest that silencing DVL1 sensitized A2780/Taxol cells to paclitaxel, by down‐regulating AKT/GSK‐3β/β‐catenin signalling, providing a novel strategy for chemosensitization of ovarian cancer to paclitaxel‐induced cytotoxicity.
Introduction
Ovarian cancer remains one of the most lethal gynaecological malignancies, and is a leading cause of cancer‐related deaths in females 1, 2, 3. Although advances in treatment of ovarian cancer have been made over recent decades, prognosis for patients with advanced ovarian tumours remains poor, due to chemoresistance 4, 5. Paclitaxel (Taxol) is currently used as the first‐line chemotherapeutic agent for several types of cancer, including ovarian carcinoma. However, it frequently induces drug resistance, which leads to treatment failure 6, 7, 8. Understanding the mechanisms involved and thus overcoming drug resistance, are critical to ovarian cancer treatment.
Mechanisms of chemoresistance are complex, and differ between cancers. The most common conflict with anticancer drugs is due to over‐expression of one or more energy‐dependent ATP binding cassette (ABC) transporters in cancer cells, such as P‐glycoprotein (P‐gp), multidrug resistance‐associated protein and breast cancer resistance protein (BCRP), which eject anticancer drugs from cells, and thereby reduce their cytotoxic effects 9, 10, 11, 12. Other proteins that affect apoptosis, growth factor and cytokine signalling, and cell cycle behaviour, also play important roles in drug resistance 13, 14, 15, 16. It has been reported that paclitaxel resistance is associated with altered cell signalling, including AKT and Wnt/β‐catenin pathways 17, 18. The aim of the current study was to examine the role of dishevelled (DVL), a critical regulator of Wnt/β‐catenin, in paclitaxel‐resistant ovarian cancer cells.
Dishevelled, a key link that bridges receptors and downstream components of the Wnt signalling pathway, inhibits activation of GSK‐3β and degradation of β‐catenin. This increases translocation of β‐catenin to the nucleus, where it interacts with the transcription factor, T‐cell factor (TCF)/lymphoid enhancer factor (LEF), to induce expression of target genes, including P‐gp, BCRP and Bcl‐2 19, 20, 21, 22, 23. DVL not only regulates canonical Wnt/β‐catenin signalling but also mediates crosstalk between Wnt/β‐catenin and other signalling molecules such as AKT 24, 25. Recently, DVL1, a DVL homologous protein (DVL1–3), has been identified in many tumours, and has been suggested to be responsible for tumour growth, progression and metastasis 26, 27, 28. However, its effect on drug resistance in cancer chemotherapy has remained unknown.
The current study demonstrated that DVL1 was overexpressed in the paclitaxel‐resistant human ovarian cancer cell line A2780/Taxol compared to parental A2780 cells. Silencing DVL1 reduced expression of P‐gp, BCRP and Bcl‐2, and increased sensitivity of A2780/Taxol cells to paclitaxel. Furthermore, overexpressing or silencing DVL1, promoted or inhibited AKT/GSK‐3β/β‐catenin signalling, respectively. These results suggest that silencing DVL1 seemed to sensitize paclitaxel‐resistant ovarian cancer cells by down‐regulating the AKT/GSK‐3β/β‐catenin pathway; this provides a novel strategy for improving chemosensitivity of ovarian cancer.
Materials and methods
Reagents
RPMI‐1640, foetal bovine serum (FBS), penicillin and streptomycin were obtained from HyClone Laboratories (Logan, UT, USA). 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide (MTT) and dimethyl sulphoxide (DMSO) were purchased from Sigma‐Aldrich (St Louis, MO, USA). Paclitaxel was purchased from the National Institutes for Food and Drug Control (Beijing, China). DVL inhibitor compound 3289‐8625 was obtained from Merck (Darmstadt, Germany) and AKT inhibitor MK‐2206 was purchased from Selleck Chemicals (Houston, TX, USA). siDVL1 (target sequence, 5′‐GGAGGAGATCTTTGATGAC‐3′) and siControl (non‐specific siRNA) were from Genepharma (Shanghai, China). cDNA encoding DVL1 was cloned into pcDNA3.1 to generate DVL1 expression vector pcDNA3.1‐DVL1. Rabbit antibodies anti‐P‐gp, ‐BCRP, ‐Bcl‐2, ‐GSK‐3β, ‐phospho‐GSK‐3β (Ser‐9), ‐AKT and ‐phospho‐AKT (Ser‐473) were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse antibodies anti‐DVL1, ‐β‐catenin and ‐GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
A2780 human ovarian cancer cells and their paclitaxel‐resistant derivative A2780/Taxol cells were obtained from KeyGEN Biotech Co. (Nanjing, Jiangsu, China). Cells were cultured in RPMI‐1640 supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin sulphate, and incubated at 37 °C with 5% CO2. For A2780/Taxol cells, 0.3 μmol/l of paclitaxel was included in the medium to maintain paclitaxel‐resistance.
Transient transfection
A2780/Taxol cells were cultured in 96‐ or 6‐well plates overnight, and were then transfected with siDVL1, siControl, pcDNA3.1‐DVL1 or pcDNA3.1 empty vector, using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Seventy‐two hours after transfection, cells were collected and analysed using western blotting or MTT assays.
Drug sensitivity assay
Drug sensitivity was assessed using the MTT assay. Briefly, cells (5 × 103/well) were seeded in 100 μl RPMI 1640 with 5% FBS in 96‐well plates. Twenty‐four hours after incubation, they were either treated with 100 μm DVL inhibitor compound 3289‐8625 or transfected with siDVL1 for 24 h, and were then treated with a range of concentrations of paclitaxel, for an additional 48 h. Then, MTT was added to each well at final concentration of 0.5 mg/ml. After 4 h, media were discarded, and formazan blue that forms in live cells was dissolved in 150 μl of DMSO. Absorbance at 490 nm was measured using an Ultra Microplate Reader (Bio‐Tek Instruments, Winooski, VT, USA). Paclitaxel concentrations that achieved 50% growth inhibition (IC50) were calculated from survival curves using the Bliss method 29.
Western blotting
After the indicated treatments, cells were washed three times in PBS, lysed in RIPA buffer (Beyotime, Nantong, China) on ice for 30 min, and centrifuged at 15 000 g for 15 min. Supernatants were collected, and protein concentrations were determined using a BCA protein assay kit (Beyotime, Nantong, China). Forty micrograms of protein were then analysed using western blotting following standard protocols. An ECL chemiluminescent detection system (Thermo Scientific, Barrington, IL, USA) was used to develop immunoreactive bands, which were then visualized using a Bio‐Rad Molecular Imager (Hercules, CA, USA). Relative protein levels (means ± SD) from three separate experiments were determined by densitometry using Image J software (National Institutes of Health, Bethesda, MD, USA) according to the manufacturer's instructions.
Immunofluorescence staining
Cells were cultured on confocal dishes, and treated as indicated. They were washed three times in PBS, fixed in 4% paraformaldehyde for 20 min, then permeabilized using 0.3% Triton X‐100 for 10 min. After blocking with 10% goat serum for 2 h at room temperature, cells were incubated with anti‐β‐catenin antibodies at 4 °C overnight, followed by Alexa Fluor 488 secondary antibodies. Cells were counterstained with 5 mg/ml DAPI for 10 min, and images were acquired using a Zeiss confocal microscope.
Statistical analysis
All experiments were repeated in triplicate, and results are expressed as means ± SD, n = 3. Data were analysed using two‐tailed unpaired Student's t‐test between any two groups. One‐way ANOVA analysis of variance was used to assess difference of means between groups. All analyses were performed using GraphPad Prism Software Version 5.0 (GraphPad Software Inc, La Jolla, CA, USA); level of significance was set at a P < 0.05.
Results
Silencing DVL1 enhanced sensitivity of A2780/Taxol ovarian cancer cells to paclitaxel
Dishevelled‐1 plays an important role in cancer progression 26, 30, however, up to now its role in cancer chemoresistance has remained unknown. To determine whether DVL1 is related to paclitaxel‐induced resistance of ovarian cancer, its expression was compared in paclitaxel‐resistant A2780/Taxol and parental A2780 ovarian cancer cells. Western blotting showed that DVL1 was overexpressed in A2780/Taxol cells (Fig. 1a), suggesting that it might be involved in resistance of A2780/Taxol cells to paclitaxel. Next, effects of DVL on paclitaxel cytotoxicity was examined in A2780/Taxol cells treated with or without the DVL small molecule inhibitor compound, 3289‐8625. As shown in Fig. 1b and 1c, MTT assay revealed that IC50 for paclitaxel was significantly (P < 0.05) greater in A2780/Taxol cells (1.79 μm) compared to A2780 cells (0.18 μm), indicating that A2780/Taxol cells were more resistant than were A2780 cells, to paclitaxel. In the presence of compound 3289‐8625, IC50 of A2780/Taxol cells was significantly reduced, to 0.33 μm (P < 0.05). Similarly, silencing DVL1 using siDVL1 also sensitized A2780/Taxol cells to paclitaxel, and reduced IC50 of A2780/Taxol (0.49 μm) cells significantly compared to siControl (1.43 μm; P < 0.05) (Fig. 1d,e). These results show that overexpression of DVL1 was related to paclitaxel resistance in A2780/Taxol cells, and that down‐regulating DVL1 enhanced cytotoxicity of paclitaxel in otherwise resistant ovarian cancer cells.
Figure 1.
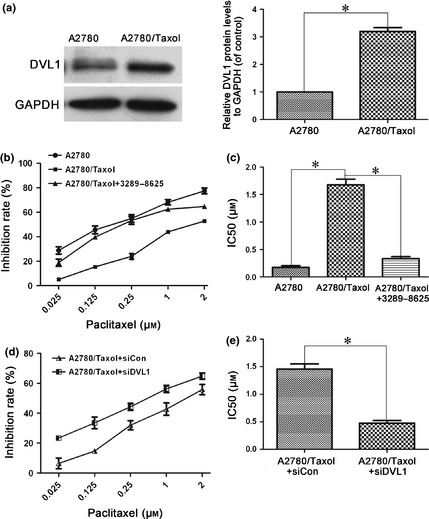
Silencing DVL1 enhanced the sensitivity of A2780/Taxol ovarian cancer cells to paclitaxel. (a) Western blotting of DVL1 expression in A2780 and A2780/Taxol cells. GAPDH was used as the internal control. The left panel shows representative Western blots, and the right panel shows quantitation of the Western blots to show the relative DVL1 expression normalized to GAPDH control. (b) The drug sensitivity of A2780, A2780/Taxol and compound 3289‐8625‐treated A2780/Taxol cells was assessed using MTT assays to measure cell viability. (c) The IC50 of paclitaxel in A2780, A2780/Taxol and compound 3289‐8625‐treated A2780/Taxol cells. The IC50 of paclitaxel was calculated from the survival curves generated in (b) using the Bliss method. (d) The drug sensitivity of A2780/Taxol cells transfected with siDVL1 or siControl. (e) The IC50 of paclitaxel in transfected A2780/Taxol cells, which was calculated from the survival curves in (d) using the Bliss method. Each experiment was performed in triplicate. *P < 0.05.
DVL1 positively regulated expression of P‐gp, BCRP and Bcl‐2
Next, expression of drug resistance‐related and anti‐apoptotic proteins, including P‐gp, BCRP and Bcl‐2, was compared in A2780 and A2780/Taxol cells to assess the role of DVL1 in resistance of ovarian cancer cells to paclitaxel. Protein expression levels of P‐gp, BCRP and Bcl‐2 were up‐regulated significantly in A2780/Taxol cells compared to A2780 cells (Fig. 2a). As DVL1 was also overexpressed in A2780/Taxol cells, we assessed whether it would regulate expression of P‐gp, BCRP and Bcl‐2. A2780 cells were transfected with pcDNA3.1‐DVL1, and western blotting then revealed that levels of P‐gp, BCRP and Bcl‐2 were significantly increased (P < 0.05) compared to control‐transfected cells (Fig. 2b).
Figure 2.
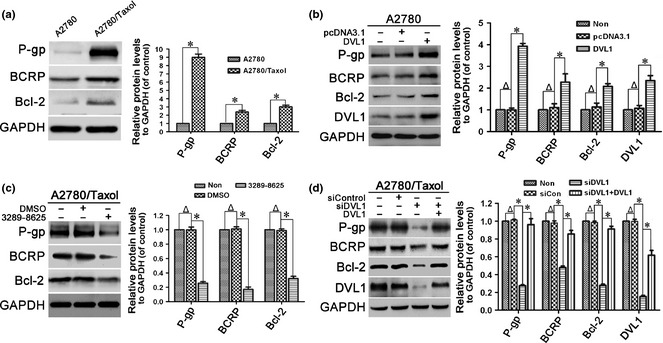
Effect of DVL1 on drug resistance‐related proteins. Western blotting for the drug resistance‐related proteins P‐gp, BCRP and Bcl‐2 in (a) A2780 and A2780/Taxol cells, (b) A2780 cells transfected with pcDNA3.1‐DVL1 or pcDNA3.1 for 72 h, (c) A2780/Taxol cells treated with or without 100 μM compound 3289‐8625 for 72 h, and (d) A2780/Taxol cells transfected with siControl, siDVL1 or siDVL1 plus pcDNA3.1‐DVL1 for 72 h. GAPDH was used as the internal control. The left panels show representative Western blots, and the right panels show relative quantitation of the blots after normalization to GAPDH. In B and D, DVL1 blots were included to confirm either the overexpression of DVL1 from pcDNA3.1‐DVL1 or the silencing of DVL1 by siDVL1. Each experiment was performed in triplicate. Δ P > 0.05; *P < 0.05.
To confirm that increased expression of P‐gp, BCRP and Bcl‐2 was mediated by DVL1, we inhibited DVL1 in A2780/Taxol cells using compound 3289‐8625, and then examined expression of P‐gp, BCRP and Bcl‐2. As expected, treatment with compound 3289‐8625 reduced expression of P‐gp, BCRP and Bcl‐2 (Fig. 2c). Similarly, down‐regulation of DVL1 using siDVL1 also reduced expression of P‐gp, BCRP and Bcl‐2. Moreover, siDVL1‐induced reduction in P‐gp, BCRP and Bcl‐2 expression was rescued by overexpressing DVL1 (Fig. 2d). Collectively, these results suggest that DVL1 up‐regulated expression of P‐gp, BCRP and Bcl‐2, mediating paclitaxel resistance in ovarian cancer.
DVL1 increased accumulation and nuclear translocation of β‐catenin
Previous studies demonstrated that P‐gp, BCRP and Bcl‐2 are β‐catenin target genes 19, 20, 21, 31, 32, 33. Thus, effects of DVL1 on expression and subcellular translocation of β‐catenin were investigated. β‐catenin protein levels were increased by overexpression of DVL1 in A2780 cells (Fig. 3a), and reduced by treatment with compound 3289‐8625 in A2780/Taxol cells (Fig. 3b). Also, β‐catenin protein levels were down‐regulated in siDVL1‐transfected A2780/Taxol cells, which were rescued by overexpression of DVL1 (Fig. 3c). These results indicate that DVL1 increased accumulation of β‐catenin in ovarian cancer cells. To confirm this, we demonstrated that β‐catenin levels were higher in A2780/Taxol compared to A2780 cells, and that silencing DVL1 reduced β‐catenin levels significantly (Fig. 3d). Immunofluorescence staining of A2780 cells revealed that β‐catenin was localized primarily in cell membranes and cytoplasm, and that little was found in the nucleus. Conversely in A2780/Taxol cells, more cytoplasmic accumulation and nuclear translocation were observed. However, β‐catenin levels in both cytoplasm and nucleus were reduced in A2780/Taxol cells transfected with siDVL1 (Fig. 3e). Taken together, these results show that DVL1 increased accumulation and nuclear translocation of β‐catenin.
Figure 3.
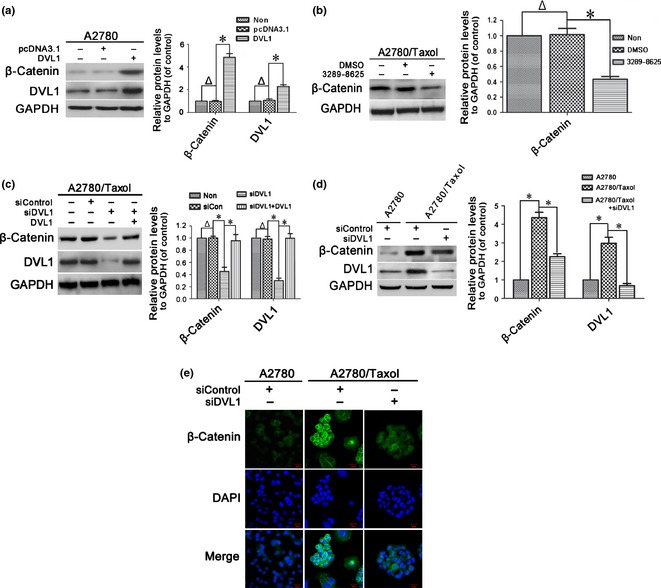
The effect of DVL1 on the accumulation and nuclear translocation of β‐catenin. (a–d) Western blotting for β‐catenin in (a) A2780 cells transfected with pcDNA3.1‐DVL1 or pcDNA3.1 for 72 h, (b) A2780/Taxol cells treated with or without 100 μm compound 3289‐8625 for 72 h, (c) A2780/Taxol cells transfected with siControl, siDVL1 or siDVL1 plus pcDNA3.1‐DVL1 for 72 h, and (d) A2780 cells transfected with siControl and A2780/Taxol cells transfected with siControl or siDVL1 for 72 h. GAPDH was used as the internal control. Left panels show representative Western blots, and right panels show the relative quantification after normalization to GAPDH. In (a, c and d), DVL1 blots were included to confirm either the overexpression of DVL1 from pcDNA3.1‐DVL1 or the silencing of DVL1 by siDVL1. Each experiment was performed in triplicate. Δ P > 0.05; *P < 0.05. (e) Immunofluorescence analysis of the cellular distribution of β‐catenin in the cytoplasm and nucleus of A2780 and A2780/Taxol cells transfected with siControl or siDVL for 72 h. Green, β‐catenin; blue, nuclear DNA (Scale bar = 20 μm).
DVL1 inhibited GSK3β by activating AKT
Activated AKT can inactivate GSK‐3β via phosphorylation at Ser‐9/21. Inactivation of GSK‐3β prevents phosphorylation and degradation of β‐catenin, leading to its accumulation and nuclear translocation 34, 35. To explore molecular mechanisms underlying DVL1‐regulated β‐catenin accumulation, we assessed effects of DVL1 on AKT and GSK‐3β activity. As shown in Fig. 4a, overexpression of DVL1 significantly up‐regulated phosphorylation of AKT at Ser‐473 and GSK‐3β at Ser‐9 in A2780 cells. In contrast, levels of phosphorylated AKT (Ser‐473) and GSK‐3β (Ser‐9) were reduced in compound 3289‐8625‐treated and siDVL1‐transfected A2780/Taxol cells. reduced phosphorylation induced by DVL1‐silencing was rescued by DVL1‐overexpression (Fig. 4b,c). These data suggest that DVL1 increased AKT and inhibited GSK‐3β activity. Furthermore, inactivation of AKT using inhibitor MK‐2206 attenuated DVL1‐mediated inhibition of GSK‐3β and accumulation of β‐catenin in A2780 and A2780/Taxol cells (Fig. 4d,e). These results imply that DVL1 inhibited GSK3β by activating AKT to increase accumulation of β‐catenin.
Figure 4.
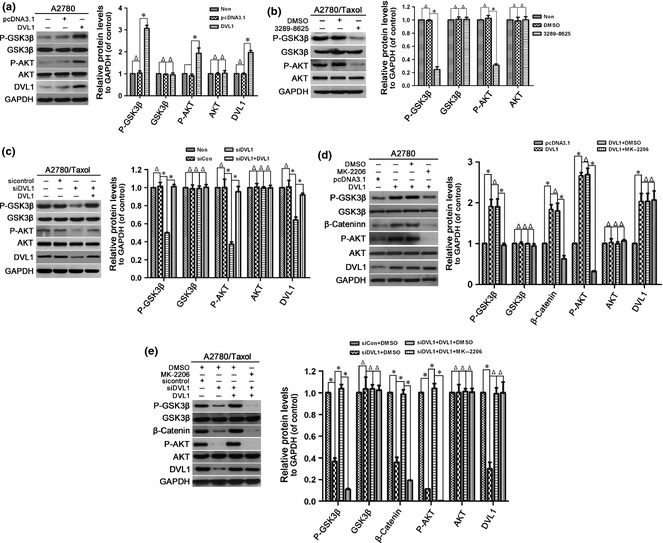
The effect of DVL1 on AKT/GSK‐3β/β‐catenin signalling. Western blotting for phospho‐GSK‐3β (Ser‐9), GSK‐3β, phospho‐AKT (Ser‐473), AKT and DVL1 (when pcDNA3.1‐DVL1‐ or siDVL‐transfected cells were included) in (a) A2780 cells transfected with pcDNA3.1‐DVL1 or pcDNA3.1 for 72 h, (b) A2780/Taxol cells treated with or without compound 3289‐8625 for 72 h, (c) A2780/Taxol cells transfected with siControl, siDVL1 or siDVL1 plus pcDNA3.1‐DVL1 for 72 h, (d) A2780 cells transfected with pcDNA3.1 or pcDNA3.1‐DVL1 for 72 h, and then treated with the AKT inhibitor MK‐2206 for 5 h, and (e) A2780/Taxol cells transfected with siControl, siDVL1 or siDVL1 plus pcDNA3.1‐DVL1 for 72 h, and then treated with MK‐2206 for 5 h. GAPDH was used as the internal control. Left panels show representative Western blots, and right panels show the relative quantification after normalization to GAPDH. Each experiment was performed in triplicate. Δ P > 0.05; *P < 0.05.
Discussion
Paclitaxel (Taxol) is a front‐line chemotherapeutic agent used to treat a number of human carcinomas, including ovarian cancer 36, 37. However, it frequently induces drug resistance, which is a major obstacle to successful clinical treatment of ovarian cancer 8, 38. Thus, understanding mechanisms contributing to drug resistance is urgent for development of efficient therapeutic strategies and overcoming chemoresistance. Cancer‐derived cell lines, in which paclitaxel resistance is induced by intermittent drug treatment, are used commonly as models to explore this phenomenon. Thus, stable paclitaxel‐resistant ovarian cancer cell line A2780/Taxol was used in the present study and was compared to its parental cell line A2780. A2780/Taxol cells exhibited strong resistance to paclitaxel, with an IC50 value that was almost 10‐fold of that of A2780 cells (Fig. 1b,c).
Accumulating evidence has shown that altered activity of the Wnt/β‐catenin signalling pathway, responsible for a diverse set of biological processes (such as cell proliferation, differentiation, migration, survival/apoptosis and stem cell self‐renewal), is involved in development of paclitaxel‐resistance in cancers 17, 39. In addition, abnormal activation of Wnt/β‐catenin signalling has been implicated in development of a broad spectrum of tumours, including ovarian cancer 40, 41. DVL is a key regulator of Wnt/β‐catenin signalling, and relays Wnt signals from their receptors to downstream effectors. Upon Wnt stimulation, DVL is recruited to the cell surface receptor frizzled (FZ), resulting in disassembly of β‐catenin destruction complex (that contains GSK‐3β), with inhibition of GSK‐3β catalytic activity and β‐catenin degradation. This leads to cytoplasmic accumulation and nuclear translocation of β‐catenin, where it ultimately activates transcription of target genes 22, 42. Notably, DVL overexpression can be sufficient to promote Wnt/β‐catenin pathway activity in the absence of Wnt stimulation by inhibiting GSK‐3β activity 43, 44. It has been reported that DVL is involved in development of several cancers 27, 45. However, mechanisms by which DVL plays a role in cancer chemoresistance has remained unknown. For this purpose the current study examined the relationship between DVL and paclitaxel‐resistance in ovarian cancer.
Data acquired revealed that DVL1 was overexpressed in A2780/Taxol compared to A2780 cells (Fig. 1a), indicating that DVL1 was related to paclitaxel resistance in ovarian cancer. To determine the role of DVL1 in paclitaxel‐resistance, DVL1 function was blocked using the small molecule inhibitor compound 3289‐8625, a cell‐permeable amidobenzanilide compound that disrupts FZ‐DVL interaction by targeting the PDZ domain of DVL 46, 47. Compound 3289‐8625 increased sensitivity of A2780/Taxol cells to paclitaxel, and reduced the IC50 value of the drug (Fig. 1b,c). To further examine the role of DVL1 in chemoresistance, its expression was down‐regulated using siDVL1. Consistent with the above observations, siDVL1 increased sensitivity of A2780/Taxol cells to paclitaxel and rreduced the IC50 value (Fig. 1d,e). These data imply that DVL1 is positively correlated to resistance of ovarian cancer to paclitaxel, and that down‐regulation of DVL1 restores sensitivity of ovarian cancer to paclitaxel.
We then focused on proteins involved in drug resistance. Membrane transporters in the ABC protein superfamily can reduce intracellular drug concentrations and cytotoxicity via drug efflux. Overexpression of these proteins is positively correlated with resistance to drugs, including paclitaxel 10, 48, 49. Anti‐apoptotic proteins are also related to drug resistance. Abnormal up‐regulation of anti‐apoptotic proteins such as Bcl‐2 also contributes to drug resistance 50. The currently acquire data demonstrated that two important ABC transporter proteins, P‐gp and BCRP, and anti‐apoptotic protein Bcl‐2 were overexpressed in A2780/Taxol cells compared to A2780 (Fig. 2a). In addition, levels of these proteins were increased when DVL1 was up‐regulated (Fig. 2b), but reduced when DVL1 was down‐regulated using compounds 3289‐8625 or siDVL1 (Fig. 2c,d). Meanwhile, reduction in siDVL1 was reversed by ectopic DVL1 expression (Fig. 2d). These data indicate that silencing DVL1 enhanced sensitivity of A2780/Taxol cells to paclitaxel by down‐regulating P‐gp, BCRP and Bcl‐2.
P‐gp, BCRP and Bcl‐2 are target genes of the Wnt/β‐catenin pathway. The role of β‐catenin in drug resistance of mixed‐lineage leukaemia stem cells and cholangiocarcinoma has been reported previously 51, 52. In the current study, we demonstrated that β‐catenin was overexpressed in A2780/Taxol paclitaxel‐resistant human ovarian cancer cells compared to parental A2780 cells (Fig. 3d), showing that β‐catenin was involved in resistance of ovarian cancer cells to paclitaxel. As DVL1 was also overexpressed in A2780/Taxol cells (Fig. 1a) and recent studies suggested that DVL might be sufficient to activate Wnt/β‐catenin signalling 43, 44, we thought that altered DVL1 protein levels could modulate expression of β‐catenin. Our hypothesis was confirmed by results revealing that DVL1 positively regulated accumulation and nuclear translocation of β‐catenin in A2780 and A2780/Taxol cells (Fig. 3), implying that DVL1 regulated expression of P‐gp, BCRP, and Bcl‐2 by activating β‐catenin.
β‐Catenin is activated after an event triggered by DVL‐mediated inactivation of GSK‐3β 19, 22. In the current study, overexpression and silencing of DVL1 inhibited or promoted GSK‐3β activity, respectively (Fig. 4a–c). Two mechanisms have been proposed to account for DVL‐mediated inhibition of GSK‐3β: inhibition of catalytic activity of GSK‐3β by serine phosphorylation 22, 53, 54, and disruption of the interaction between GSK‐3β and β‐catenin 55. However, the process whereby DVL stimulates serine phosphorylation of GSK‐3β is not well understood. It has been reported that activated AKT (phosphorylated at Thr‐308 and Ser‐473) phosphorylates GSK‐3β at Ser‐9/21, inhibiting its activity 56. As both DVL and AKT regulate GSK‐3β activity, these signalling cascades could converge at GSK‐3β to regulate β‐catenin. Here, we demonstrated that overexpression of DVL1 enhanced AKT activity (Fig. 4a), whereas inhibiting DVL1 using compound 3289‐8625 or siDVL1 reduced it (Fig. 4b,c). Conversely, inactivation of AKT using MK‐2206 attenuated DVL1‐mediated inhibition of GSK‐3β and accumulation of β‐catenin (Fig. 4d,e). These results show that DVL1 inhibited GSK‐3β by activating AKT to increase accumulation of β‐catenin.
To the best of our knowledge, this is the first study demonstrating roles for DVL1 in ovarian cancer resistant to paclitaxel. DVL1 was overexpressed in paclitaxel‐resistant human ovarian cancer cells, where it inhibited GSK‐3β activity by activating AKT. This increased accumulation of β‐catenin and enhanced expression of P‐gp, BCRP and Bcl‐2. Silencing DVL1 sensitized paclitaxel‐resistant human ovarian cancer cells by down‐regulating AKT/GSK‐3β/β‐catenin signalling, providing a novel insight into chemosensitization of ovarian cancer to paclitaxel‐induced cytotoxicity.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant nos 81301919, 81301854 and 81200917).
References
- 1. Rasool N, LaRochelle W, Zhong H, Ara G, Cohen J, Kohn EC (2010) Secretory leukocyte protease inhibitor antagonizes paclitaxel in ovarian cancer cells. Clin. Cancer Res. 16, 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Permuth‐Wey J, Sellers TA (2009) Epidemiology of ovarian cancer. Methods Mol. Biol. 472, 413–437. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal R, Kaye SB (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 3, 502–516. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Zhang J, Zhang Z, Li H, Cheng W, Liu J (2013) Cancer stem cells, epithelial‐mesenchymal transition, and drug resistance in high‐grade ovarian serous carcinoma. Hum. Pathol. 44, 2373–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li N, Hou JL, Shi ZZ, Li XG, Sun YC, Xu X et al (2014) Copy number changes of 4‐gene set may predict early relapse in advanced epithelial ovarian cancer after initial platinum‐paclitaxel chemotherapy. Am. J. Cancer Res. 4, 285–292. [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan SL, Medina JE, Taylor MM, Dinulescu DM (2014) Targeting platinum resistant disease in ovarian cancer. Curr. Med. Chem. 21, 3009–3020. [DOI] [PubMed] [Google Scholar]
- 8. Vergara D, Tinelli A, Iannone A, Maffia M (2012) The impact of proteomics in the understanding of the molecular basis of Paclitaxel‐resistance in ovarian tumors. Curr. Cancer Drug Targets 12, 987–997. [DOI] [PubMed] [Google Scholar]
- 9. Sedlakova I, Laco J, Tosner J, Caltova K, Cervinka M, Rezac A et al (2012) Proteins of resistance and drug resistance in ovarian carcinoma patients. Klin Onkol. 25, 457–463. [PubMed] [Google Scholar]
- 10. Kovalev AA, Tsvetaeva DA, Grudinskaja TV (2013) Role of ABC‐cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer. Exp. Oncol. 35, 287–290. [PubMed] [Google Scholar]
- 11. Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F (2006) The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr. Drug Targets 7, 893–909. [DOI] [PubMed] [Google Scholar]
- 12. Lopez J, Banerjee S, Kaye SB (2013) New developments in the treatment of ovarian cancer – future perspectives. Ann. Oncol. 24(Suppl 10), x69–x76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suh DH, Kim MK, No JH, Chung HH, Song YS (2011) Metabolic approaches to overcoming chemoresistance in ovarian cancer. Ann. N. Y. Acad. Sci. 1229, 53–60. [DOI] [PubMed] [Google Scholar]
- 14. Malaguarnera R, Belfiore A (2014) The emerging role of insulin and insulin‐like growth factor signaling in cancer stem cells. Front. Endocrinol. (Lausanne) 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haass NK, Beaumont KA, Hill DS, Anfosso A, Mrass P, Munoz MA et al (2014) Real‐time cell cycle imaging during melanoma growth, invasion, and drug response. Pigment Cell Melanoma Res. 27, 764–776. [DOI] [PubMed] [Google Scholar]
- 16. Kapse‐Mistry S, Govender T, Srivastava R, Yergeri M (2014) Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 5, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chau WK, Ip CK, Mak AS, Lai HC, Wong AS (2013) c‐Kit mediates chemoresistance and tumor‐initiating capacity of ovarian cancer cells through activation of Wnt/beta‐catenin‐ATP‐binding cassette G2 signaling. Oncogene 32, 2767–2781. [DOI] [PubMed] [Google Scholar]
- 18. Kim SH, Juhnn YS, Song YS (2007) Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann. N. Y. Acad. Sci. 1095, 82–89. [DOI] [PubMed] [Google Scholar]
- 19. MacDonald BT, Tamai K, He X (2009) Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H et al (2010) Inhibition of Wnt signaling pathway decreases chemotherapy‐resistant side‐population colon cancer cells. Anticancer Res. 30, 2041–2048. [PubMed] [Google Scholar]
- 21. Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH (2007) Bcl‐2 overexpression in PhIP‐induced colon tumors: cloning of the rat Bcl‐2 promoter and characterization of a pathway involving beta‐catenin, c‐Myc and E2F1. Oncogene 26, 6194–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aschenbach WG, Ho RC, Sakamoto K, Fujii N, Li Y, Kim YB et al (2006) Regulation of dishevelled and beta‐catenin in rat skeletal muscle: an alternative exercise‐induced GSK‐3beta signaling pathway. Am. J. Physiol. Endocrinol. Metab. 291, E152–E158. [DOI] [PubMed] [Google Scholar]
- 23. Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L et al (2008) Activation of beta‐catenin signalling by GSK‐3 inhibition increases p‐glycoprotein expression in brain endothelial cells. J. Neurochem. 106, 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sutton LP, Rushlow WJ (2012) The dopamine D2 receptor regulates Akt and GSK‐3 via Dvl‐3. Int. J. Neuropsychopharmacol. 15, 965–979. [DOI] [PubMed] [Google Scholar]
- 25. Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH et al (2001) Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276, 17479–17483. [DOI] [PubMed] [Google Scholar]
- 26. Zhao Y, Yang ZQ, Wang Y, Miao Y, Liu Y, Dai SD et al (2010) Dishevelled‐1 and dishevelled‐3 affect cell invasion mainly through canonical and noncanonical Wnt pathway, respectively, and associate with poor prognosis in nonsmall cell lung cancer. Mol. Carcinog. 49, 760–770. [DOI] [PubMed] [Google Scholar]
- 27. You L, Xu Z, Punchihewa C, Jablons DM, Fujii N (2008) Evaluation of a chemical library of small‐molecule Dishevelled antagonists that suppress tumor growth by down‐regulating T‐cell factor‐mediated transcription. Mol. Cancer Ther. 7, 1633–1638. [DOI] [PubMed] [Google Scholar]
- 28. Mizutani K, Miyamoto S, Nagahata T, Konishi N, Emi M, Onda M (2005) Upregulation and overexpression of DVL1, the human counterpart of the Drosophila dishevelled gene, in prostate cancer. Tumori 91, 546–551. [DOI] [PubMed] [Google Scholar]
- 29. He L, Zhao C, Yan M, Zhang LY, Xia YZ (2009) Inhibition of P‐glycoprotein function by procyanidine on blood‐brain barrier. Phytother. Res. 23, 933–937. [DOI] [PubMed] [Google Scholar]
- 30. Nagahata T, Shimada T, Harada A, Nagai H, Onda M, Yokoyama S et al (2003) Amplification, up‐regulation and over‐expression of DVL‐1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 94, 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metcalfe C, Mendoza‐Topaz C, Mieszczanek J, Bienz M (2010) Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX‐dependent polymerization. J. Cell Sci. 123, 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D et al (2009) The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta‐catenin pathway. Oncogene 28, 2245–2256. [DOI] [PubMed] [Google Scholar]
- 33. Hong Y, Yang J, Wu W, Wang W, Kong X, Wang Y et al (2008) Knockdown of BCL2L12 leads to cisplatin resistance in MDA‐MB‐231 breast cancer cells. Biochim. Biophys. Acta 1782, 649–657. [DOI] [PubMed] [Google Scholar]
- 34. Cheng L, Zhang C, Li D, Zou J, Wang J (2014) Transforming growth factor‐beta1 (TGF‐beta1) induces mouse precartilaginous stem cell proliferation through TGF‐beta receptor II (TGFRII)‐Akt‐beta‐catenin signaling. Int. J. Mol. Sci. 15, 12665–12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu X, Wang S, Yu L, Yang H, Tan R, Yin K et al (2014) TL‐2 attenuates beta‐amyloid induced neuronal apoptosis through the AKT/GSK‐3beta/beta‐catenin pathway. Int. J. Neuropsychopharmacol. 17, 1511–1519. [DOI] [PubMed] [Google Scholar]
- 36. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S et al (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354, 34–43. [DOI] [PubMed] [Google Scholar]
- 37. Suh DH, Kim JW, Kang S, Kim HJ, Lee KH (2014) Major clinical research advances in gynecologic cancer in 2013. J. Gynecol. Oncol. 25, 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steffensen KD, Smoter M, Waldstrom M, Grala B, Bodnar L, Stec R et al (2014) Resistance to first line platinum paclitaxel chemotherapy in serous epithelial ovarian cancer: the prediction value of ERCC1 and Tau expression. Int. J. Oncol. 44, 1736–1744. [DOI] [PubMed] [Google Scholar]
- 39. Shang D, Liu Y, Xu X, Han T, Tian Y (2011) 5‐aza‐2'‐deoxycytidine enhances susceptibility of renal cell carcinoma to paclitaxel by decreasing LEF1/phospho‐beta‐catenin expression. Cancer Lett. 311, 230–236. [DOI] [PubMed] [Google Scholar]
- 40. Arend RC, Londono‐Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B et al (2014) Inhibition of Wnt/beta‐catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecol. Oncol. 134, 112–120. [DOI] [PubMed] [Google Scholar]
- 41. Rosenbluh J, Wang X, Hahn WC (2014) Genomic insights into WNT/beta‐catenin signaling. Trends Pharmacol. Sci. 35, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao C, Chen YG (2010) Dishevelled: the hub of Wnt signaling. Cell. Signal. 22, 717–727. [DOI] [PubMed] [Google Scholar]
- 43. Schwarz‐Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y et al (2007) The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492. [DOI] [PubMed] [Google Scholar]
- 44. Schwarz‐Romond T, Metcalfe C, Bienz M (2007) Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120, 2402–2412. [DOI] [PubMed] [Google Scholar]
- 45. Pulvirenti T, Van Der Heijden M, Droms LA, Huse JT, Tabar V, Hall A (2011) Dishevelled 2 signaling promotes self‐renewal and tumorigenicity in human gliomas. Cancer Res. 71, 7280–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D et al (2003) Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C‐terminal region of Frizzled. Mol. Cell 12, 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Punchihewa C, Ferreira AM, Cassell R, Rodrigues P, Fujii N (2009) Sequence requirement and subtype specificity in the high‐affinity interaction between human frizzled and dishevelled proteins. Protein Sci. 18, 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ieiri I (2012) Functional significance of genetic polymorphisms in P‐glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab. Pharmacokinet. 27, 85–105. [DOI] [PubMed] [Google Scholar]
- 49. Choudhuri S, Klaassen CD (2006) Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 25, 231–259. [DOI] [PubMed] [Google Scholar]
- 50. Dai H, Ding H, Meng XW, Lee SH, Schneider PA, Kaufmann SH (2013) Contribution of Bcl‐2 phosphorylation to Bak binding and drug resistance. Cancer Res. 73, 6998–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen DY, Zhang W, Zeng X, Liu CQ (2013) Inhibition of Wnt/beta‐catenin signaling downregulates P‐glycoprotein and reverses multi‐drug resistance of cholangiocarcinoma. Cancer Sci. 104, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D et al (2010) β‐Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18, 606–618. [DOI] [PubMed] [Google Scholar]
- 53. Ruel L, Stambolic V, Ali A, Manoukian AS, Woodgett JR (1999) Regulation of the protein kinase activity of Shaggy(Zeste‐white3) by components of the wingless pathway in Drosophila cells and embryos. J. Biol. Chem. 274, 21790–21796. [DOI] [PubMed] [Google Scholar]
- 54. Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC (1996) Wingless inactivates glycogen synthase kinase‐3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 15, 4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 55. Arend RC, Londono‐Joshi AI, Straughn JM Jr, Buchsbaum DJ (2013) The Wnt/beta‐catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 131, 772–779. [DOI] [PubMed] [Google Scholar]
- 56. Bishnupuri KS, Sainathan SK, Bishnupuri K, Leahy DR, Luo Q, Anant S et al (2014) Reg4‐induced mitogenesis involves Akt‐GSK3beta‐beta‐Catenin‐TCF‐4 signaling in human colorectal cancer. Mol. Carcinog. 53(Suppl 1), E169–E180. [DOI] [PMC free article] [PubMed] [Google Scholar]


