Abstract
Objectives
Frizzled‐7 (FZD7) receptor‐dependent activation of the canonical Wnt/β‐catenin pathway plays a crucial role in epithelial‐to‐mesenchymal transition (EMT) and breast cancer metastasis. FZD7 and its co‐receptor, low‐density lipoprotein receptor‐related protein 6 (LRP6), are highly expressed in MDA‐MB‐231 and T‐47D breast cancer cells, and endogenous ligands for FZD7 include Wnt3a and Wnt5a/b. γ‐Tocotrienol, a natural isoform of vitamin E, inhibits human breast cancer cell proliferation and EMT. Here, studies have been conducted to investigate the role of the canonical Wnt pathway in mediating inhibitory effects of γ‐tocotrienol on EMT in human breast cancer cells.
Materials and methods
MDA‐MB‐231, T‐47D and MCF‐10A cells were maintained in serum‐free defined media containing selected doses of γ‐tocotrienol. Cell viability was determined using the MTT colorimetric assay, Western blot analysis was used to measure protein expression and the wound‐healing assay was employed to study cell mobility and migration. Immunohistochemical fluorescence staining visualized expression and localization of EMT cell markers.
Results
γ‐Tocotrienol was found to induce dose‐responsive inhibition of MDA‐MB‐231 and T‐47D cell growth at doses that had no effect on immortalized normal MCF‐10A mammary epithelial cells. These growth inhibitory effects were associated with suppression in canonical Wnt signalling, reversal of EMT and significant reduction in breast cancer cell motility.
Conclusions
γ‐Tocotrienol suppression of metastatic breast cancer cell proliferation and EMT was associated with suppression of the canonical Wnt/β‐catenin signalling pathway.
1. Introduction
Breast cancer is the most dominant malignancy among women with more than a million cases diagnosed annually worldwide.1, 2 Furthermore, metastatic breast cancer is the primary cause for breast cancer mortality.3 The metastatic process involves epithelial cells losing their cellular polarity, adhesion to the basement membrane, and acquiring migratory and invasive properties. Metastatic epithelial cells take on a mesenchymal phenotype, hence this process is termed epithelial‐to‐mesenchymal transition (EMT).4, 5, 6, 7 During EMT, epithelial cells also display a reduction in epithelial cell marker expression (E‐cadherin and cytokeratin), and an increased expression of mesenchymal cell markers (snail, slug, vimentin, twist and fibronectin).8 Various signalling pathways have been identified that are associated with the promotion of EMT, particularly the canonical Wnt pathway.3 Targeting such pathways to inhibit EMT is currently attracting great interest as a promising approach in the treatment of metastatic breast cancer.
The canonical Wnt pathway, commonly known as Wnt/β‐catenin pathway, regulates several biological processes such as cell proliferation and stem cell maintenance.9 The fundamental steps in the regulation of this pathway involve the phosphorylation, ubiquitination and subsequent degradation of β‐catenin by the β‐catenin destruction complex of proteins that is comprised of axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 alpha/beta (GSK3α/β) and casein kinase 1 (CK1), and sequentially proteasomes.10 Various types of cancers such as breast, colon, liver and skin cancer are characterized by aberrant Wnt/β‐catenin signalling and/or dysfunction of the β‐catenin destruction complex.11, 12 Naked1/2 are cytosolic proteins that act as negative regulators of the Wnt/β‐catenin pathway.
In normal cells, Wnt ligand levels are very low and this promotes β‐catenin forming a complex with APC and axin.13, 14 This complex is then targeted by various kinases such as CK1 and GSK3β that phosphorylate β‐catenin. Phosphorylated β‐catenin then becomes a target for ubiquitination and subsequent proteasomal degradation.13, 14 This constant elimination of β‐catenin from the cytosol prevents nuclear translocation, and ultimately decreases the expression of Wnt target genes such as cyclin D1.15 However, in many types of cancer, Wnt ligands are characteristically overexpressed and their activation of frizzled (FZD) receptors and low‐density lipoprotein receptor‐related protein 5/6 (LRP5/6) co‐receptors leads to the recruitment and activation of dishevelled (DVL) proteins that inhibit GSK3β. Inhibition of GSK3β prevents the phosphorylation and degradation of β‐catenin, thereby increasing β‐catenin nuclear translocation and binding to T‐cell factor/lymphoid enhancer factor (TCL/LEF), which ultimately leads to an increased expression of Wnt target genes.13, 16 Enhanced or overexpression of Wnt target genes, including c‐Myc and cyclin D1, play a critical role in the promotion of cancer cell survival, proliferation, migration and metastasis.16
Tocotrienols and tocopherols represent the subgroups that make up the vitamin E family of compounds.17 Although both tocotrienols and tocopherols are potent antioxidants,18 only tocotrienols display potent anti‐proliferative, apoptotic and autophagic effects against breast cancer cells at a treatment dose which displays little or no effect on normal cell viability.17, 19, 20, 21 Tocotrienol anti‐cancer effects were found to be associated with a suppression in growth factor receptor mitogenic signalling.22, 23, 24, 25, 26 Furthermore, tocotrienol treatment was also found to reverse EMT in highly malignant mouse mammary tumour cells.27 Recently, studies have provided evidence that explains, at least in part, the mechanism(s) by which γ‐tocotrienol is able to inhibit the activation and signalling of a wide variety of membrane bound receptors.28 Specifically, γ‐tocotrienol was found to accumulate in and disrupt the integrity of the lipid raft domain within the plasma membrane of breast cancer cells, and this disruption of lipid raft integrity was associated with a reduction in receptor activation and signalling.28 As LRP6 is also localized in the lipid raft domain of the plasma membrane, and Wnt ligands are responsible for the phosphorylation and activation of this co‐receptor,29 studies were conducted to investigate whether γ‐tocotrienol‐induced anti‐cancer effects and reversal of EMT in highly metastatic MDA‐MB‐231 and T‐47D human breast cancer cells are mediated by a suppression in Wnt/β‐catenin signalling.
2. Materials and methods
2.1. Reagents and antibodies
All reagents were purchased from Sigma‐Aldrich (St. Louis, MO, USA), unless otherwise stated. Purified γ‐tocotrienol was generously provided by First Tech International Ltd. (Hong Kong). The Wnt/β‐catenin kit, β‐catenin, E‐cadherin and vimentin antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody for frizzled 7 (FZD7) was purchased from Abcam (Cambridge, MA, USA). Antibody for GSK3β was purchased from Gene Tex, Inc. (Irvine, CA, USA). Antibodies for cyclin D1, cytokeratin 8/18 and fibronectin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488‐conjugated anti‐rabbit antibody and EGF were purchased from Invitrogen Corporation (Carlsbad, CA, USA).
2.2. Cell culture conditions
Cell culture conditions were previously described in detail.30, 31 Briefly, the highly invasive and metastatic human MDA‐MB‐231 and T‐47D breast cancer cell lines, and the immortalized MCF‐10A normal human mammary epithelial cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). For maintaining stock cultures and seeding subcultures, MDA‐MB‐231 and T‐47D human breast cancer cells were cultured in complete growth media composed of RPMI‐1640 supplemented with 10% foetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin and 10 mg/mL insulin, whereas MCF‐10A cells were maintained in DMEM/F12 supplemented with 5% horse serum, 0.5 mg/mL hydrocortisone, 20 ng/mL EGF, 100 U/mL penicillin, 0.1 mg/mL streptomycin and 10 mg/mL insulin. For subculturing cell lines, cultures were rinsed in a sterile Ca2+‐ and Mg2+‐free phosphate‐buffered saline (PBS) and incubated in 0.05% trypsin containing 0.025% EDTA in PBS for 3–15 minutes at 37°C. Released cells were centrifuged, resuspended in medium and counted using a haemocytometer. All cells were maintained at 37°C in an environment of 95% air and 5% CO2 in a humidified incubator.
2.3. Experimental treatments
γ‐Tocotrienol is a highly lipophilic compound that is insoluble in water. In order to prepare a stock solution of γ‐tocotrienol, a measurable amount of γ‐tocotrienol was suspended in 100 μL of absolute ethyl alcohol and then added to a small volume of sterile 10% BSA in water and incubated at 37°C overnight with gentle shaking, as previously described.17 This stock solution was used to prepare various concentrations of the serum‐free defined treatment media used in experimentation that was composed of RPMI‐1640 (MDA‐MB‐231 and T‐47D) or DMEM/F12 (MCF‐10A) supplemented 5 mg/mL bovine serum albumin (BSA), 10 μg/mL transferrin, 100 U/mL soya bean trypsin inhibitor, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 10 μg/mL insulin and 10 ng/mL EGF as a mitogen.17
2.4. Measurement of viable cell number
MDA‐MB‐231 and MCF‐10A cells were initially seeded at a density of 1 × 104 cells/well, whereas T‐47D were seeded at a density of 5 × 103 cells/well in 96‐well culture plates (six replicates/group) in complete growth media and then allowed to attach overnight. The next day, cells were divided into different treatment groups, media was removed and cells were maintained on their respective serum‐free defined media containing various concentrations (0–30 μmol/L) of γ‐tocotrienol. Cells were fed fresh media every other day for a 4‐day treatment period. Afterwards, viable cell number in each well was determined using the 3‐(4,5‐dimethylthiazol‐2yl)‐2,5‐diphenyl tetrazolium bromide (MTT) colorimetric assay as described previously.17, 32, 33 Briefly, the media in various treatment groups was replaced with fresh media containing 0.5 mg/mL MTT. After a 4‐hour incubation period, media was removed and MTT crystals were dissolved in DMSO (100 μL/well). Optical density of each sample was measured at 570 nm on a microplate reader (SpectraCount; Packard BioScience Company, Meriden, CT, USA). The number of cells/well was calculated against a standard curve prepared by plating known cell densities, as determined by a haemocytometer, in triplicate at the beginning of each experiment.
2.5. Western blot analysis
At the end of a given experiment, MDA‐MB‐231 and T‐47D cells were isolated from each treatment group using trypsin, and then the whole cell lysates were prepared as previously described.34 Protein concentrations in each sample were determined using the Bio‐Rad protein assay kit (Bio‐Rad, Hercules, CA, USA). Equal amounts of protein (35 μg/lane) from each sample were then subjected to electrophoresis through SDS‐polyacrylamide minigels. Proteins from gels were transblotted at 30 V for 16–20 hours at 4°C onto a single polyvinylidene fluoride (PVDF) membrane (Dupont, Boston, MA, USA). Membranes were then blocked with 2% BSA in 10 mmol/L Tris‐HCl containing 50 mmol/L NaCl and 0.1% Tween 20, pH 7.4 (TBST), and then incubated with specific primary antibodies diluted 1:1000 to 1:3000 in TBST/2% BSA overnight at 4°C. The membranes were then washed five times with TBST and then incubated with their respective horseradish peroxide‐conjugated secondary antibodies diluted 1:5000 in TBST/2% BSA for 1 hour at room temperature. Membranes were then rinsed five times with TBST. Chemiluminescence reagent (Pierce, Rockford, IL, USA) was used according to the manufacturer's instructions for visualizing the protein bands. Images of protein bands from all treatment groups within an experiment were acquired using Syngene Imaging System (Fredrick, MD, USA). The visualization of α‐tubulin was used to ensure equal sample loading in each lane. Images of the protein bands were acquired and scanning densitometric analysis was performed with Kodak Molecular Imaging Software 4.5 (Carestream Health Inc., New Haven, CT, USA). All experiments were repeated at least three times, and representative Western blot images from each experiment are shown in the Figures.
2.6. Migration assay
The in vitro wound‐healing assay was used to quantify cell motility in two dimensions as previously described.27 Briefly, MDA‐MB‐231 and T‐47D cells were seeded at a density of 5 × 105 cells/well in sterile flat‐bottom 24‐well plates (six replicates/group) and allowed to attach overnight to form a subconfluent cell monolayer. Wounds were then scratched in each cell monolayer using a sterile plastic 200 μL micropipette tip. Medium was removed and cells were washed twice in sterile PBS and then again in fresh serum‐free medium to remove the unattached floating cells. Cells were then incubated in serum‐free media containing vehicle or approximate IC50 doses of γ‐tocotrienol and incubated for a 24‐hour period. Afterwards, media was removed and cells were washed in ice‐cold PBS, fixed in −20°C methanol, and stained with Giemsa reagent. Images were taken using a phase‐contrast Nikon Eclipse TS100 inverted microscope (Nikon Instruments Inc., Melville, NY, USA). Cell migration was determined by measuring the difference between wound width at the start of the experiment and after the 24‐hour treatment period. Values obtained were expressed as % migration. Each experiment was performed in triplicate and distance migrated was assessed in three randomly selected fields per treatment group.
2.7. Immunocytochemical fluorescence staining
MDA‐MB‐231 and T‐47D cells were initially seeded at a density of 3 × 104 cells/chamber in eight‐chamber glass culture slides (BD Falcon, San Jose, CA, USA) and allowed to attach overnight in complete growth media. Afterwards, cells were washed in PBS and incubated with their respective serum‐free defined treatment media for 4 days. The IC50 treatment doses of γ‐tocotrienol were determined to be 5 μmol/L and 4 μmol/L for MDA‐MB‐231 and T‐47D breast cancer cells, respectively. Afterwards, cells were washed in ice‐cold PBS, fixed in 4% formaldehyde/PBS for 6 minutes and permeabilized with 0.2% triton X‐100 in PBS for 6 minutes. Fixed cells were then washed in PBS and blocked with 5% goat serum in PBS for 1 hour at room temperature. Cells in each treatment group were then subdivided further and incubated with specific primary antibodies for cytokeratin 18 (1:200 dilution, 48 hours incubation), vimentin (1:250 dilution, 24 hours incubation) or total β‐catenin (1:200 dilution, 24 hours incubation) at 4°C in PBS containing 5% goat serum. Cells were then washed five times in ice‐cold PBS, and then incubated with Alexa Fluor‐488‐conjugated secondary antibody (1:2000) in PBS containing 5% goat serum for 1 hour at room temperature. All cells were then washed three times in ice‐cold PBS and mounted with Vectashield medium containing DAPI (Vector Laboratories Inc., CA, USA). The fluorescence images were captured using LSM Pascal confocal microscope (Carl Zeiss Microimaging Inc., Thornwood, NY, USA). The percentage of cells displaying positive labelling for cytokeratin 18, vimentin and total level of β‐catenin was determined by counting the number of positive staining cells as a proportion of total number of cells counted (stained with DAPI). Cells in every treatment group were counted manually in five photomicrographs taken randomly in each chamber as previously described.31
2.8. Statistical analysis
Differences between the groups were analysed by one‐way analysis of variance (ANOVA) followed by Dunnett's t test. A difference of P<.05 was considered statistically significant as compared to the vehicle‐treated control group or as defined in the figures legends. IC50 values (50% growth inhibition) were determined using non‐linear regression curve fit analysis using GraphPad Prism software version 5 (GraphPad Software, Inc., LaJolla, CA, USA).
3. Results
3.1. Effects of γ‐tocotrienol on the growth of normal and neoplastic human mammary epithelial cells
γ‐Tocotrienol treatment significantly inhibited the growth of MDA‐MB‐231 and T‐47D breast cancer cells in a dose‐response manner as compared to cells in their respective vehicle‐treated control group (Fig. 1). The IC50 values were determined to approximately 5 μmol/L and 4 μmol/L for MDA‐MB‐231 and T‐47D cells respectively. These IC50 doses were then used in the treatment of their respective breast cancer cells in subsequent experiments. However, treatment with higher doses of γ‐tocotrienol had little or no effect on growth of immortalized MCF‐10A normal mammary epithelial cells (Fig. 1). As the anti‐proliferative effects of γ‐tocotrienol was relatively selective for neoplastic, but not to normal mammary epithelial cells, subsequent experiments were conducted using only the MDA‐MB‐231 and T‐47D breast cancer cell lines.
Figure 1.
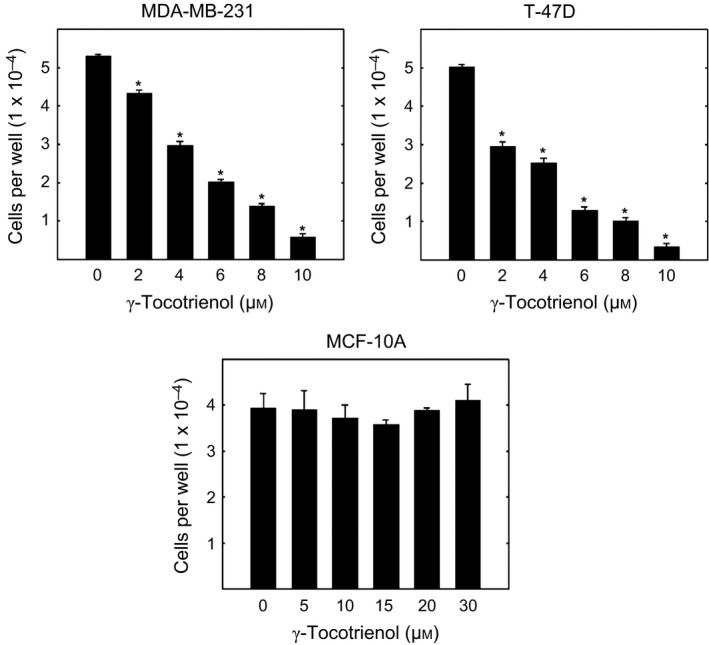
Anti‐proliferative effects of γ‐tocotrienol on MDA‐MB‐231, T‐47D and MCF‐10A human breast cancer cells. MDA‐MB‐231, T‐47D and MCF‐10A cells were initially plated at a density of 1 × 104, 5 × 103 and 1 × 104 cells/well (six wells/group), respectively, in 96‐well culture plates and maintained in serum‐free defined media containing 0–30 μmol/L γ‐tocotrienol. Viable cell number was determined using the MTT colorimetric assay. Vertical bars indicate mean cell number±SEM in each treatment group. *P<.05 as compared with their respective vehicle‐treated control group
3.2. Effects of γ‐tocotrienol on canonical Wnt/β‐catenin receptor and co‐receptor levels
Total levels of the FZD7 receptor, LRP6 co‐receptor and phosphorylated‐LRP6 (activated) were highly expressed in the vehicle‐treated MDA‐MB‐231 and T‐47D breast cancer cell lines (Fig. 2a). Treatment with γ‐tocotrienol induced a dose‐responsive decrease in FZD7, LRP6 and phosphorylated‐LRP6 protein levels, as compared to their respective vehicle‐treated MDA‐MB‐231 and T‐47D control groups (Fig. 2a). Scanning densitometric analysis of Western blots showed that treatment with 3–7 μmol/L (MDA‐MB‐231) and 4–6 μmol/L (T‐47D) γ‐tocotrienol induced a significant decrease in FZD7, LRP6 and phosphorylated‐LRP6 protein levels as compared to their respective vehicle‐treated control groups (Fig. 2b).
Figure 2.
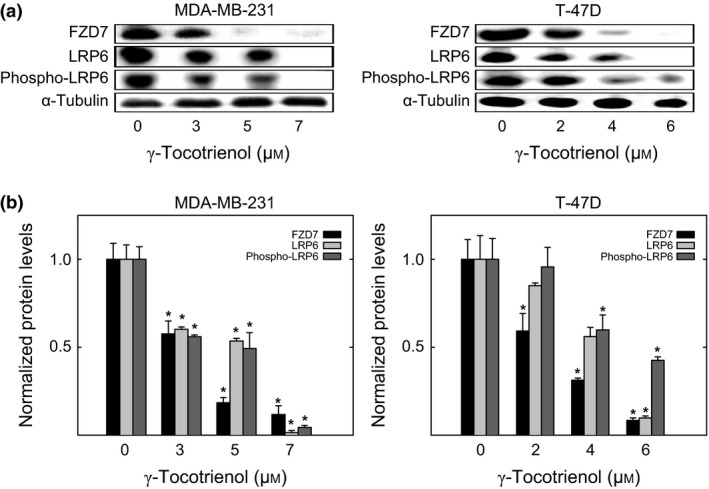
Western blot analysis of γ‐tocotrienol effects on Wnt/β‐catenin receptor/co‐receptor levels. (a) Whole cell lysates were prepared from cells in each treatment group for subsequent separation by polyacrylamide gel electrophoresis (35 μg/lane) followed by Western blot analysis for FZD7, LRP6 and phospho‐LRP6. (b) Scanning densitometric analysis was performed for each blot to visualize relative protein levels. Integrated optical density of each band was normalized with their corresponding α‐tubulin and control treatment bands. Vertical bars indicate the fold‐change in protein levels in each treatment groups±SEM as compared with their respective vehicle‐treated control group. *P<.05 as compared to their respective vehicle‐treated control group
3.3. Effects of γ‐tocotrienol on Wnt ligand levels
Wnt3a and Wnt5a/b are ligands that activate the Wnt/β‐catenin signalling pathway. Wnt3a and Wnt5a/b levels were relatively high in both MDA‐MB‐231 and T‐47D cells, and treatment with γ‐tocotrienol induced a dose‐responsive decrease in the levels of these ligands in both breast cancer cell lines (Fig. 3a). Scanning densitometric analysis of Western blots showed that treatment with 3–7 μmol/L (MDA‐MB‐231) and 2–6 μmol/L (T‐47D) γ‐tocotrienol induced a significant decrease in Wnt3a and Wnt5a/b protein levels as compared to their respective vehicle‐treated control groups (Fig. 3b).
Figure 3.
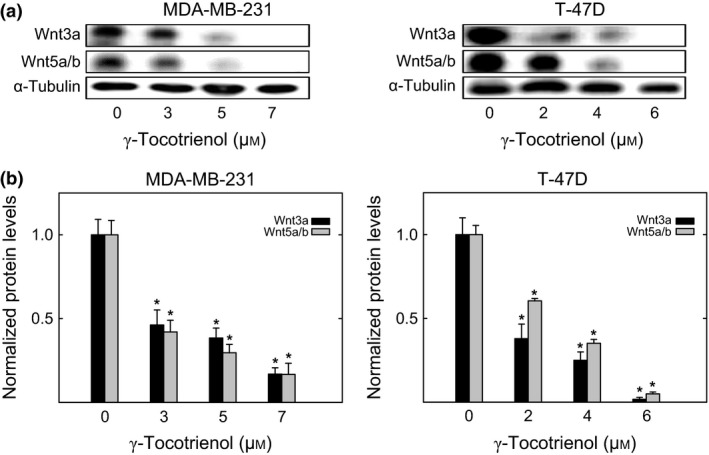
Effects of γ‐tocotrienol on Wnt ligand levels. (a) Whole cell lysates were prepared from cells in each treatment group for subsequent separation by polyacrylamide gel electrophoresis (35 μg/lane) followed by Western blot analysis for Wnt3a and Wnt5a/b. (b) Scanning densitometric analysis was performed for each blot to visualize relative protein levels. Integrated optical density of each band was normalized with their corresponding α‐tubulin and control treatment bands. Vertical bars indicate the fold‐change in protein levels in each treatment group±SEM as compared with their respective vehicle‐treated control group. *P<.05 as compared to their respective vehicle‐treated control group
3.4. Effects of γ‐tocotrienol on Wnt/β‐catenin pathway downstream signalling proteins
Western blot analysis shows that treatment with 3–7 μmol/L (MDA‐MB‐231) or 2–6 μmol/L (T‐47D) γ‐tocotrienol induced a dose‐dependent decrease in DVL2, DVL3 and cyclin D1 levels, and a corresponding increase in naked1 and naked2 (negative regulators of the Wnt/β‐catenin pathway) levels, as compared to cells in their respective vehicle‐treated control groups (Fig. 4a). These same treatments were also found to induce a dose‐responsive decrease in the cytosolic complex proteins axin1 and GSK3β, as compared to cells in their respective vehicle‐treated control groups (Fig. 4a). Scanning densitometric analysis of Western blots showed that γ‐tocotrienol treatment induced a significant and dose‐responsive decrease in the relative levels of DVL2, DVL3, axin1, GSK3β and cyclin D1, and corresponding significant and dose‐responsive increase in naked1 and naked2, as compared to cells in their respective MDA‐MB‐231 and T‐47D vehicle‐treated control groups (Fig. 4b).
Figure 4.
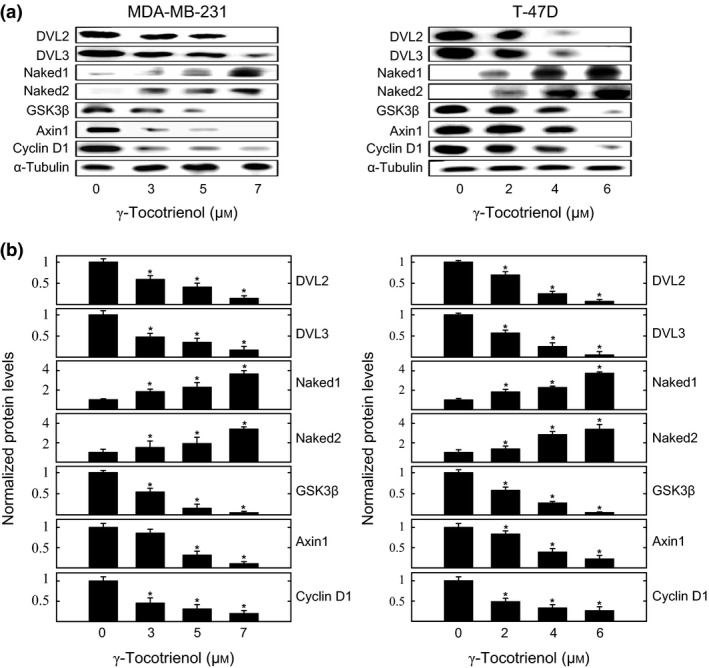
Effects of γ‐tocotrienol on Wnt/β‐catenin pathway downstream signalling protein levels. (a) Whole cell lysates were prepared from cells in each treatment group for subsequent separation by polyacrylamide gel electrophoresis (35 μg/lane) followed by Western blot analysis for DVL2, DVL3, naked1, naked2, GSK3β, axin1 and cyclin D1. (b) Scanning densitometric analysis was performed for each blot to visualize relative protein levels. Integrated optical density of each band was normalized with their corresponding α‐tubulin and control treatment bands. Vertical bars indicate the fold‐change in protein levels in various treatment groups±SEM as compared with their respective vehicle‐treated control group. *P<.05 as compared to their respective vehicle‐treated control group
3.5. Effects of γ‐tocotrienol on MDA‐MB‐231 and T‐47D breast cancer cell migration
Studies were conducted to investigate whether the inhibitory effects of γ‐tocotrienol treatment on Wnt/β‐catenin signalling is directly correlated with a reduction in breast cancer cell mobility, as demonstrated with the wound‐healing cell migration assay. Results showed nearly complete wound closure in both the MDA‐MB‐231 and T‐47D vehicle‐treated groups, whereas treatment with 5 μmol/L (MDA‐MB‐231) or 4 μmol/L (T‐47D) γ‐tocotrienol resulted in a large reduction in cell migration, after a 24‐hour incubation period (Fig. 5a). Quantitative analysis determined that γ‐tocotrienol treatment significantly inhibited migration by 50% and 33.3% for MDA‐MB‐231 and T‐47D breast cancer cells respectively (Fig. 5b).
Figure 5.
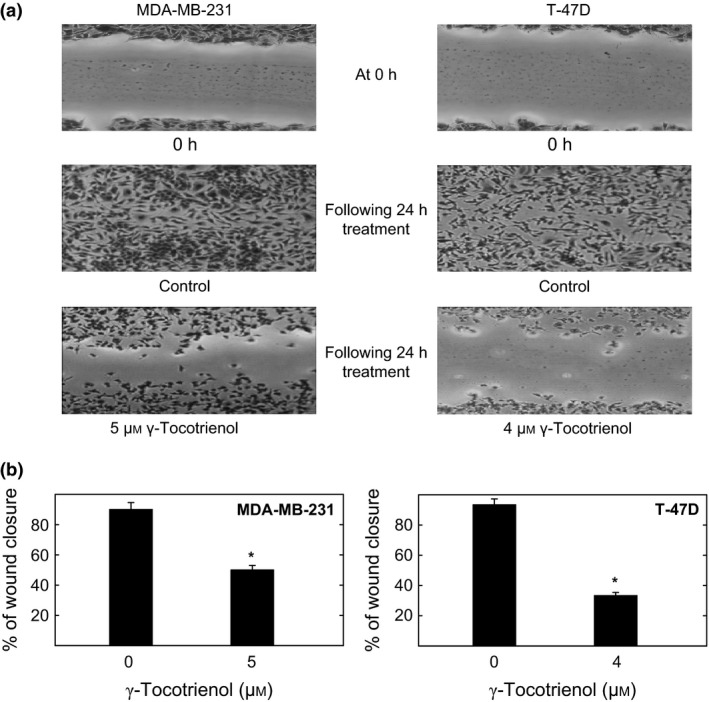
Photomicrographs of γ‐tocotrienol treatment effects on MDA‐MB‐231 and T‐47D breast cancer cell migration. (a) Cells were initially plated at a density of 5 × 105 cells/well in 24‐well plates (six replicates/group) and allowed cells to attach and form a subconfluent monolayer. A wound was scratched in each well using a sterile 200 μL micropipette and cells were exposed to their respective treatment treatments for a 24‐hour culture period. Photomicrographs (100× magnification) were taken at the beginning and end of the treatment period. (b) Quantitative analysis of wound closure in each treatment group was calculated relatively to wound distance at time zero. Vertical bar represents the per cent migration±SEM. Each experiment was performed in triplicate and the distance migrated was calculated in three randomly selected fields per treatment group. *P<.05 compared to their respective vehicle‐treated control group
3.6. Effects of γ‐tocotrienol on epithelial and mesenchymal cell marker expression
Western blot analysis shows that MDA‐MB‐231 and T‐47D cells in their respective vehicle‐treated control groups displayed relatively low levels of expression for the epithelial cell markers cytokeratin 8, cytokeratin 18 and E‐cadherin, and a corresponding high level of expression for the mesenchymal cell markers vimentin, fibronectin and total β‐catenin (Fig. 6a). Treatment with 3–7 μmol/L (MDA‐MB‐231) or 2–6 μmol/L (T‐47D) γ‐tocotrienol induced a dose‐responsive reversal in epithelial vs mesenchymal cell marker expression (Fig. 6a). Scanning densitometric analysis of protein bands in each blot shows that γ‐tocotrienol treatment resulted in a significant dose‐responsive increase in epithelial, and corresponding dose‐responsive decrease in mesenchymal cell marker levels, as compared to cells in their respective MDA‐MB‐231 or T‐47D vehicle‐treated control groups (Fig. 6b).
Figure 6.
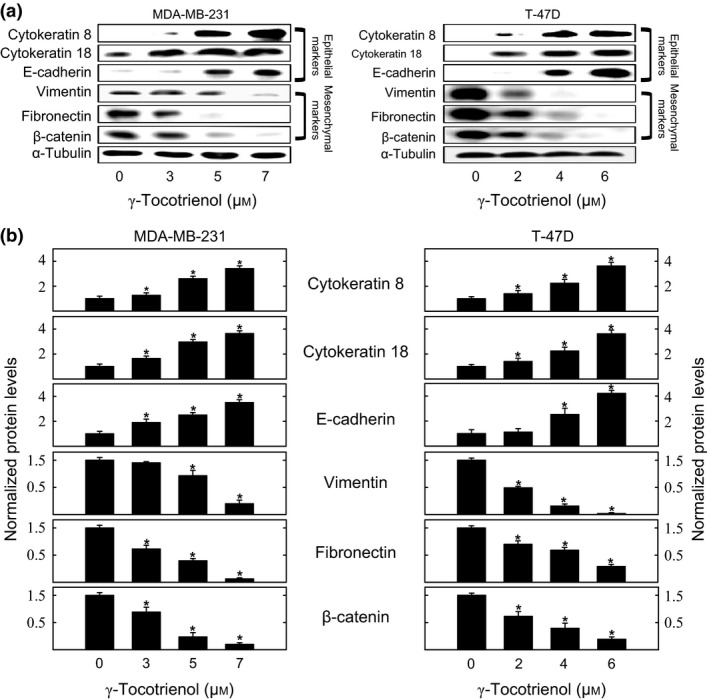
Effects of γ‐tocotrienol on epithelial vs mesenchymal cell markers expression. (a) Whole cell lysates were prepared from cells in each treatment group for subsequent separation by polyacrylamide gel electrophoresis (35 μg/lane) followed by Western blot analysis for cytokeratin 8, cytokeratin 18, E‐cadherin, fibronectin, vimentin and total level of β‐catenin. (b) Scanning densitometric analysis was performed for each blot to visualize relative protein levels. Integrated optical density of each band was normalized with their corresponding α‐tubulin and control treatment bands and then shown in bar graphs. Vertical bars indicate the fold‐change in protein levels in each treatment groups±SEM as compared with their respective vehicle‐treated control group. *P<.05 compared to their respective vehicle‐treated control group
3.7. Effects of γ‐tocotrienol on immunofluorescence staining of epithelial vs mesenchymal cell marker expression
MDA‐MB‐231 and T‐47D cells in their respective vehicle‐treated control groups displayed a relatively low level of positive immunofluorescence staining for the epithelial cell marker cytokeratin 18, and a relatively high level of positive immunofluorescence staining for the mesenchymal markers vimentin and β‐catenin (Fig. 7a). Treatment with 5 μmol/L or 4 μmol/L γ‐tocotrienol resulted in a reversal of positive immunofluorescence staining of epithelial vs mesenchymal cell markers in MDA‐MB‐231 and T‐47D breast cancer cells respectively (Fig. 7a). Image analysis of fluorescence photomicrographs shows that treatment with 5 μmol/L and 4 μmol/L T‐47D γ‐tocotrienol resulted in a significant increase in positive epithelial cell marker (cytokeratin 18) staining, and significant decrease in positive mesenchymal cell marker (vimentin and total β‐catenin) staining in MDA‐MB‐231 and T‐47D breast cancer cells respectively (Fig. 7b).
Figure 7.
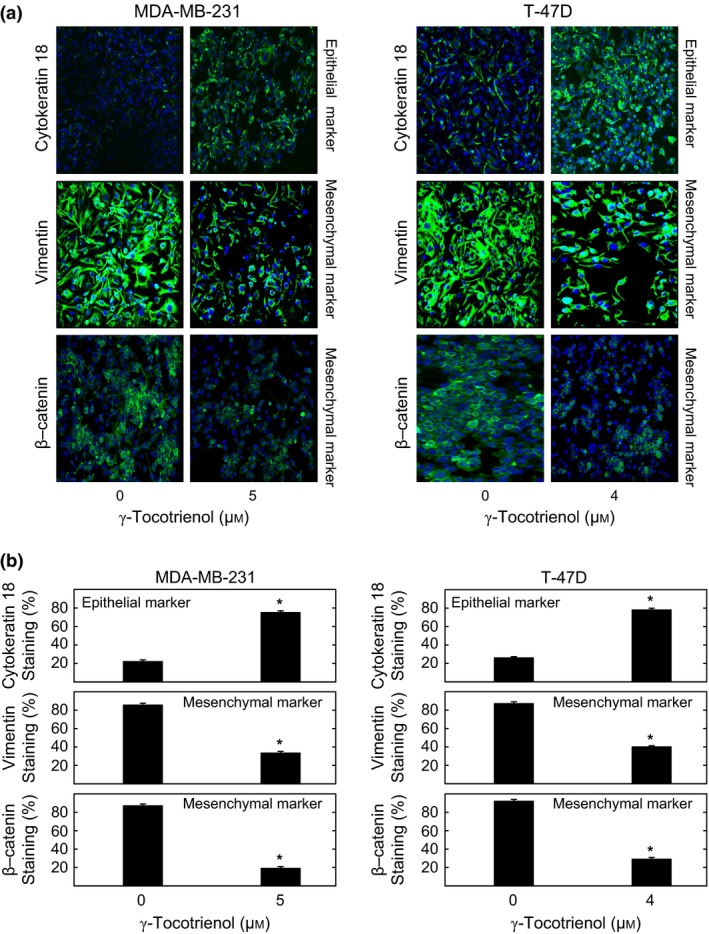
Effect of γ‐tocotrienol on immunocytochemical fluorescence staining of epithelial vs mesenchymal cell markers expression. (a) Cells in the various treatment groups were fixed, blocked and incubated with specific primary antibodies for cytokeratin 18, vimentin and total level of β‐catenin followed by incubation with Alexa Fluor 488‐conjugated secondary antibody. Green staining in the photomicrographs (magnification 200×) indicates positive fluorescence staining for target proteins and the blue colour represents counter staining of the cell nuclei with DAPI. (b) The percentage of cells displaying positive labelling for cytokeratin 18, vimentin and total level of β‐catenin was determined by counting the number of positive staining cells as a proportion of total number of cells counted (stained with DAPI). Vertical bars indicate the fold‐change in protein staining in each treatment groups±SEM as compared with their respective vehicle‐treated control group. *P<.05 compared to their respective vehicle‐treated control group
4. Discussion
Results in the present study demonstrate that γ‐tocotrienol‐induced anti‐cancer effects and reversal of EMT are associated with a significant reduction in Wnt/β‐catenin signalling in highly metastatic breast cancer cells. Following a 4‐day γ‐tocotrienol treatment period, MDA‐MB‐231 and T‐47D breast cancer cells displayed a dose‐dependent reduction in growth, cellular motility and reversal in EMT characterized by a significant reduction in the expression of mesenchymal cellular markers vimentin, fibronectin and total β‐catenin, and corresponding significant increase in the expression of epithelial cell markers cytokeratin 8, cytokeratin 18 and E‐cadherin. In addition, these γ‐tocotrienol‐induced anti‐cancer effects were also shown to be associated with a significant reduction in FZD7 receptor and LRP6 co‐receptor levels, a significant reduction in Wnt3a and Wnt5a/b ligand levels, and significant reduction in the expression of downstream Wnt/β‐catenin signalling proteins DVL2, DVL3, GSK3β, axin1 and cyclin D1, and corresponding increase in the expression of the Wnt/β‐catenin pathway inhibitory proteins naked1/2. Taken together, these findings provide strong evidence that the inhibition effects of γ‐tocotrienol on metastatic breast cancer cell proliferation and EMT may be mediated, at least in part, by a suppression in the Wnt/β‐catenin signalling pathway.
The present findings confirm and extend previous investigations that showed highly metastatic mouse mammary tumour cells were characterized by EMT as evidenced by the expression of high levels of mesenchymal cell markers and low levels of epithelial cell markers, and γ‐tocotrienol treatment induced a reversal in EMT.27 However, findings in the present study clearly demonstrate that γ‐tocotrienol‐induced reversal in EMT is associated with a significant suppression in Wnt/β‐catenin signalling. Although investigating Wnt/β‐catenin signalling can be complicated because of the possible interactions between at least 19 Wnt ligands, 10 frizzled receptors (FZDs) and 2 low‐density lipoprotein receptor‐related proteins (LRP5/6),9 Wnt3A and Wnt5a are two important ligands that activate the Wnt/β‐catenin signalling pathway by inducing β‐catenin translocation and accumulation in the nucleus.35, 36 Furthermore, blocking the activation of the FZD7 receptor using the recombinant soluble FZD7 peptide (sFZD7) has been shown to significantly decrease Wnt/β‐catenin signalling, and subsequent tumour cell proliferation and tumourigenesis.37 LRP5/6 co‐receptors are very similar in structure and both play an important role in Wnt‐dependent signal transduction.36
Studies have shown that aberrant activation of the Wnt/β‐catenin pathway plays a critical role in the development and progression of metastasis.38 Briefly, Wnt ligands activate FZD receptors and/or LRP co‐receptors to initiate downstream signalling.10, 39 The initial cytosolic proteins recruited and phosphorylated are DVL2/3, which acts to inhibit GSK3β and subsequently the formation of the cytosolic protein complex that consists of various proteins include axin, APC, CK1α and GSK3β.10, 38, 39 Inhibition of the formation of the β‐catenin destruction complex promotes the stabilization and translocation of β‐catenin into the nucleus and leads to an increase in the expression of Wnt target genes that include cyclin D, c‐Myc and matrix metalloproteinase‐7 (MMP7), which all play a role in increasing cell proliferation and motility.38, 40 Furthermore, naked1/2 are other proteins induced by the inhibition of Wnt signalling and act as through a negative feedback inhibition mechanism to inhibit DVL2/3 and promote the destruction of β‐catenin and cause a subsequent reduction in Wnt target gene expression.41 It is well established that β‐catenin can be found within cells in three different forms, including the form attached to the c‐terminal of E‐cadherin in the cell membrane, as well as the nuclear and cytoplasmic forms. The present study focused on β‐catenin expression in the entire cell as an established mesenchymal marker, which was significantly reduced following exposure to anti‐proliferative doses of γ‐tocotrienol. However, as the nuclear form of β‐catenin plays a role in regulating gene transcription, it is possible that the anti‐cancer action of γ‐tocotrienol may also involve inhibitory effects on nuclear β‐catenin and subsequent gene regulation and expression.42, 43 Additional studies are required to further explore this possibility.
Findings in the present study show that γ‐tocotrienol treatment resulted in a significant reduction in Wnt pathway FZD7 receptor and LRP6 co‐receptor levels, and activation (phosphorylated‐LRP6), as well as a large decrease in Wnt ligand (Wnt3a and Wnt5a/b) levels in MDA‐MB‐231 and T‐47D human breast cancer cells. These γ‐tocotrienol‐induced effects were also associated with a large reduction in downstream Wnt signalling proteins (DVL2/3), cytosolic protein complex proteins (GSK3β and axin1), β‐catenin and ultimately the expression of the Wnt pathway target gene protein (cyclin D1). In addition, γ‐tocotrienol treatment was found to significantly increase the overexpression of Wnt signalling pathway inhibitory proteins (naked1/2). These findings demonstrate that γ‐tocotrienol significantly inhibits Wnt/β‐catenin pathway signalling in metastatic breast cancer cells.
Epithelial‐to‐mesenchymal transition is characterized by a cell losing its epithelial characteristics and acquiring mesenchymal phenotypic traits that display migratory and metastatic characteristics that can be triggered by various stimuli including aberrant growth factor signalling, alterations in cellular microenvironment, cell‐to‐cell communication and/or hypoxia.44 Various signalling pathways are associated with the promotion of EMT including Wnt/β‐catenin, Hedgehog, Notch, MAPK, PI3K/Akt and integrins.45 Furthermore, a reduction in E‐cadherin levels within adherens junctions has also been shown to enhance the activation of β‐catenin leading to deregulation of Wnt/β‐catenin signalling and the increased expression of mesenchymal proteins such as vimentin that act to modify cellular morphology and motility.5, 45, 46, 47 Metastatic cancer cells have also been shown to remodel the extracellular matrix (ECM) by depositing high levels of fibronectin in the ECM, and these effects are directly associated with the promotion of EMT.45, 48, 49, 50 Cytokeratins are epithelium‐specific intermediate filament proteins that serve as a cellular marker for epithelial differentiation. EMT is characterized by the replacement of cytokeratins with vimentin, an intermediate filament‐protein found in mesenchymal cells.51 Results show that γ‐tocotrienol treatment induced a reversal in EMT characterized by an increase in cytokeratin 8/18 and E‐cadherin (epithelial markers) levels and corresponding decreases in the expression of fibronectin, vimentin and total β‐catenin (mesenchymal markers) in MDA‐MB‐231 and T‐47D breast cancer cell. Figure 8 summarizes the effects of γ‐tocotrienol on Wnt/β‐catenin signalling and the reversal of EMT in these highly metastatic breast cancer cell lines.
Figure 8.
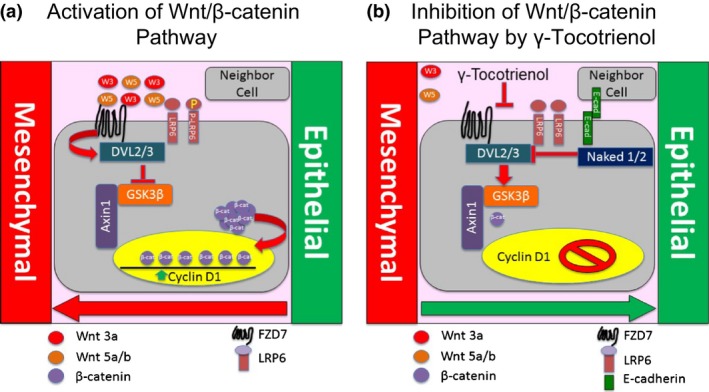
Schematic representation of γ‐tocotrienol effects on the Wnt/β‐catenin signalling and EMT. (a) Wnt/β‐catenin pathway is constitutively active in human breast cancer cell lines. Wnt3a and Wnt5a/b initiate Wnt‐induced FZD7/LRP6 complex activation, which then recruits DVL2/3 proteins and inhibits axin1‐GSK3β phosphorylation of β‐catenin. Consequently, β‐catenin phosphorylation and degradation is inhibited. These events bring about an accumulation and activation of β‐catenin to promote Wnt‐responsive genes expression associated with EMT. (b) γ‐Tocotrienol inhibits Wnt/β‐catenin signalling by decreasing the expression of Wnt3a and Wnt5a/b ligands, FZD7/LRP6 complex activation and a corresponding increase in naked1/2 levels resulting in a reduction in DVL2/3 recruitment. A reduction in DVL2/3 results in the activation of GSK3β and phosphorylation and subsequent degradation of β‐catenin in the cytosolic complex. These events ultimately lead to a reduction in β‐catenin accumulation and suppression gene expression associated with EMT
Lipid rafts within the plasma membrane are small areas primarily composed of cholesterol, glycosphingolipids and proteins, and many lipid raft proteins play a role in mediating receptor signal transduction.52, 53 Studies have shown that the anti‐cancer effects of γ‐tocotrienol are associated with its accumulation and disruption of the lipid raft integrity and suppression of receptor activation and signalling in SKBR3 and BT474 human breast cancer cells.28 As LRP6 is localized in the lipid raft domain,29 γ‐tocotrienol disruption of lipid raft integrity would explain a possible mechanism by which γ‐tocotrienol acts to inhibit Wnt/β‐catenin signalling. Additional studies are required to determine if this hypothesis is correct.
In conclusion, these findings demonstrate that γ‐tocotrienol‐induced inhibitory effects on MDA‐MB‐231 and T‐47D proliferation and EMT are associated with a suppression in canonical Wnt/β‐catenin signalling. These findings also suggest that γ‐tocotrienol therapy may provide some benefit in the treatment of highly malignant breast cancers that are characterized by aberrant canonical Wnt/β‐catenin signalling.
Conflict of interest
There is no conflict of interest.
Acknowledgements
This work was supported by grants from First Tech International Ltd. (Hong Kong) and the Louisiana Cancer Foundation. The authors thank the First Tech International Ltd. for generously providing γ‐tocotrienol for use in these studies. The authors also thank Dr. Karen P. Briski for her generous technical assistance and use of the laser confocal microscope.
References
- 1. Dougall WC, Qian X, Greene MI. Interaction of the neu/p185 and EGF receptor tyrosine kinases: implications for cellular transformation and tumor therapy. J Cell Biochem. 1993;53:61–73. [DOI] [PubMed] [Google Scholar]
- 2. Gao N, Xu H, Liu C, et al. Nestin: predicting specific survival factors for breast cancer. Tumour Biol. 2014;35:1751–1755. [DOI] [PubMed] [Google Scholar]
- 3. Flemban A, Qualtrough D. The potential role of hedgehog signaling in the luminal/basal phenotype of breast epithelia and in breast cancer invasion and metastasis. Cancers (Basel). 2015;7:1863–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium‐mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. [DOI] [PubMed] [Google Scholar]
- 5. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest. 2009;119:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozdamar B, Bose R, Barrios‐Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. [DOI] [PubMed] [Google Scholar]
- 7. Savagner P. The epithelial‐mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(suppl 7):vii89–vii92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Micalizzi DS, Farabaugh SM, Ford HL. Epithelial‐mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA‐MB‐231 xenograft growth. Breast Cancer Res. 2009;11:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clevers H, Nusse R. Wnt/beta‐catenin signaling and disease. Cell. 2012;149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 11. Clevers H. Wnt/beta‐catenin signaling in development and disease. Cell. 2006;127:469–480. [DOI] [PubMed] [Google Scholar]
- 12. Halbedl S, Kratzer MC, Rahm K, et al. Synthesis of novel inhibitors blocking Wnt signaling downstream of beta‐catenin. FEBS Lett. 2013;587:522–527. [DOI] [PubMed] [Google Scholar]
- 13. Bouteille N, Driouch K, Hage PE, et al. Inhibition of the Wnt/beta‐catenin pathway by the WWOX tumor suppressor protein. Oncogene. 2009;28:2569–2580. [DOI] [PubMed] [Google Scholar]
- 14. Murillo G, Peng X, Torres KE, Mehta RG. Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway. Cancer Prev Res (Phila). 2009;2:942–950. [DOI] [PubMed] [Google Scholar]
- 15. MacDonald BT, Tamai K, He X. Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laezza C, D'Alessandro A, Paladino S, et al. Anandamide inhibits the Wnt/beta‐catenin signalling pathway in human breast cancer MDA MB 231 cells. Eur J Cancer. 2012;48:3112–3122. [DOI] [PubMed] [Google Scholar]
- 17. McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224:292–301. [DOI] [PubMed] [Google Scholar]
- 18. Serbinova EA, Packer L. Antioxidant properties of alpha‐tocopherol and alpha‐tocotrienol. Methods Enzymol. 1994;234:354–366. [DOI] [PubMed] [Google Scholar]
- 19. McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids. 2000;35:171–180. [DOI] [PubMed] [Google Scholar]
- 20. Parajuli P, Tiwari RV, Sylvester PW. Anti‐proliferative effects of gamma‐tocotrienol are associated with suppression of c‐Myc expression in mammary tumour cells. Cell Prolif. 2015;48:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sylvester PW, Akl MR, Malaviya A, et al. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors. 2014;40:49–58. [DOI] [PubMed] [Google Scholar]
- 22. Bachawal SV, Wali VB, Sylvester PW. Combined gamma‐tocotrienol and erlotinib/gefitinib treatment suppresses Stat and Akt signaling in murine mammary tumor cells. Anticancer Res. 2010;30:429–437. [PubMed] [Google Scholar]
- 23. Bachawal SV, Wali VB, Sylvester PW. Enhanced antiproliferative and apoptotic response to combined treatment of gamma‐tocotrienol with erlotinib or gefitinib in mammary tumor cells. BMC Cancer. 2010;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parajuli P, Tiwari RV, Sylvester PW. Anticancer effects of gamma‐tocotrienol are associated with a suppression in aerobic glycolysis. Biol Pharm Bull. 2015;38:1352–1360. [DOI] [PubMed] [Google Scholar]
- 25. Shah SJ, Sylvester PW. Gamma‐tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp Biol Med (Maywood). 2005;230:235–241. [DOI] [PubMed] [Google Scholar]
- 26. Shirode AB, Sylvester PW. Synergistic anticancer effects of combined gamma‐tocotrienol and celecoxib treatment are associated with suppression in Akt and NFkappaB signaling. Biomed Pharmacother. 2010;64:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ayoub NM, Akl MR, Sylvester PW. Combined gamma‐tocotrienol and Met inhibitor treatment suppresses mammary cancer cell proliferation, epithelial‐to‐mesenchymal transition and migration. Cell Prolif. 2013;46:538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alawin OA, Ahmed RA, Ibrahim BA, Briski KP, Sylvester PW. Antiproliferative effects of gamma‐tocotrienol are associated with lipid raft disruption in HER2‐positive human breast cancer cells. J Nutr Biochem. 2016;27:266–277. [DOI] [PubMed] [Google Scholar]
- 29. Feng Q, Gao N. Keeping Wnt signalosome in check by vesicular traffic. J Cell Physiol. 2015;230:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plonka J, Latocha M, Kusmierz D, Zielinska A. Expression of proapoptotic BAX and TP53 genes and antiapoptotic BCL‐2 gene in MCF‐7 and T‐47D tumour cell cultures of the mammary gland after a photodynamic therapy with photolon. Adv Clin Exp Med. 2015;24:37–46. [DOI] [PubMed] [Google Scholar]
- 31. Tiwari RV, Parajuli P, Sylvester PW. gamma‐Tocotrienol‐induced autophagy in malignant mammary cancer cells. Exp Biol Med (Maywood). 2014;239:33–44. [DOI] [PubMed] [Google Scholar]
- 32. Shah S, Gapor A, Sylvester PW. Role of caspase‐8 activation in mediating vitamin E‐induced apoptosis in murine mammary cancer cells. Nutr Cancer. 2003;45:236–246. [DOI] [PubMed] [Google Scholar]
- 33. Tiwari RV, Parajuli P, Sylvester PW. Synergistic anticancer effects of combined gamma‐tocotrienol and oridonin treatment is associated with the induction of autophagy. Mol Cell Biochem. 2015;408:123–137. [DOI] [PubMed] [Google Scholar]
- 34. Wali VB, Sylvester PW. Synergistic antiproliferative effects of gamma‐tocotrienol and statin treatment on mammary tumor cells. Lipids. 2007;42:1113–1123. [DOI] [PubMed] [Google Scholar]
- 35. Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P‐cadherin, beta‐catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–8614. [DOI] [PubMed] [Google Scholar]
- 36. He X, Semenov M, Tamai K, Zeng X. LDL receptor‐related proteins 5 and 6 in Wnt/beta‐catenin signaling: arrows point the way. Development. 2004;131:1663–1677. [DOI] [PubMed] [Google Scholar]
- 37. Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled‐7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai J, Guan H, Fang L, et al. MicroRNA‐374a activates Wnt/beta‐catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple‐negative breast cancer cells. J Transl Med. 2013;11:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Z, Lu P, Zhang H, et al. Nestin positively regulates the Wnt/beta‐catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Raay TJ, Fortino NJ, Miller BW, et al. Naked1 antagonizes Wnt signaling by preventing nuclear accumulation of beta‐catenin. PLoS ONE. 2011;6:e18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prasad CP, Gupta SD, Rath G, Ralhan R. Wnt signaling pathway in invasive ductal carcinoma of the breast: relationship between beta‐catenin, dishevelled and cyclin D1 expression. Oncology. 2007;73:112–117. [DOI] [PubMed] [Google Scholar]
- 43. Xu W, Du M, Zhao Y, Wang Q, Sun W, Chen B. gamma‐Tocotrienol inhibits cell viability through suppression of beta‐catenin/Tcf signaling in human colon carcinoma HT‐29 cells. J Nutr Biochem. 2012;23:800–807. [DOI] [PubMed] [Google Scholar]
- 44. Foroni C, Broggini M, Generali D, Damia G. Epithelial‐mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev. 2012;38:689–697. [DOI] [PubMed] [Google Scholar]
- 45. Abdel Bar FM, Khanfar MA, Elnagar AY, et al. Design and pharmacophore modeling of biaryl methyl eugenol analogs as breast cancer invasion inhibitors. Bioorg Med Chem. 2010;18:496–507. [DOI] [PubMed] [Google Scholar]
- 46. Demirkan B. The roles of epithelial‐to‐mesenchymal transition (EMT) and mesenchymal‐to‐epithelial transition (MET) in breast cancer bone metastasis: potential targets for prevention and treatment. J Clin Med. 2013;2:264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Katsumoto T, Mitsushima A, Kurimura T. The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer‐graphic reconstruction. Biol Cell. 1990;68:139–146. [DOI] [PubMed] [Google Scholar]
- 48. Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wright JA, Richer JK, Goodall GJ. microRNAs and EMT in mammary cells and breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:213–223. [DOI] [PubMed] [Google Scholar]
- 51. Cimpean AM, Suciu C, Ceausu R, Tatucu D, Muresan AM, Raica M. Relevance of the immunohistochemical expression of cytokeratin 8/18 for the diagnosis and classification of breast cancer. Rom J Morphol Embryol. 2008;49:479–483. [PubMed] [Google Scholar]
- 52. Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. [DOI] [PubMed] [Google Scholar]
- 53. Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(suppl):S323–S328. [DOI] [PMC free article] [PubMed] [Google Scholar]


