Since the first isolation of Candida auris in 2009, scientific community has witnessed an exponential emergence of infection episodes and outbreaks in different world regions [1]. According to the Centers for Disease Control and Prevention (CDC), 560 cases of C. auris infections have been notified in the United States as 31 January 2019. It is likely that many cases are missed, due to its misidentification with other non-albicans Candida spp. (e.g., C. haemulonii) by common microbiological diagnostic methods (https://www.cdc.gov/fungal/diseases/candidiasis/tracking-c-auris.html). Most of the reports occurred in critically ill adults, with risk factors for invasive fungal infections, such as immunosuppression, surgery, or indwelling catheters. The most common form of infection was candidemia, with a crude mortality of nearly 30%, but up to 70% in some reports [2].
Despite implementation of countermeasures to limit colonization and infections in intensive care units (ICUs), cases continue to be reported, with a tendency to an endemic pattern [3]. This reflects the ability of C. auris to persist in clinical environment, facilitating its transmission within critical care setting. Multidrug-resistant (MDR) pattern and has been frequently observed (around 40%) with serious and complex consequences for antifungal therapy [4].
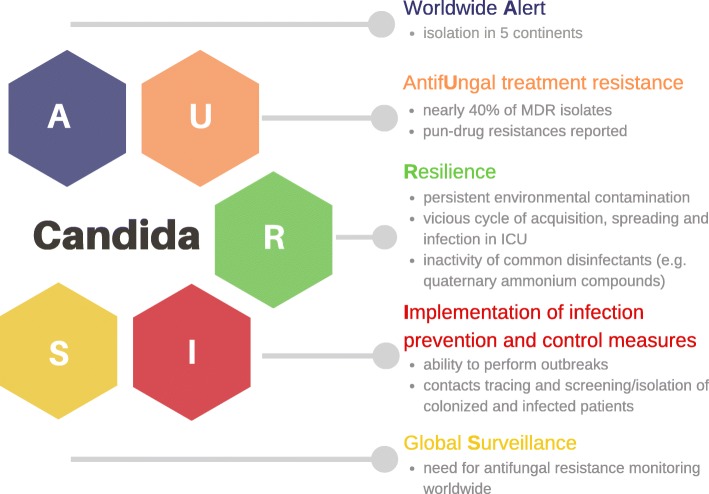
In view of C. auris progressive spread and treatment concerns, attention should be focused on the following A.U.R.I.S. major issues (Fig. 1):
Worldwide Alert
AntifUngal treatment resistance
Resilience and mechanisms of transmission
Implementation of infection prevention and control measures
Surveillance
Fig. 1.
Major issues related to Candida auris. Major issues related to Candida auris described with A.U.R.I.S. outline. MDR, multidrug resistant; ICU, intensive care unit
Worldwide Alert
Following the first isolation in Japan, cases have been reported in several countries in five continents. Although uncommon for fungi, C. auris has the ability to cause outbreaks, as seen in India, the UK, Spain, the USA, Venezuela, Colombia, and South Africa [1]. It is still debated whether C. auris emerged in one region with subsequent spreading to others, or if it emerged independently across different countries. Evidence from genomic sequencing demonstrates different clades of C. auris show strong geographic structure, with independent emergence in East and South Asia, Africa, and South America [1, 5, 6].
AntifUngal treatment resistance
To date, there are not established minimum inhibitory concentrations (MICs) breakpoints for susceptibility testing of C. auris. Antifungal susceptibility data from three continents demonstrated that nearly 40% were MDR, with strains being resistant to fluconazole (90%), amphotericin B (30–40%) and echinocandins (5–10%). Moreover, a small percentage were also resistant to all antifungals actually available [4, 6]. C. auris demonstrates a high propensity to develop antifungal resistance under selective pressure. Recent studies demonstrated mutations in ERG11 (encoding lanosterol demethylase, the target of azoles) and FKS1 genes (encoding 1,3-beta-glucan synthase, the target of echinocandins) [1, 7].
The recommended antifungals for C. auris treatment are mainly based on in vitro testing and on the most frequently retrieved resistance profiles. Echinocandins are the recommended first-line treatment, pending specific susceptibility testing. Lipid formulation of amphotericin B should be an alternative in patients not responding to echinocandins. Close monitoring to early detect therapeutic failures and evolution of antifungal resistance is needed. New antifungals (e.g., SCY-078, APX001A/APX001, and rezafungin) have been tested with success but they are not available to date for clinical use [1].
Resilience and mechanisms of transmission
Unlike others Candida species, C. auris can colonize different anatomical sites (e.g., skin, skin, rectum, axilla, stool) and contaminate hospital equipment and surfaces, creating a vicious cycle of acquisition, spreading, and infection, particularly in ICUs. Indeed, bed, chairs, and monitoring tools (e.g., pulse oximeters, temperature probes) were contaminated during outbreaks [8]. Recently, Eyre et al. [9] published the results of a patients’ and hospital environmental screening program in Oxford, UK, after 70 patients (66 admitted to a neuro-ICU) were identified as being colonized or infected by C. auris. Seven patients developed an invasive infection during hospital stay. C. auris was detected mainly on skin-surface axillary temperature probes and other reusable tools. In patients monitored with skin-surface temperature probes, the risk of C. auris infection/colonization was seven times higher. Adoption of specific bundles of infection control had no significant effects until removal of the temperature probes [9].
Recent studies have confirmed that C. auris can form biofilms, with a high variation of capacity of production depending on the C. auris strain considered [10]. Biofilm may present reduced susceptibility to hydrogen peroxide and chlorhexidine [11].
Quaternary ammonium compounds and cationic surface-active products seem to be ineffective against C. auris. Chlorine-based products appear to be the most effective for environmental surface disinfection [12]. Chlorine-based disinfectants (at a concentration of 1000 ppm), hydrogen- peroxide, or other disinfectants with documented fungicidal activity are recommended for environmental cleaning by the European CDC (ECDC) [13].
Implementation of infection prevention and control measures
CDC and ECDC released recommendations for C. auris case and outbreak management [13]. Usually, outbreaks follow an exponential increase in the number of affected patients. It is mandatory to trace contacts with the aim to achieve early identification and screening of possible colonized patients that might be responsible for persistence of C. auris. Patients potentially or already colonized should be placed in single rooms with contact isolation precautions. Screening should be applied for contacts and patients previously hospitalized in healthcare settings where C. auris isolation was confirmed. Hand hygiene (with alcohol or chlorhexidine hand rubs), wearing of protective clothing, and skin and environmental/equipment decontamination should be performed to prevent ongoing transmission.
Global Surveillance
Aiming to support implementation measures on global surveillance on antimicrobial resistances, in 2016, the World Health Organization [14] launched the Global Resistance Surveillance System (GLASS). The emergence of C. auris and progressive spread of infections caused by other resistant pathogens has strengthened the need for a surveillance network for antimicrobial resistance globally for critically ill patients’ safety.
It is hard to predict future C. auris diffusion. There will be outbreaks also in countries in which C. auris has been not reported yet? Will new MDR clones continue to emerge? Will we be able to apply effective antifungal stewardship programs and control measures? By now, global surveillance, improving knowledge, and taking care of the A.U.R.I.S. major issues may be the best ways to face C. auris challenge.
Acknowledgements
None.
Funding
None.
Availability of data and materials
Not applicable.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- ECDC
European Center for Disease Prevention and control
- GLASS
Global Resistance Surveillance System
- ICU
Intensive care unit
- MDR
Multidrug resistant
- MIC
Minimum inhibitory concentrations
Authors’ contributions
AC, GM, AG, MB, and DE conceived the content, wrote the manuscript, and approved the last version.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AC is a member of the Advisory Board of Critical Care. GM and DE declare to have no competing interests. AG received grants, fees for educational presentation, and advisory board membership, without any relationship to the submitted work, from Pfizer, Merck Sharp, and Gilead. Outside the submitted work, MB has received funding for scientific advisory boards, travel and speaker honoraria from Angelini, AstraZeneca, Bayer, Biomerieux, Cidara, Cubist, Gilead, Pfizer, Melinta Therapeutics, Menarini, MSD, Nabriva, Paratek, Roche, Shionogi, Tetraphase, The Medicines Company and Astellas Pharma Inc.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrea Cortegiani, Email: andrea.cortegiani@unipa.it.
Giovanni Misseri, Email: giovannimisseri1987@gmail.com.
Antonino Giarratano, Email: antonino.giarratano@unipa.it.
Matteo Bassetti, Email: matteo.bassetti@asuiud.sanita.fvg.it.
David Eyre, Email: david.eyre@ndm.ox.ac.uk.
References
- 1.Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6:69. doi: 10.1186/s40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osei Sekyere J. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen. 2018;7:e00578. doi: 10.1002/mbo3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Gaitan A, Moret AM, Tasias-Pitarch M, Aleixandre-Lopez AI, Martinez-Morel H, Calabuig E, et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 4.Cortegiani Andrea, Misseri Giovanni, Chowdhary Anuradha. What’s new on emerging resistant Candida species. Intensive Care Medicine. 2018;45(4):512–515. doi: 10.1007/s00134-018-5363-x. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes J, Abdolrasouli A, Farrer RA, Cuomo CA, Aanensen DM, Armstrong-James D, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7:43. doi: 10.1038/s41426-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kordalewska M, Lee A, Park S, Berrio I, Chowdhary A, Zhao Y, et al. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrob Agents Chemother. 2018;62:e00238-18. [DOI] [PMC free article] [PubMed]
- 8.Madder H, Moir I, Moroney R, Butcher L, Newnham R, Sunderland M, et al. Multiuse patient monitoring equipment as a risk factor for acquisition of Candida auris. bioRxiv. 2017:149054. 10.1101/149054.
- 9.Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, et al. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379:1322–1331. doi: 10.1056/NEJMoa1714373. [DOI] [PubMed] [Google Scholar]
- 10.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, et al. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis. 2017;23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kean R, McKloud E, Townsend EM, Sherry L, Delaney C, Jones BL, et al. The comparative efficacy of antiseptics against Candida auris biofilms. Int J Antimicrob Agents. 2018. [DOI] [PubMed]
- 12.Ku TSN, Walraven CJ, Lee SA. Candida auris: disinfectants and implications for infection control. Front Microbiol. 2018;9:726. doi: 10.3389/fmicb.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://ecdc.europa.eu/en/publications-data/rapid-risk-assessment-candida-auris-healthcare-settings-europe. Accessed 20 Mar 2019.
- 14.https://www.who.int/glass/en/. Accessed 20 Mar 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.